Abstract
Non-invasive ventilation (NIV) has assumed an important role in the management of acute respiratory failure (ARF). NIV, compared with standard medical therapy, improves survival and reduces complications in selected patients with ARF. NIV represents the first-line intervention for some forms of ARF, such as chronic obstructive pulmonary disease (COPD) exacerbations and acute cardiogenic pulmonary edema. The use of NIV is also well supported for immunocompromised patients who are at high risk for infectious complications from endotracheal intubation. Selection of appropriate patients is crucial for optimizing NIV success rates. Appropriate ventilator settings, a well-fitting and comfortable interface, and a team skilled and experienced in managing NIV are key components to its success. In a recent issue of the Journal of the American Medical Association, Patel et al. reported the results of their single-center trial of 83 patients with acute respiratory distress syndrome (ARDS) who were randomly assigned to NIV delivered via a helmet or face mask. Patients assigned to the helmet group exhibited a significantly lower intubation rate and were more likely to survive through 90 days. This perspective reviews the findings of this trial in the context of current clinical practice and in light of data from the literature focused on the potential reasons for success of NIV delivered through a helmet compared to face mask. The implications for early management of patients with ARDS are likewise discussed.
Keywords: Acute respiratory distress syndrome (ARDS), acute respiratory failure (ARF), non-invasive ventilation (NIV), helmet, face mask
In 1990, when Brochard et al. initially published their report regarding the benefits of using non-invasive ventilation (NIV) delivered by face mask as a potential alternative to endotracheal intubation in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (1), few clinicians thought that this technique would become a first-line intervention for certain forms of acute respiratory failure (ARF), such as COPD exacerbations and acute cardiogenic pulmonary edema (2). NIV refers to the delivery of mechanical ventilation with techniques [e.g., continuous positive airway pressure (CPAP) or pressure support ventilation (PSV) with positive end-expiratory pressure (PEEP)] that do not require an invasive endotracheal airway (2). Therefore, NIV should not be used when patients cannot protect their airway (2,3). Compared with invasive mechanical ventilation, NIV achieves the same physiological benefits of reduced work of breathing and improved gas exchange (2). Furthermore, NIV avoids the complications of intubation and reduces the risk of ventilator-associated pneumonia, especially in patients who are immunosuppressed or have other comorbidities (2,3). Compared with standard medical therapy, NIV improves survival (4-6) and reduces complications in selected patients with ARF (2). In a meta-analysis of 78 randomized controlled trials (RCTs) published in 2015, Cabrini et al. found that mortality was reduced when NIV was used to treat [risk ratio (RR), 0.72; 95% confidence interval (CI), 0.63 to 0.81] or prevent ARF (RR, 0.64; 95% CI, 0.46 to 0.90), with survival being improved in patients with COPD exacerbation, pulmonary edema, ARF of mixed etiologies, and postoperative ARF (4).
On June 14, 2016, Patel and colleagues published an article in the Journal of the American Medical Association, reporting that PSV plus PEEP delivered non-invasively (non-invasive positive pressure ventilation, NPPV) by means of a helmet (helmet NPPV) was more effective than NPPV delivered by a face mask (face mask NPPV) in reducing intubation rates and mortality in patients with acute respiratory distress syndrome (ARDS) (7). This RCT was conducted at the medical intensive care unit (ICU) at the University of Chicago (Chicago, IL, USA) and recruited 83 patients to receive either helmet NPPV or face mask NPPV, following initial period of face mask NPPV for at least 8 h. The helmet consisted of a transparent plastic hood that covers the entire head of the patient and a rubber collar neck seal, assembled to produce a breathing circuit closed off from the outside environment (Figure 1). The helmet was secured by padded axillary braces attached to two hooks on the front and back of a plastic ring of the helmet. The RCT originally planned to randomize 206 patients (103 per group). However, based on predefined criteria for efficacy, the study was terminated early, after 39 patients were randomized to the face mask NPPV group and 44 to the helmet NPPV group. The main results were the lower intubation rate (18.2% vs. 61.5%, P<0.001), higher number of ventilator-free days within 28 days (28 vs. 12.5 days, P<0.001), and lower mortality rate within 90 days (34.1% vs. 56.4%, P=0.02) in the helmet NPPV group compared with the face mask NPPV group. Adverse events included interface-related skin ulcers in each group: 7.6% of patients in the face mask NPPV group developed nose ulcers and 6.8% of patients in the helmet NPPV group developed neck ulcers. The results of this study thereby suggest that success or failure of NIV in patients with ARDS depends not only on the severity of ARDS, but also on the type of patients recruited, the ventilatory approach and device used for NPPV, and the skills of the team.
Figure 1.
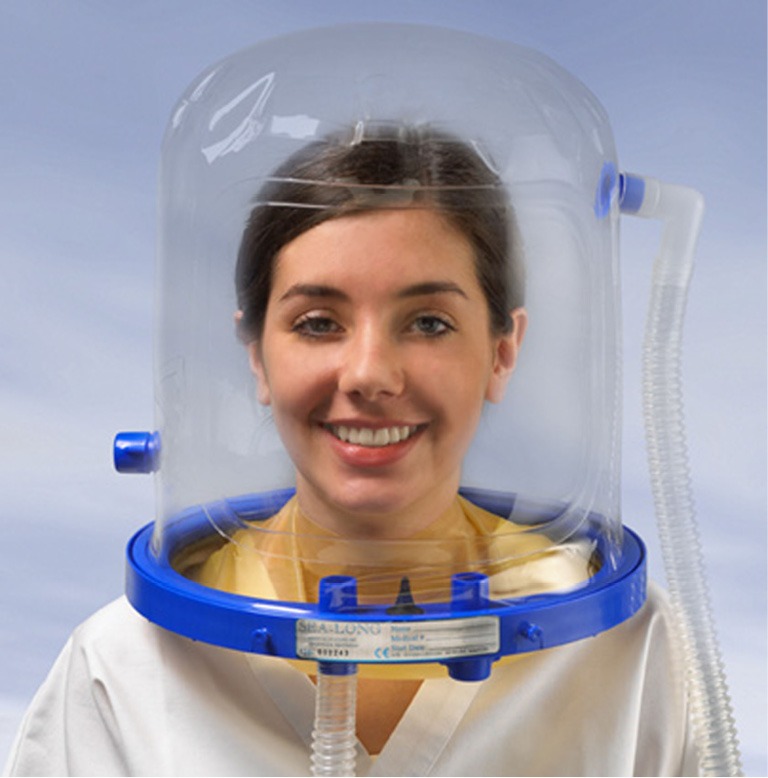
Non-invasive ventilation delivered by means of a helmet. In the study of Patel et al., forty-four patients admitted to ICU with ARDS received non-invasive mechanical ventilation (Engström Carestation, GE Healthcare) via a helmet (Sea-Long) (7). With permission from Sea-Long, Louisville, Kentucky, USA. ICU, intensive care unit; ARDS, acute respiratory distress syndrome.
Among patients with ARDS, the use of NIV is still controversial because of the generally high likelihood of failure and the risks associated with a delay in starting invasive mechanical ventilation (6,8,9). Based on an analysis of 13 studies involving 540 patients, Agarwal et al. found that, in ARDS patients treated with NIV, the intubation rate ranged from 30% to 86%, with a pooled intubation rate of 48% (95% CI, 39% to 58%), and the mortality rate ranged from 15% to 71%, with a pooled mortality rate of 35% (95% CI, 26% to 45%) (9). Increasing the severity of ARDS increases the risk of failure, with 100% NIV failure in patients with ARDS presenting with shock (4,6,9). However, in a meta-analysis of six RCTs involving 227 patients, the use of NIV in patients with ARDS reduced the risks of endotracheal intubation (pooled RR, 0.59; 95% CI, 0.44 to 0.80), ICU mortality (pooled RR, 0.69; 95% CI, 0.45 to 1.07), and hospital mortality (pooled RR, 0.52; 95% CI, 0.17 to 1.58), compared with standard medical therapy (10).
In the study of Patel et al., approximately one-half of the patients in each group were immunocompromised by virtue of cancer or transplantation therapy, and approximately one-third in each group were diagnosed with pneumonia due to immunosuppression (7). There is convincing evidence supporting the benefits of NIV as a treatment for ARF in patients with immunosuppression (2). Antonelli et al. showed that the use of face mask NPPV in 20 recipients of solid organ transplantation with hypoxemic ARF was associated with a significant reduction in the rate of endotracheal intubation (20% vs. 70%, P=0.002), rate of fatal complications (20% vs. 50%, P=0.05), length of stay in the ICU by survivors (mean ± standard deviation: 5.5±3 vs. 9±4 days; P=0.03), and ICU mortality (20% vs. 50%, P=0.05), compared with standard treatment using supplemental oxygen administration (11). Hilbert et al. found that fewer immunosuppressed patients treated with face mask NPPV for ARF required endotracheal intubation (12 vs. 20 patients, P=0.03), had serious complications (13 vs. 21 patients, P=0.02), died in the ICU (10 vs. 18 patients, P=0.03), or died in the hospital (13 vs. 21 patients, P=0.02), compared with patients who received supplemental oxygen and no ventilatory support (12). Squadrone et al. reported that immunocompromised patients who received early CPAP had less need for ICU admission for mechanical ventilation (4 vs. 16 patients, P=0.0002) and a lower risk of requiring ventilatory support (RR, 0.25; 95% CI, 0.10 to 0.62) (13). Among patients admitted to the ICU, the intubation rate was lower in the CPAP group than in the control group (2 vs. 14 patients, P=0.0001), resulting in a lower risk of intubation with early CPAP (RR, 0.46; 95% CI, 0.27 to 0.78).
However, beneficial effects of NIV in immunocompromised patients have not been observed in all RCTs. Lemiale et al. showed that among 374 critically ill immunocompromised patients admitted to the ICU with hypoxemic ARF, early face mask NPPV (191 patients) did not reduce 28-day mortality, compared with oxygen therapy alone (183 patients) (14). By day 28 after randomization, 46 deaths (24.1%) had occurred in the face mask NPPV group vs. 50 (27.3%) in the standard care group (P=0.47). The relatively low mortality rate with standard care may have been influenced by the higher number of patients in that group receiving heated, humidified high-flow oxygen delivered by nasal cannula (HFNC), compared with the face mask NPPV group (44.3% vs. 31.4%, P=0.01). In a multicenter, open-label RCT, treatment with HFNC improved survival among patients with ARF without hypercapnia, as compared with standard oxygen therapy or face mask NPPV (15). The support provided by HFNC in the control group may have remarkably reduced the need for invasive mechanical ventilation, thus masking a potentially beneficial effect of NPPV in this patient population (14). Despite the lack of clear benefit of NPPV over other approaches reported by some authors (14,16), NPPV remains an attractive modality for ARF in immunocompromised patients (17) and the first-line treatment for selected patients (Level 1 evidence) (2).
NPPV was the main ventilatory approach chosen by Patel et al. in their RCT (7). The physiologic goals of NPPV are to unload the respiratory muscles and relieve dyspnea by using PSV, as well as to improve oxygenation and recruit alveoli with the appropriate application of PEEP. In recent years, PEEP has assumed a primary role in the framework of lung-protective strategies (6). Adequate PEEP may prevent atelectrauma by avoiding intratidal opening and closing of alveoli and decreasing lung inhomogeneities, and it may also attenuate the dispersion of edema to previously spared regions within the lungs, thereby lowering the risk of lung injury (6). In the RCT by Patel et al., patients receiving helmet NPPV, compared with those treated with face mask NPPV, tolerated higher levels of PEEP (8.0 vs. 5.1 cm H2O, P=0.006) and lower driving pressures (7); these settings are more consistent with a lung-protective strategy recommended for ARDS (6). Titration of PEEP to higher levels in the face mask NPPV group was limited because of patient intolerance and excessive air leakage. Lung-protective strategies during NPPV are not possible in the presence of substantial air leakage (6). Indeed, an inadequate level of PEEP or transient loss of PEEP during mechanical ventilation can compromise lung recruitment and gas exchange, thus increasing the risk of ventilation-induced lung injury and negatively affect outcomes of patients with ARDS. Thus, the inability to up-titrate the applied PEEP and the intermittent mask removal noted in the study of Patel et al. may have coexisted to decrease the efficacy of face mask NPPV (7). By contrast, the use of a helmet may have played a role in improving outcomes (7) primarily by providing prolonged continuous NPPV and minimizing air leakage (3,18).
In their study assessing the efficacy of helmet NPPV compared to face mask NPPV as first-line treatment for patients with hypoxemic ARF, Antonelli et al. found that the helmet was associated with continuous use of NPPV for a longer period of time (P=0.05) (19). Furthermore, no patient failed NPPV because of intolerance to the technique in the helmet NPPV group, whereas 8 patients (38%) in the mask NPPV group were unable to tolerate their device (P=0.047). Rocco et al. compared the efficacy of helmet NPPV with face mask NPPV in 19 immunocompromised patients with hypoxemic ARF, fever, and lung infiltrates (20). The use of helmet NPPV was as effective as face mask NPPV in avoiding endotracheal intubation (intubation rate, 37% vs. 47%, P=0.37) and improving gas exchange, but it was associated with fewer discontinuations of NPPV in the first 24 h of application (P<0.001), better arterial oxygen tension/inspired oxygen concentration (PaO2/FiO2) ratio at treatment discontinuation (P=0.02), and fewer NPPV-related complications (e.g., skin necrosis, P=0.01). The helmet may thus represent a valid alternative to a face mask in patients with hypoxemic ARF, increasing tolerance (number of hours of continuous NPPV use without interruptions), and decreasing the rate of complications directly related to the administration of NPPV (20). Many other studies support these observations that, compared to a face mask, the helmet is better tolerated, thereby allowing for longer use (18), and it is associated with less air leakage and discomfort and fewer complications (3).
An appropriate setting of helmet NPPV is highly recommended for successful NIV. Although the helmet is an effective interface for applying NPPV, it may be associated with more carbon dioxide (CO2) rebreathing and patient-ventilator asynchrony than face mask NPPV because of its high inner volume and low elastance (21,22). Specific adjustments of the ventilator settings (e.g., high flow rates, short inspiratory rise time, cycling to 50% of peak inspiratory flow), the ventilator circuit (e.g., short ventilator circuit without filters), and the helmet itself (e.g., accurate inflation of the helmet’s internal cushion when possible) greatly improve mechanical performance of the helmet and patient-ventilator synchrony, as well as reduce CO2 rebreathing and air leaks during NPPV (22,23). Patel et al. incorporated many of these aspects into their study protocol (7).
Last but not least, the study by Patel and colleagues was conducted in a single center by a skilled team, which may have played an important role in improving the outcomes of their patients with ARDS receiving NPPV. It is well recognized that the experience and skill of the personnel who manage NIV are key components of its success (24). Physicians must be adept at selecting patients who are likely to succeed with NIV and promptly intubating those who are likely to fail (24). Careful patient selection according to available guidelines is highly recommended (3-6). Identification of predictors of NPPV success or failure in patients with ARF may help clinicians recognize those patients who are appropriate candidates for NPPV and those in whom the technique is unlikely to be effective, thus avoiding its inappropriate use and unnecessary delays in providing invasive ventilation (25). In patients with hypoxemic ARF with known risk factors and predictors for NIV failure (e.g., advanced age, high acuity illness on admission [e.g., Simplified Acute Physiology Score II score >34], ARDS, community-acquired pneumonia with or without sepsis, multi-organ system failure), a short NIV trial may be justified in the presence of an experienced ICU team. However, a PaO2/FiO2 ratio that does not improve or that worsens during a 1-h NPPV trial, particularly when associated with persistent dyspnea, tachypnea, or use of accessory muscles of respiration, accurately predicts NIV failure and suggests the need for proceeding with intubation (3,8,25). Furthermore, once NIV is begun, patients should be closely monitored, paying attention not only to vital signs and gas exchange but also to tolerance, comfort, air leaks, and patient-ventilator interaction (3).
The study by Patel et al. leaves us with some important messages. Helmet NPPV appears to be more effective than face mask NPPV in patients with ARDS, especially in those who are immunocompromised. To optimize outcomes, careful patient selection is recommended, and helmet NPPV should be applied by a trained and experienced team. Appropriate settings of the device and ventilator are necessary to optimize the benefits and minimize the risks of helmet NPPV. Future studies are highly desirable to confirm the findings of Patel et al. and to more conclusively define the role of helmet NPPV in the routine management of select patients with ARF due to ARDS. In particular, a comparison with HFNC would be beneficial to establish whether helmet NPPV may represent a first-line intervention for some patients with ARF from ARDS, leading to improved survival. The findings of Patel et al. have established a new horizon for the use of NIV in the acute care setting.
Acknowledgements
None.
Footnotes
Provenance: This is a Guest Perspective commissioned by Section Editor Jianrong Zhang, MD (Department of Thoracic Surgery, First Affiliated Hospital of Guangzhou Medical University, Guangzhou Institute of Respiratory Disease, Guangzhou, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990;323:1523-30. 10.1056/NEJM199011293232204 [DOI] [PubMed] [Google Scholar]
- 2.Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet 2009;374:250-9. 10.1016/S0140-6736(09)60496-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carron M, Freo U, BaHammam AS, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth 2013;110:896-914. 10.1093/bja/aet070 [DOI] [PubMed] [Google Scholar]
- 4.Cabrini L, Landoni G, Oriani A, et al. Noninvasive ventilation and survival in acute care settings: a comprehensive systematic review and metaanalysis of randomized controlled trials. Crit Care Med 2015;43:880-8. 10.1097/CCM.0000000000000819 [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Yuan B, Liang Q, et al. Noninvasive ventilation for acute lung injury a meta-analysis of randomized controlled trials. Heart Lung 2016;45:249-57. 10.1016/j.hrtlng.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 6.Chiumello D, Brioni M. Severe hypoxemia: which strategy to choose. Crit Care 2016;20:132. 10.1186/s13054-016-1304-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel BK, Wolfe KS, Pohlman AS, et al. Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2016;315:2435-41. 10.1001/jama.2016.6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonelli M, Conti G, Moro ML, et al. Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med 2001;27:1718-28. 10.1007/s00134-001-1114-4 [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care 2010;55:1653-60. [PubMed] [Google Scholar]
- 10.Luo J, Wang MY, Zhu H, et al. Can non-invasive positive pressure ventilation prevent endotracheal intubation in acute lung injury/acute respiratory distress syndrome? A meta-analysis. Respirology 2014;19:1149-57. 10.1111/resp.12383 [DOI] [PubMed] [Google Scholar]
- 11.Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 2000;283:235-41. 10.1001/jama.283.2.235 [DOI] [PubMed] [Google Scholar]
- 12.Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 2001;344:481-7. 10.1056/NEJM200102153440703 [DOI] [PubMed] [Google Scholar]
- 13.Squadrone V, Massaia M, Bruno B, et al. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med 2010;36:1666-74. 10.1007/s00134-010-1934-1 [DOI] [PubMed] [Google Scholar]
- 14.Lemiale V, Mokart D, Resche-Rigon M, et al. Effect of Noninvasive Ventilation vs Oxygen Therapy on Mortality Among Immunocompromised Patients With Acute Respiratory Failure: A Randomized Clinical Trial. JAMA 2015;314:1711-9. 10.1001/jama.2015.12402 [DOI] [PubMed] [Google Scholar]
- 15.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 16.Frat JP, Ragot S, Girault C, et al. Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: a post-hoc analysis of a randomised trial. Lancet Respir Med 2016;4:646-52. 10.1016/S2213-2600(16)30093-5 [DOI] [PubMed] [Google Scholar]
- 17.Hill NS, Brennan J, Garpestad E, et al. Noninvasive ventilation in acute respiratory failure. Crit Care Med 2007;35:2402-7. 10.1097/01.CCM.0000284587.36541.7F [DOI] [PubMed] [Google Scholar]
- 18.Esquinas Rodriguez AM, Papadakos PJ, Carron M, et al. Clinical review: Helmet and non-invasive mechanical ventilation in critically ill patients. Crit Care 2013;17:223. 10.1186/cc11875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonelli M, Conti G, Pelosi P, et al. New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet--a pilot controlled trial. Crit Care Med 2002;30:602-8. 10.1097/00003246-200203000-00019 [DOI] [PubMed] [Google Scholar]
- 20.Rocco M, Dell'Utri D, Morelli A, et al. Noninvasive ventilation by helmet or face mask in immunocompromised patients: a case-control study. Chest 2004;126:1508-15. 10.1378/chest.126.5.1508 [DOI] [PubMed] [Google Scholar]
- 21.Vargas F, Thille A, Lyazidi A, et al. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med 2009;37:1921-8. 10.1097/CCM.0b013e31819fff93 [DOI] [PubMed] [Google Scholar]
- 22.Mojoli F, Iotti GA, Currò I, et al. An optimized set-up for helmet noninvasive ventilation improves pressure support delivery and patient-ventilator interaction. Intensive Care Med 2013;39:38-44. 10.1007/s00134-012-2686-x [DOI] [PubMed] [Google Scholar]
- 23.Moerer O, Harnisch LO, Herrmann P, et al. Patient-Ventilator Interaction During Noninvasive Ventilation in Simulated COPD. Respir Care 2016;61:15-22. 10.4187/respcare.04141 [DOI] [PubMed] [Google Scholar]
- 24.Hill NS. Where should noninvasive ventilation be delivered? Respir Care 2009;54:62-70. [PubMed] [Google Scholar]
- 25.Bello G, De Pascale G, Antonelli M. Noninvasive ventilation for the immunocompromised patient: always appropriate? Curr Opin Crit Care 2012;18:54-60. 10.1097/MCC.0b013e32834e7c21 [DOI] [PubMed] [Google Scholar]


