Abstract
Laparoscopic surgery is a good choice for surgical treatment of hiatal hernia because of its mini-invasive nature and intraperitoneal view and operating angle. This article will talk about the surgical procedures, technical details, precautions and complications about laparoscopic hernioplasty of hiatal hernia.
Keywords: Laparoscopic hernioplasty, hiatal hernia, mesh, fundoplication
Introduction
Laparoscopic hernioplasty of hiatal hernia has been confirmed effective and safe in recent years and performed more due to its mini-invasive nature and intraperitoneal view and operating angle (1,2). However, it is still a kind of difficult surgery in the field of hernia surgery that requires a good understanding of the surrounding anatomy of the gastroesophageal junction and esophageal hiatus as well as precise operation (3). Sufficient dissection and exposure of the operating field, application of mesh and fundoplication are some key issues in these operations (4-11).
Classification of hiatal hernia
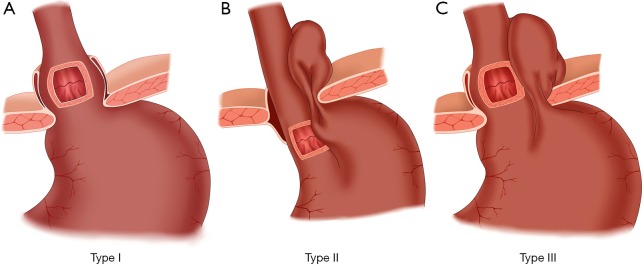
The classification of a hiatal hernia is essential in the diagnosis and treatment. According to the 2013 American Gastrointestinal Endoscopic Surgery Society guidelines, hiatal hernias can be divided into four types (Figure 1).
Figure 1.
Classification of hiatal hernia.
Type I: sliding hiatal hernias are the most common type in clinics and account for 95% of all hiatal hernias. The gastroesophageal junction of this type of hernia moves up to the chest, usually with a small hiatus, and the hernia can slide up and down. The hernia occurs in the supine position and disappears while standing. Because there is no defect in the phrenic esophageal ligament covering the hiatus and the lower esophagus, there is usually no true hernial sac. Due to a loose phrenic esophageal ligament, the subphrenic esophageal segment and the cardiac end slide out through the esophageal hiatus and into the chest, which causes the normally acute esophagus—gastroesophageal junction angle (angle of his) to become an obtuse angle, leading to the destruction of the normal anti-reflux mechanism of the lower esophagus. Therefore, this type usually has complications of varying degrees of gastroesophageal reflux.
Type II: paraesophageal hiatal hernias are rare. The gastroesophageal junction is still located below the diaphragm, while part of the gastric fundus or gastric body enters into the chest through the weak point of an enlarged esophageal hiatus. Due to the presence of esophageal ligament diaphragmatic defects, this type usually has a complete hernial sac. The subphrenic esophageal segment and esophagus—stomach transition angle remains in the normal anatomical position and plays the normal role of a physiological sphincter. The anti-reflux mechanism is not damaged; therefore, gastroesophageal reflux rarely occurs with this type. Approximately one-third of large paraesophageal hiatal hernias are prone to incarceration.
Type III: mixed hiatal hernias involve the coexistence of the previous two types. The previous two types of hernia could develop into a mixed hiatal hernia in later stages. A type III hernia has the gastroesophageal junction and the greater curvature side of the gastric fundus shifted to be located on the diaphragm. The part herniated into the stomach is large and can be one-third or one-half of the stomach. This type of hernia often has acute abdominal symptoms, such as incarceration, strangulation and perforation.
Type IV: a giant hernia not only has the stomach herniated into the chest but also has other abdominal internal organs inside the hernial sac, including omentum, colon and small intestine.
Some scholars combine type III and IV hernias into one type, which is collectively called a mixed hernia. These types account for the greatest number of hernias except type I hernias (95% of the remaining 5%), while the true type II hernia is very rare. The common type I hernia exhibits greatly different clinical manifestations, laboratory findings and treatment principles compared with type II, III and IV hernias.
Indications
❖ Patients with type II, III, or IV hernia accompanied by symptoms;
❖ Type I hernia with severe symptoms affecting everyday life, symptoms that are refractory to medical therapy or the patient cannot tolerate adverse drug reactions;
❖ Type I hernia that responds to medical therapy, but the symptoms repeat if medication is discontinued, and the patient does not want long-term medication therapy;
❖ Type I hernia that already exhibits serious complications of gastroesophageal reflux, including (I) esophagitis above grade B (Los Angeles classification); (II) severe esophageal stenosis and bleeding, etc.; and (III) serious lesions external to the gastrointestinal tract, such as aspiration pneumonia and asthma.
Contraindications
❖ Type II, III, or IV hernia without any symptoms;
❖ Type I hernia without any symptoms or mild symptoms that can be controlled with acid-suppressing drugs;
❖ The patient has combined heart and lung disease and cannot tolerate general anesthesia;
❖ History of abdominal surgery or severe abdominal adhesions.
Surgical instruments and materials
Ultrasonic dissector, laparoscopic surgical liver retractor, antiblocking mesh or biological mesh, and tack fixation device.
Preoperative preparation
❖ Anemia and hypoalbuminemia should be corrected, and water and electrolyte balance should be regulated;
❖ Give laxatives, such as lactulose, before the operation to drain the stool.
Anesthesia
General anesthesia with endotracheal intubation.
Surgical positioning and trocar placement
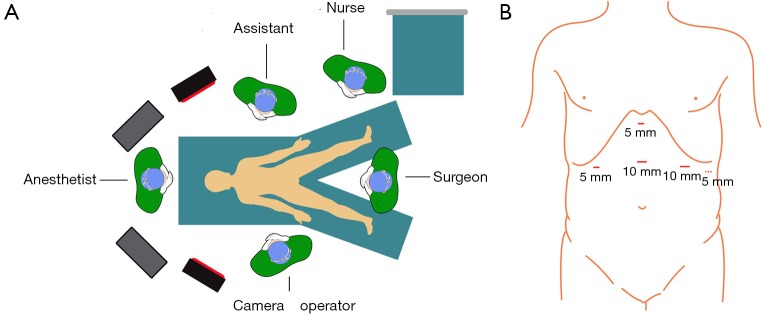
An indwelling urine catheter after anesthesia as well as an indwelling gastric tube should be placed. Patients are in a supine position with both legs separated; Trendelenburg shows a 30 degree angle. A 10-mm trocar is placed at the midpoint between the xiphoid and the umbilicus; the laparoscope is positioned. A 5-mm trocar is placed under the xiphoid and used as a liver retractor placement hole. A 5-mm trocar is placed at a 2-fingers-width distance below the costal margin of the right upper quadrant mid-clavicular line and used as the auxiliary operation hole. A 10-mm trocar is placed at a 2-fingers-width distance below the costal margin of the left upper quadrant mid-clavicular line and used as the major operation hole. If necessary, one 5-mm trocar can be added at the left anterior axillary. The surgeon stands between the legs of the patient; the assistant stands on the left side of the patient, and the camera operator stands on the right side of the patient (Figure 2).
Figure 2.
Surgical positioning and trocar placement. (A) Operating room layout; (B) trocar positions.
Surgical anatomy (Figures 3,4)
Figure 3.
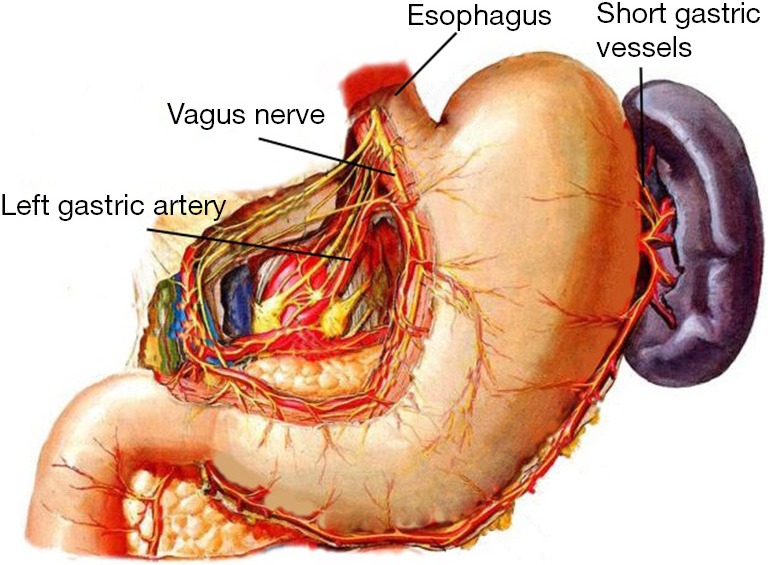
Anatomy of the gastroesophageal junction and adjacent organs.
Figure 4.
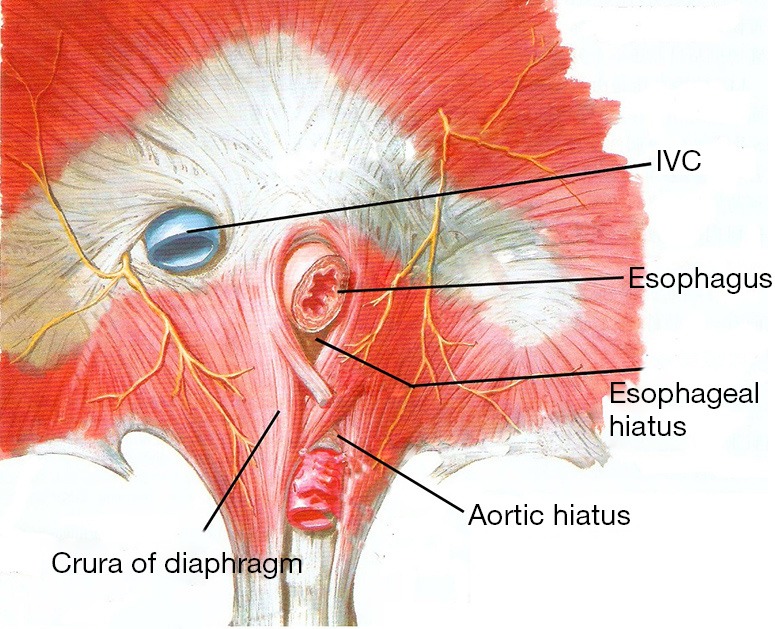
Anatomy around the esophageal hiatus.
It is very important to know anatomic acknowledge around esophageal hiatus before performance of laparoscopic repair of hiatal hernia. The aorta position is especially important when the mesh is fixed with tacks.
Surgical procedure (Figure 5)
Figure 5.

Laparoscopic repair of hiatal hernia and Nissen fundoplication (Chief Surgeon: Qiyuan Yao) (12). Available online: http://www.asvide.com/articles/1148
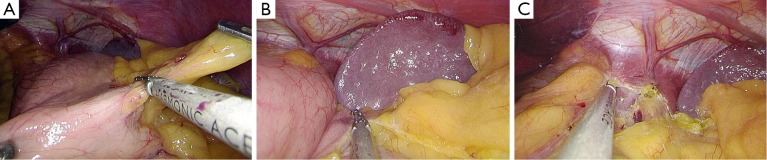
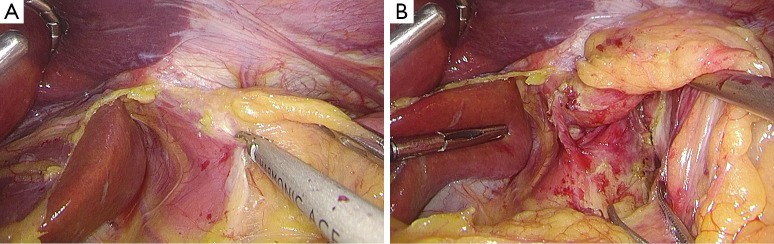
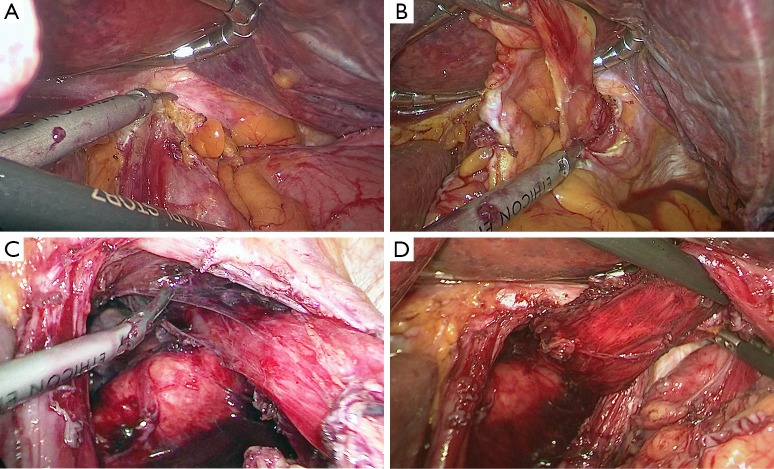
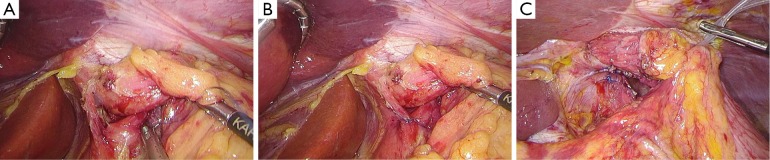
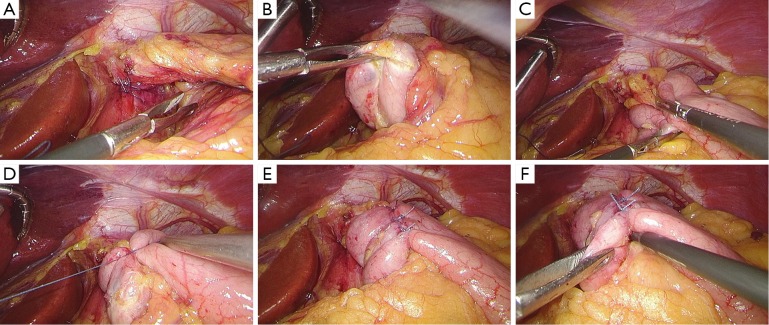
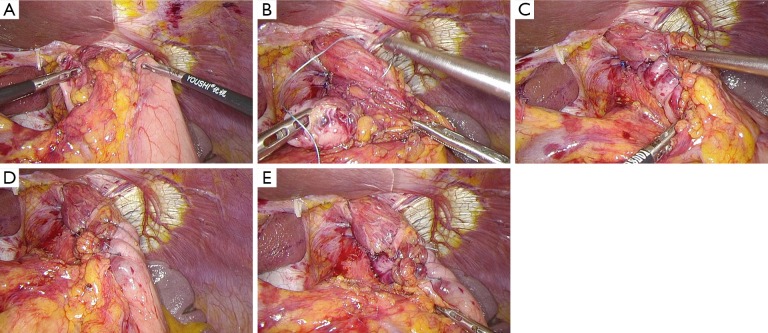
After establishing pneumoperitoneum, the liver retractor blocks the liver to observe the esophageal hiatus. Reduce the hernia contents back to a satisfactory position as much as possible (Figure 6). Use an ultrasonic dissector to disarticulate the left gastroepiploic artery, gastric wall blood vessels and the short gastric vessels starting from the greater curvature of the stomach. Open the left phrenic esophageal ligament, and completely mobilize the gastric fundus, revealing the left crura diaphragmatis (Figure 7). Then, dissect the hepatogastric ligament along the edge of the liver; dissect the right phrenicoesophageal ligament, revealing the right crura diaphragmatis (Figure 8). Mobilize the right crura diaphragmatis, and expose the diaphragmatic bilateral interchange behind the esophagus (Figure 9). Dissect the phrenicoesophageal ligament in front of the abdominal esophagus; mobilize the hernial sac through the esophageal hiatus and resect; reduce the hernia contents. Mobilize the esophagus in the mediastinum; make certain that the ventral esophagus length is at least 3 cm; protect the vagus nerve (Figure 10). Lift the esophagus to the left; perform interrupted sutures of the bilateral crura diaphragmatis with non-absorbable thread behind the esophagus; narrow the esophageal hiatus to 1.5–2 cm (Figure 11). If the crura diaphragmatis are obviously weak or the esophageal hiatus diameter is >5 cm, the repair can be strengthened by mesh. The mesh may be stapled or sutured. Currently, it is usually recommended to strengthen the crura using meshes after suturing to narrow the hiatus (Figure 12). Next, perform fundoplication; the short, loose-type 360° Nissen fundoplication is most commonly used. Use the no-damage grip clamp to hold the gastric fundus around the right side of the esophagus via the rear esophagus, generally with 2–3 sutures. One proximal suture is fixed to the esophageal muscular layer. After fundoplication, examine the folding loop to allow the separation clamp to pass through (Figure 13). Other fundoplication methods include Toupet fundoplication (270° fundoplication) and Dor fundoplication (180° fundoplication). In Toupet fundoplication (270° fundoplication), the gastric fundus does not completely encircle the esophagus; make 3–4 sutures each for both sides of the front esophageal wall (Figure 14). Dor fundoplication (180° fundoplication) uses the gastric fundus to cover the abdominal esophagus from the front esophagus, and 3–4 sutures are placed between the esophagus and gastric fundus. These two fundoplication methods can also be used in appropriate patients. A drainage tube can be placed through the infrahepatic area to the splenic fossa and drawn from the right upper quadrant trocar hole. Alternatively, a drainage tube may not need to be placed. Finally, observe and ensure that there is no bleeding in the surgical field or each trocar hole; close the incisions (Figure 15).
Figure 6.
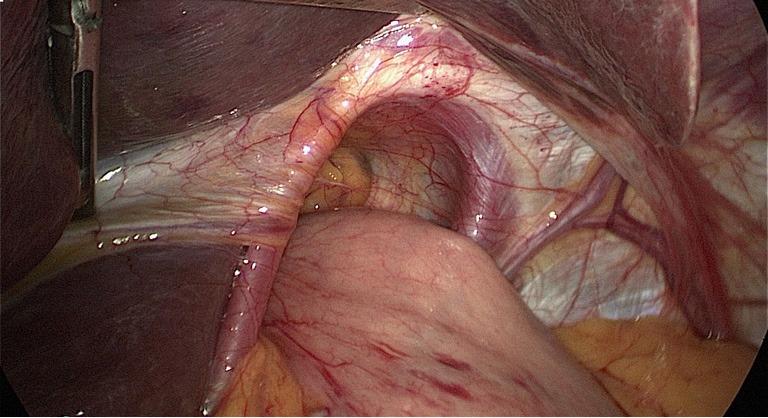
Observe the esophageal hiatus and reduce the hernia contents.
Figure 7.
Starting from the greater curvature of the stomach, the left gastroepiploic artery and the short gastric vessels were transected to expose the left crura diaphragmatis.
Figure 8.
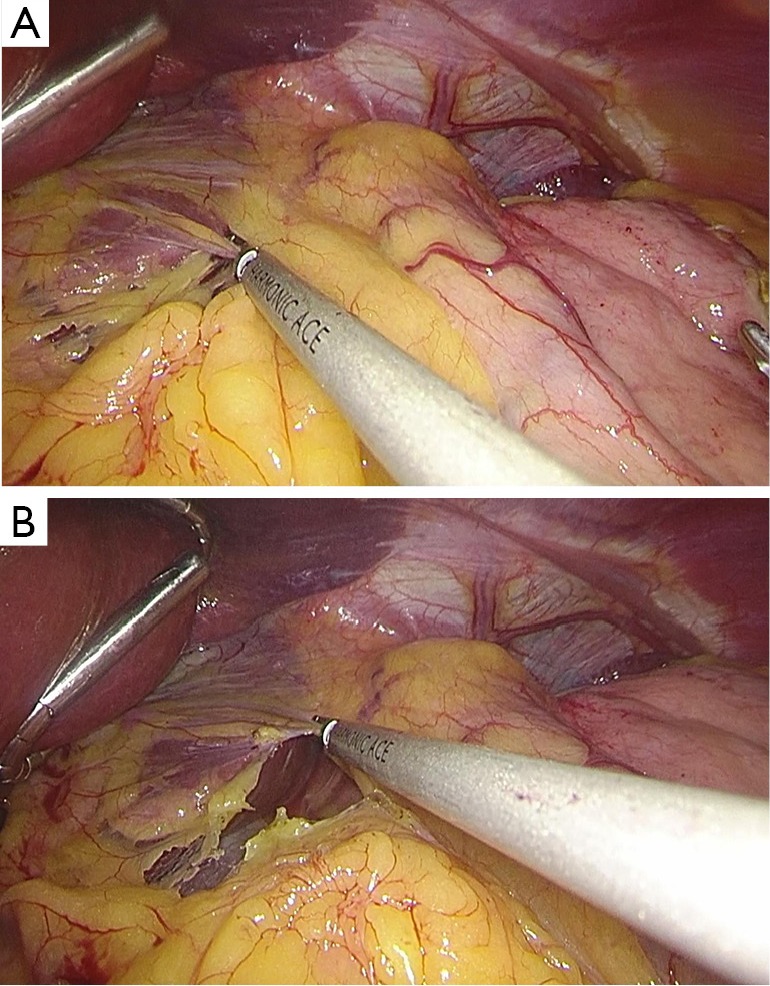
The hepatogastric ligament was cut to expose the right crura diaphragmatis.
Figure 9.
The interchange of bilateral crura diaphragmatis was exposed.
Figure 10.
Mobilize and try to remove the sac, and mobilize the abdominal esophagus and ensure a length of at least 3 cm.
Figure 11.
Interrupted sutures of the bilateral crura diaphragmatis.
Figure 12.
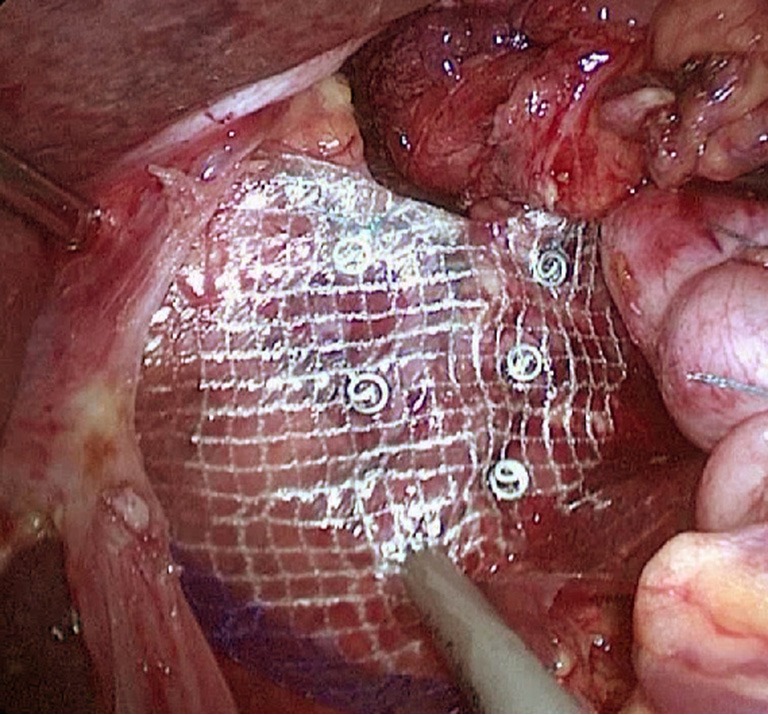
Mesh repair.
Figure 13.
Short, loose-type 360° Nissen fundoplication.
Figure 14.
Toupet fundoplication (270° fundoplication).
Figure 15.
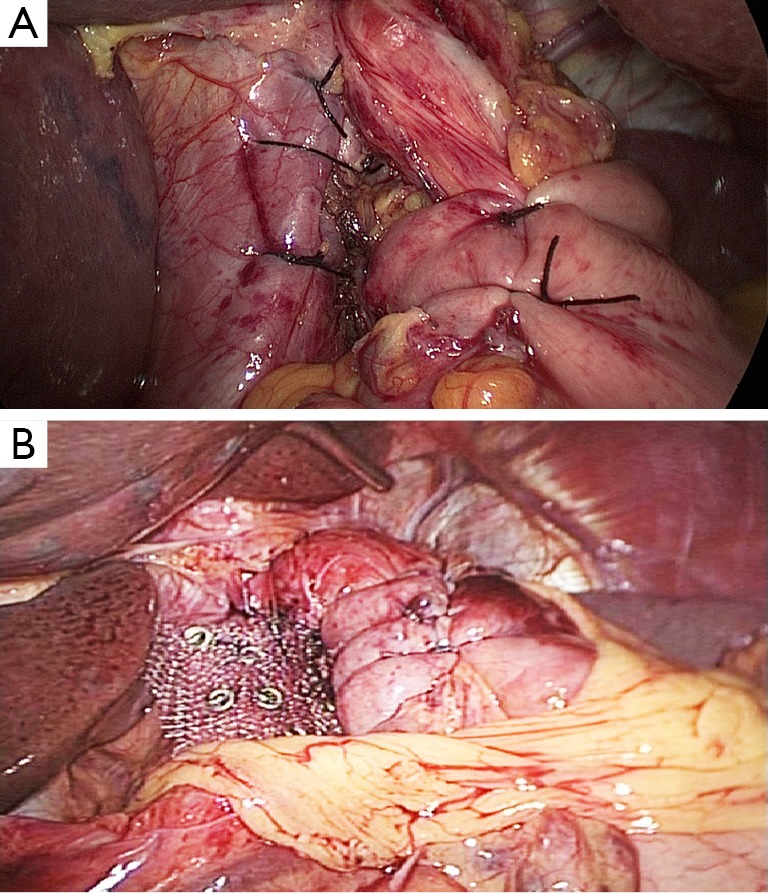
View inside the abdominal cavity after surgery.
Key point analysis
The most important step in the laparoscopic hiatal hernia repair is to reveal bilateral crura diaphragmatis. The enlarged esophageal hiatus is repaired and reconstructed to the appropriate size. If the crura are particularly weak or the esophageal hiatus is too large, the tension of a simple interrupted suture is often too high, and the hernia is prone to postoperative recurrence. A mesh repair is required for these situations. A mesh repair involves strengthening the repair based on the crura suture instead of the bridge repair. The mesh should use anti-adhesion composite materials or a bio-mesh to avoid causing postoperative mesh-related complications. In addition, the part of the mesh near the esophagus should not be trimmed in a manner that produces rough edges causing erosion of the esophagus. The size of the esophageal hiatus after the reconstruction should be approximately 1.5–2 cm. If the hiatus is too large, it is easy to have a recurrence; if it is too small, it may cause postoperative eating difficulties.
After the hiatal hernia repair, a fundoplication should be routinely performed because the surgery itself can damage the support structure around the gastroesophageal junction, thereby causing or aggravating gastroesophageal reflux disease. If a short, loose-type 360° Nissen fundoplication is performed, always start from the left side to disarticulate all the short gastric vessels. With this approach, the tension during fundoplication is not too high. If Toupet fundoplication or Dor fundoplication is performed, the procedure can also be started from the right side, but try to maintain the short gastric vessels. Because these two fundoplication methods do not break blood vessels and the gastric fundus tension is often not large, fundoplication can be completed. A preferred fundoplication method is not yet conclusive. However, short, loose-type 360° Nissen fundoplication is currently more commonly used. Preoperative or intraoperative esophageal manometry data will help guide the fundoplication method choice.
Precautions
The patient must take a supine position with both lower limbs separated, which is conducive for the operating surgeon. The surgeon should stand between the patient’s legs; both separations and sutures are thus very convenient. Due to the high position of the surgical field and the blockage of ribs, both open transthoracic and abdominal surgeries have difficulties in exposure. The difficulty of the operation is great.
Laparoscopic hiatal hernia repair has advantages of good visibility as well as operative space. Trendelenburg is more helpful for exposure and suturing.
The vagus nerve should be identified and protected during the surgery to minimize postoperative bloating, belching and other symptoms as well as to avoid gastroparesis. The free portion of the abdominal esophagus should be long enough so that the loop can be folded in the correct position, reducing postoperative hernia recurrence.
The hernial sac should be removed. However, when the sac is very large, forced separation and removal can easily cause pleural injury. In these cases, if it does not affect the crura diaphragmatic sutures or fundoplication, part of the sac can be retained.
Postoperative care
Analgesia: postoperative pain is often mild, and no special treatment is required. Give pain medication as necessary.
Diet: the patient may begin taking small amounts of water at 6 hours after the operation; gradually transition to a liquid and semi-liquid food diet. Because early postoperative consumption of an obstruction is commonly seen in patients, it is generally recommended to have a semi-liquid or soft-food-based diet in the first month after surgery and to avoid both eating too fast and eating large pieces of food. If the patients can smoothly transition to a semi-liquid diet, they can be discharged.
Complications and treatment
Intraoperative complications
Bleeding: properly handle the short gastric vessels during surgery. Pay attention to protect the spleen; otherwise, it can cause uncontrolled bleeding. If bleeding occurs, promptly convert to laparotomy. The removal of the spleen is even sometimes required.
Thoracic organ injuries: screw nails are recommended for fixed meshes to avoid puncturing the diaphragm and damaging thoracic organs. If not certain, it is safer to suture.
Abdominal organ injuries: apart from the spleen, which may become damaged when mobilize the gastric fundus, most abdominal organ injuries occur when the hernial contents are reduced or while withdrawing the stomach and the esophagus. Be sure to use gentle movements during the operation. When the anatomical structure is not clear, blunt dissection should be used, avoiding sharp separation and directly damaging organs.
Pneumothorax: pleural rupture is commonly seen during surgery. Usually, thoracic drainage is not required. Only positive pressure ventilation to achieve lung recruitment is necessary at the end of surgery. A large pleural rupture can be sutured.
Postoperative complications
Recurrence: the hiatal hernia recurrence rate is much higher than other common abdominal hernias, such as inguinal hernias and incisional hernias. If a postoperative type I hernia recurs without symptoms, patients can follow-up. If the recurrence causes significant obstruction and reflux symptoms, then reoperation is necessary. For experienced doctors, the surgery can be performed again via laparoscopy.
Difficulty in eating: more than half of the patients have difficulty in eating within the first month after the surgery; most patients can relieve themselves. Less than 5% of the patients still have difficulty in eating 6 months after the surgery. Very few patients need dilation or reoperation. However, corrective surgery requires careful consideration. There must be objective evidence, and factors associated with the mental disturbance of patients should be excluded.
Acknowledgements
Funding: Shenzhen government funding for scientific and technical research and development (JCYJ20140414092023238).
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Stylopoulos N, Rattner DW. The history of hiatal hernia surgery: from Bowditch to laparoscopy. Ann Surg 2005;241:185-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. 10.1007/s00464-013-3173-3 [DOI] [PubMed] [Google Scholar]
- 3.Altorki NK, Yankelevitz D, Skinner DB. Massive hiatal hernias: the anatomic basis of repair. J Thorac Cardiovasc Surg 1998;115:828-35. 10.1016/S0022-5223(98)70363-0 [DOI] [PubMed] [Google Scholar]
- 4.Tam V, Winger DG, Nason KS. A systematic review and meta-analysis of mesh vs suture cruroplasty in laparoscopic large hiatal hernia repair. Am J Surg 2016;211:226-38. 10.1016/j.amjsurg.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson DI, Thompson SK, Devitt PG, et al. Laparoscopic repair of very large hiatus hernia with sutures versus absorbable mesh versus nonabsorbable mesh: a randomized controlled trial. Ann Surg 2015;261:282-9. 10.1097/SLA.0000000000000842 [DOI] [PubMed] [Google Scholar]
- 6.Müller-Stich BP, Achtstätter V, Diener MK, et al. Repair of Paraesophageal Hiatal Hernias—Is a Fundoplication Needed? A Randomized Controlled Pilot Trial. J Am Coll Surg 2015;221:602-10. 10.1016/j.jamcollsurg.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 7.Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg 2011;213:461-8. 10.1016/j.jamcollsurg.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 8.Koch OO, Asche KU, Berger J, et al. Influence of the size of the hiatus on the rate of reherniation after laparoscopic fundoplication and refundopilication with mesh hiatoplasty. Surg Endosc 2011;25:1024-30. 10.1007/s00464-010-1308-3 [DOI] [PubMed] [Google Scholar]
- 9.Braghetto I, Korn O, Csendes A, et al. Postoperative results after laparoscopic approach for treatment of large hiatal hernias: is mesh always needed? Is the addition of an antireflux procedure necessary? Int Surg 2010;95:80-7. [PubMed] [Google Scholar]
- 10.Targarona EM, Bendahan G, Balague C, et al. Mesh in the hiatus: a controversial issue. Arch Surg 2004;139:1286-96; discussion 1296. 10.1001/archsurg.139.12.1286 [DOI] [PubMed] [Google Scholar]
- 11.Watson DI, Jamieson GG, Devitt PG, et al. A prospective randomized trial of laparoscopic Nissen fundoplication with anterior vs posterior hiatal repair. Arch Surg 2001;136:745-51. 10.1001/archsurg.136.7.745 [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Hua R, Yao Q, et al. Laparoscopic repair of hiatal hernia and Nissen fundoplication (Chief Surgeon: Qiyuan Yao). Asvide 2016;3:384. Available online: http://www.asvide.com/articles/1148