Abstract
Photobiomodulation (PBM) describes the use of red or near-infrared light to stimulate, heal, regenerate, and protect tissue that has either been injured, is degenerating, or else is at risk of dying. One of the organ systems of the human body that is most necessary to life, and whose optimum functioning is most worried about by humankind in general, is the brain. The brain suffers from many different disorders that can be classified into three broad groupings: traumatic events (stroke, traumatic brain injury, and global ischemia), degenerative diseases (dementia, Alzheimer's and Parkinson's), and psychiatric disorders (depression, anxiety, post traumatic stress disorder). There is some evidence that all these seemingly diverse conditions can be beneficially affected by applying light to the head. There is even the possibility that PBM could be used for cognitive enhancement in normal healthy people. In this transcranial PBM (tPBM) application, near-infrared (NIR) light is often applied to the forehead because of the better penetration (no hair, longer wavelength). Some workers have used lasers, but recently the introduction of inexpensive light emitting diode (LED) arrays has allowed the development of light emitting helmets or “brain caps”. This review will cover the mechanisms of action of photobiomodulation to the brain, and summarize some of the key pre-clinical studies and clinical trials that have been undertaken for diverse brain disorders.
Keywords: Photobiomodulation, Low level laser (light) therapy, Ischemic stroke, Traumatic brain injury, Alzheimer's disease, Parkinson's disease, Major depression, Cognitive enhancement
Graphical abstract
Highlights
-
•
NIR light can penetrate the head and reach the brain.
-
•
NIR is absorbed by cytochrome c oxidase in mitochondria.
-
•
Heat-gated ion channels also play a role.
-
•
Increased blood flow, energy, neuroprotection, less inflammation, brain repair
-
•
Can treat traumatic (stroke, TBI), neurodegenerative and psychiatric diseases
1. Introduction
Photobiomodulation (PBM) as it is known today (the beneficial health benefits of light therapy had been known for some time before), was accidently discovered in 1967, when Endre Mester from Hungary attempted to repeat an experiment recently published by McGuff in Boston, USA [1]. McGuff had used a beam from the recently discovered ruby laser [2], to destroy a cancerous tumor that had been experimentally implanted into a laboratory rat. However (unbeknownst to Mester) the ruby laser that had been built for him, was only a tiny fraction of the power of the laser that had previously been used by McGuff. However, instead of curing the experimental tumors with his low-powered laser, Mester succeeded in stimulating hair regrowth and wound healing in the rats, in the sites where the tumors had been implanted [3], [4]. This discovery led to a series of papers describing what Mester called “laser biostimulation”, and soon became known as “low level laser therapy” (LLLT) [5], [6], [7].
LLLT was initially primarily studied for stimulation of wound healing, and reduction of pain and inflammation in various orthopedic conditions such as tendonitis, neck pain, and carpal tunnel syndrome [8]. The advent of light emitting diodes (LED) led to LLLT being renamed as “low level light therapy”, as it became more accepted that the use of coherent lasers was not absolutely necessary, and a second renaming occurred recently [9] when the term PBM was adopted due to uncertainties in the exact meaning of “low level”.
2. Mechanisms of action of photobiomodulation
2.1. Mitochondria and cytochrome c oxidase
The most well studied mechanism of action of PBM centers around cytochrome c oxidase (CCO), which is unit four of the mitochondrial respiratory chain, responsible for the final reduction of oxygen to water using the electrons generated from glucose metabolism [10]. The theory is that CCO enzyme activity may be inhibited by nitric oxide (NO) (especially in hypoxic or damaged cells). This inhibitory NO can be dissociated by photons of light that are absorbed by CCO (which contains two heme and two copper centers with different absorption spectra) [11]. These absorption peaks are mainly in the red (600–700 nm) and near-infrared (760–940 nm) spectral regions. When NO is dissociated, the mitochondrial membrane potential is increased, more oxygen is consumed, more glucose is metabolized and more ATP is produced by the mitochondria.
2.2. Reactive oxygen species, nitric oxide, blood flow
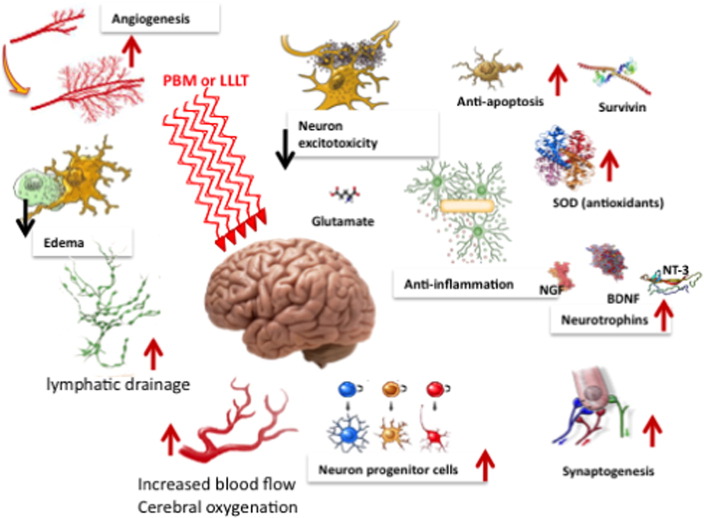
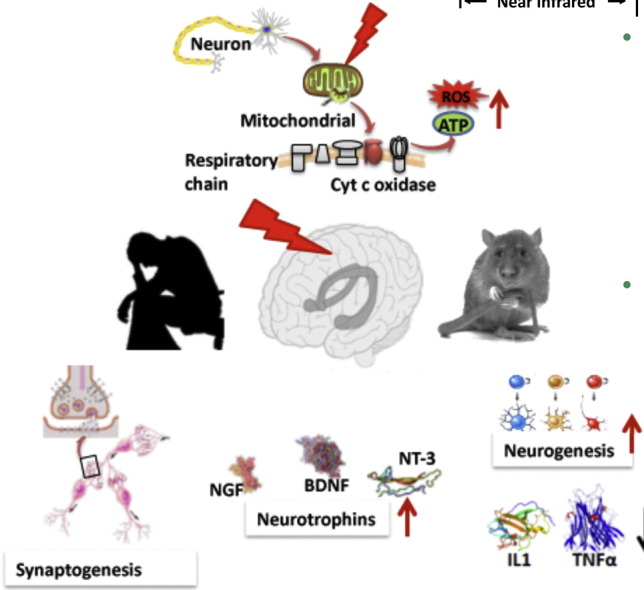
It has been shown that there is a brief increase in reactive oxygen species (ROS) produced in the mitochondria when they absorb the photons delivered during PBM. The idea is that this burst of ROS may trigger some mitochondrial signaling pathways leading to cytoprotective, anti-oxidant and anti-apoptotic effects in the cells [12]. The NO that is released by photodissociation acts as a vasodilator as well as a dilator of lymphatic flow. Moreover NO is also a potent signaling molecule and can activate a number of beneficial cellular pathways [13]. Fig. 2 illustrates these mechanisms.
Fig. 2.
Tissue specific processes that occur after PBM and benefit a range of brain disorders. BDNF, brain-derived neurotrophic factor; LLLT, low level light therapy; NGF, nerve growth factor; NT-3, neurotrophin 3; PBM, photobiomodulation; SOD, superoxide dismutase.
2.3. Light sensitive ion channels and calcium
It is quite clear that there must be some other type of photoacceptor, in addition to CCO, as is clearly demonstrated by the fact that wavelengths substantially longer than the red/NIR wavelengths discussed above, can also produce beneficial effects is some biological scenarios. Wavelengths such as 980 nm [14], [15], 1064 nm laser [16], and 1072 nm LED [17], and even broad band IR light [18] have all been reported to carry out PBM type effects. Although the photoacceptor for these wavelengths has by no means been conclusively identified, the leading hypothesis is that it is primarily water (perhaps nanostructured water) located in heat or light sensitive ion channels. Clear changes in intracellular calcium can be observed, that could be explained by light-mediated opening of calcium ion channels, such as members of the transient receptor potential (TRP) super-family [19]. TRP describes a large family of ion channels typified by TRPV1, recently identified as the biological receptor for capsaicin (the active ingredient in hot chili peppers) [20]. The biological roles of TRP channels are multifarious, but many TRP channels are involved in heat sensing and thermoregulation [21].
2.4. Signaling mediators and activation of transcription factors
Most authors suggest that the beneficial effects of tPBM on the brain can be explained by increases in cerebral blood flow, greater oxygen availability and oxygen consumption, improved ATP production and mitochondrial activity [22], [23], [24]. However there are many reports that a brief exposure to light (especially in the case of experimental animals that have suffered some kind of acute injury or traumatic insult) can have effects lasting days, weeks or even months [25]. This long-lasting effect of light can only be explained by activation of signaling pathways and transcription factors that cause changes in protein expression that last for some considerable time. The effects of PBM on stimulating mitochondrial activity and blood flow is of itself, unlikely to explain long-lasting effects. A recent review listed no less than fourteen different transcription factors and signaling mediators, that have been reported to be activated after light exposure [10].
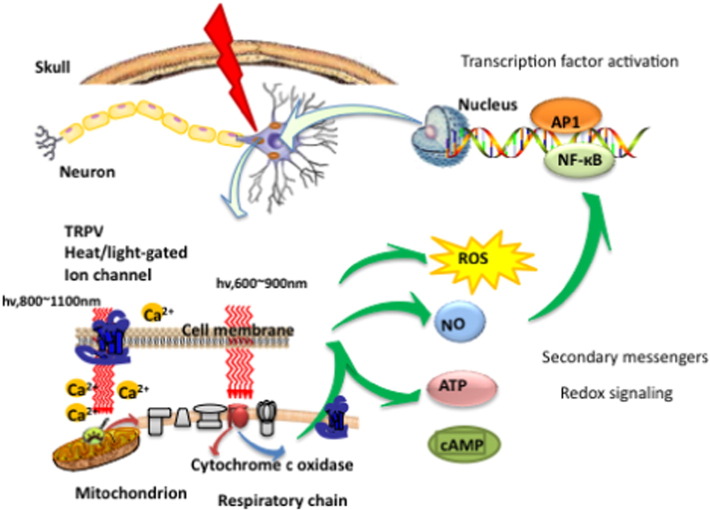
Fig. 1 illustrates two of the most important molecular photoreceptors or chromophores (cytochrome c oxidase and heat-gated ion channels) inside neuronal cells that absorb photons that penetrate into the brain. The signaling pathways and activation of transcription factors lead to the eventual effects of PBM in the brain.
Fig. 1.
Molecular and intracellular mechanisms of transcranial low level laser (light) or photobiomodulation. AP1, activator protein 1; ATP, adenosine triphosphate; Ca2 +, calcium ions; cAMP, cyclic adenosine monophosphate; NF-kB, nuclear factor kappa B; NO, nitric oxide; ROS, reactive oxygen species; TRPV, transient receptor potential vanilloid.
Fig. 2 illustrates some more tissue specific mechanisms that lead on from the initial photon absorption effects explained in Fig. 1. A wide variety of processes can occur that can benefit a correspondingly wide range of brain disorders. These processes can be divided into short-term stimulation (ATP, blood flow, lymphatic flow, cerebral oxygenation, less edema). Another group of processes center around neuroprotection (upregulation of anti-apoptotic proteins, less excitotoxity, more antioxidants, less inflammation). Finally a group of processes that can be grouped under “help the brain to repair itself” (neurotrophins, neurogenesis and synaptogenesis).
2.5. Biphasic dose response and effect of coherence
The biphasic dose response (otherwise known as hormesis, and reviewed extensively by Calabrese et al. [26]) is a fundamental biological law describing how different biological systems can be activated or stimulated by low doses of any physical insult or chemical substance, no matter how toxic or damaging this insult may be in large doses. The most well studied example of hormesis is that of ionizing radiation, where protective mechanisms are induced by very low exposures, that can not only protect against subsequent large doses of ionizing radiation, but can even have beneficial effects against diseases such as cancer using whole body irradiation [27].
There are many reports of PBM following a biphasic dose response (sometimes called obeying the Arndt-Schulz curve [28], [29]. A low dose of light is beneficial, but raising the dose produces progressively less benefit until eventually a damaging effect can be produced at very high light [30]. It is often said in this context that “more does not mean more”.
Another question that arises in the field of PBM is whether the coherent monochromatic lasers that were used in the original discovery of the effect, and whose use continued for many years, are superior to the rather recent introduction of LEDs, that are non-coherent and have a wider band-spread (generally 30 nm full-width half-maximum). Although there are one or two authors who continue to believe that coherent lasers are superior [31], most commentators feel that other parameters such as wavelength, power density, energy density and total energy are the most important determinants of efficacy [8].
3. Tissue optics, direct versus systemic effects, light sources
3.1. Light penetration into the brain
Due to the growing interest in PBM of the brain, several tissue optics laboratories have investigated the penetration of light of different wavelengths through the scalp and the skull, and to what depths into the brain this light can penetrate. This is an intriguing question to consider, because at present it is unclear exactly what threshold of power density in mW/cm2 is required in the b5rain to have a biological effect. There clearly must be a minimum value below which the light can be delivered for an infinite time without doing anything, but whether this is in the region of μW/cm2 or mW/cm2 is unknown at present.
Functional near-infrared spectroscopy (fNIRS) using 700–900 nm light has been established as a brain imaging technique that can be compared to functional magnetic resonance imaging (fMRI) [32]. Haeussinger et al. estimated that the mean penetration depth (5% remaining intensity) of NIR light through the scalp and skull was 23:6 + 0:7 mm [33]. Other studies have found comparable results with variations depending on the precise location on the head and wavelength [34], [35].
Jagdeo et al. [36] used human cadaver heads (skull with intact soft tissue) to measure penetration of 830 nm light, and found that penetration depended on the anatomical region of the skull (0.9% at the temporal region, 2.1% at the frontal region, and 11.7% at the occipital region). Red light (633 nm) hardly penetrated at all. Tedord et al. [37] also used human cadaver heads to compare penetration of 660 nm, 808 nm, and 940 nm light. They found that 808 nm light was best and could reach a depth in the brain of 40–50 mm. Lapchak et al. compared the transmission of 810 nm light through the skulls of four different species, and found mouse transmitted 40%, while for rat it was 21%, rabbit it was 11.3 and for human skulls it was only 4.2% [38]. Pitzschke and colleagues compared penetration of 670 nm and 810 nm light into the brain when delivered by a transcranial or a transphenoidal approach, and found that the best combination was 810 nm delivered transphenoidally [39]. In a subsequent study these authors compared the effects of storage and processing (frozen or formalin-fixed) on the tissue optical properties of rabbit heads [40]. Yaroslavsky et al. examined light penetration of different wavelengths through different parts of the brain tissue (white brain matter, gray brain matter, cerebellum, and brainstem tissues, pons, thalamus). Best penetration was found with wavelengths between 1000 and 1100 nm [41].
Henderson and Morries found that between 0.45% and 2.90% of 810 nm or 980 nm light penetrated through 3 cm of scalp, skull and brain tissue in ex vivo lamb heads [42].
3.2. Systemic effects
It is in fact very likely that the beneficial effects of PBM on the brain cannot be entirely explained by penetration of photons through the scalp and skull into the brain itself. There have been some studies that have explicitly addressed this exact issue. In a study of PBM for Parkinson's disease in a mouse model [43]. Mitrofanis and colleagues compared delivering light to the mouse head, and also covered up the head with aluminum foil so that they delivered light to the remainder of the mouse body. They found that there was a highly beneficial effect on neurocognitive behavior with irradiation to the head, but nevertheless there was also a statistically significant (although less pronounced benefit, referred to by these authors as an ‘abscopal effect”) when the head was shielded from light [44]. Moreover Oron and co-workers [45] have shown that delivering NIR light to the mouse tibia (using either surface illumination or a fiber optic) resulted in improvement in a transgenic mouse model of Alzheimer's disease (AD). Light was delivered weekly for 2 months, starting at 4 months of age (progressive stage of AD). They showed improved cognitive capacity and spatial learning, as compared to sham-treated AD mice. They proposed that the mechanism of this effect was to stimulate c-kit-positive mesenchymal stem cells (MSCs) in autologous bone marrow (BM) to enhance the capacity of MSCs to infiltrate the brain, and clear β-amyloid plaques [46]. It should be noted that the calvarial bone marrow of the skull contains substantial numbers of stem cells [47].
3.3. Laser acupuncture
Laser acupuncture is often used as an alternative or as an addition to traditional Chinese acupuncture using needles [48]. Many of the applications of laser acupuncture have been for conditions that affect the brain [49] such as Alzheimer's disease [50] and autism [51] that have all been investigated in animal models. Moreover laser acupuncture has been tested clinically [52].
3.4. Light sources
A wide array of different light sources (lasers and LEDs) have been employed for tPBM. One of the most controversial questions which remains to be conclusively settled, is whether a coherent monochromatic laser is superior to non-coherent LEDs typically having a 30 nm band-pass (full width half maximum). Although wavelengths in the NIR region (800–1100 nm) have been the most often used, red wavelengths have sometimes been used either alone, or in combination with NIR. Power levels have also varied markedly from Class IV lasers with total power outputs in the region of 10 W [53], to lasers with more modest power levels (circa 1 W). LEDs can also have widely varying total power levels depending on the size of the array and the number and power of the individual diodes. Power densities can also vary quite substantially from the Photothera laser [54] and other class IV lasers , which required active cooling (~ 700 mW/cm2) to LEDs in the region of 10–30 mW/cm2.
3.5. Usefulness of animal models when testing tPBM for brain disorders
One question that is always asked in biomedical research, is how closely do the laboratory models of disease (which are usually mice or rats) mimic the human disease for which new treatments are being sought? This is no less critical a question when the areas being studied include brain disorders and neurology. There now exist a plethora of transgenic mouse models of neurological disease [55], [56]. However in the present case, where the proposed treatment is almost completely free of any safety concerns, or any reported adverse side effects, it can be validly questioned as to why the use of laboratory animal models should be encouraged. Animal models undoubtedly have disadvantages such as failure to replicate all the biological pathways found in human disease, difficulty in accurately measuring varied forms of cognitive performance, small size of mice and rats compared to humans, short lifespan affecting the development of age related diseases, and lack of lifestyle factors that adversely affect human diseases. Nevertheless, small animal models are less expensive, and require much less time and effort to obtain results than human clinical trials, so it is likely they will continue to be used to test tPBM for the foreseeable future.
4. PBM for stroke
4.1. Animal models
Perhaps the most well-investigated application of PBM to the brain, lies in its possible use as a treatment for acute stroke [57]. Animal models such as rats and rabbits, were first used as laboratory models, and these animals had experimental strokes induced by a variety of methods and were then treated with light (usually 810 nm laser) within 24 h of stroke onset [58]. In these studies intervention by tLLLT within 24 h had meaningful beneficial effects. For the rat models, stroke was induced by middle cerebral artery occlusion (MCAO) via an insertion of a filament into the carotid artery or via craniotomy [59], [60]. Stroke induction in the “rabbit small clot embolic model” (RSCEM) was by injection of a preparation of small blood clots (made from blood taken from a second donor rabbit) into a catheter placed in the right internal carotid artery [61]. These studies and the treatments and results are listed in Table 1.
Table 1.
Reports of transcranial LLLT used for stroke in animal models.
| Subject | Stroke model | Parameters | Effect | References |
|---|---|---|---|---|
| Rat | MCAO | 660 nm; 8.8 mW; 2.64 J/cm2; pulse frequency of 10 kHz; laser applied at cerebrum at 1, 5 and 10 min | Suppression of NOS activity and up regulation of TGF-β1 | [59] |
| Rat | MCAO | 808 nm; 7.5 mW/cm2; 0.9 J/cm2; 3.6 J/cm2 at cortical surface; CW and pulse wave at 70 Hz, 4 mm diameter | Administration of LLLT 24 h after stroke onset induced functional benefit and neurogenesis induction | [60] |
| Rabbit | RSCEM | 808 nm ± 5 nm; 7.5 W/cm2, 2 min duration 3 h after stroke and 25 mW/cm2 10 min duration 1 or 6 h after stroke | Improved behavioral performance and durable effect after LLLT within 6 h from stroke onset | [62] |
| Rat | MCAO | 808 nm; 0.5 mW/cm2; 0.9 J/cm2 on brain 3 mm dorsal to the eye and 2 mm anterior to the ear | LLLT applied at different locations on the skull improved neurological function after acute stroke | [63] |
| Rabbit | RSCEM | 808 nm; 7.5 mW/cm2; 0.9 J/cm2; 3.6 J/cm2 at cortical surface; CW; 300 min; pulse at 1 kHz, 2 min at 100 Hz | LLLT administered 6 h after embolic stroke resulted in clinical improvements in rabbits | [64] |
CW, continuous wave; LLLT, low level light therapy; MCAO, middle cerebral artery occlusion; NOS, nitric oxide synthase; RSCEM, rabbit small clot embolic model; TGFβ1, transforming growth factor β1.
4.2. Clinical trials for acute stroke
Treatment of acute stroke was addressed in a series of three clinical trials called “Neurothera Effectiveness and Safety Trials” (NEST-1 [65], NEST-2 [66], and NEST-3 [67]) using an 810 nm laser applied to the shaved head within 24 h of patients suffering an ischemic stroke. The first study, NEST-1, enrolled 120 patients between the ages of 40 to 85 years of age with a diagnosis of ischemic stroke involving a neurological deficit that could be measured. The purpose of this first clinical trial was to demonstrate the safety and effectiveness of laser therapy for stroke within 24 h [65]. tPBM significantly improved outcome in human stroke patients, when applied at ~ 18 h post-stroke, over the entire surface of the head (20 points in the 10/20 EEG system) regardless of stroke [65]. Only one laser treatment was administered, and 5 days later, there was significantly greater improvement in the Real- but not in the Sham-treated group (p < 0.05, NIH Stroke Severity Scale). This significantly greater improvement was still present at 90 days post-stroke, where 70% of the patients treated with Real-LLLT had a successful outcome, while only 51% of Sham-controls did. The second clinical trial, NEST-2, enrolled 660 patients, aged 40 to 90, who were randomly assigned to one of two groups (331 to LLLT, 327 to sham) [68]. Beneficial results (p < 0.04) were found for the moderate and moderate-severe (but not for the severe) stroke patients, who received the Real laser protocol [68]. These results suggested that the overall severity of the individual stroke should be taken into consideration in future studies, and very severe patients are unlikely to recover with any kind of treatment. The last clinical trial, NEST-3, was planned for 1000 patients enrolled. Patients in this study were not to receive tissue plasminogen activator, but the study was prematurely terminated by the DSMB for futility (an expected lack of statistical significance) [67]. NEST-1 was considered successful, even though as a phase 1 trial, it was not designed to show efficacy. NEST-2 was partially successful when the patients were stratified, to exclude very severe strokes or strokes deep within the brain [66]. There has been considerable discussion in the scientific literature on precisely why the NEST-3 trial failed [69]. Many commentators have wondered how could tPBM work so well in the first trial, in a sub-group in the second trial, and fail in the third trial. Lapchak's opinion is that the much thicker skull of humans compared to that of the other animals discussed above (mouse, rat and rabbit), meant that therapeutically effective amounts of light were unlikely to reach the brain [69]. Moreover the time between the occurrence of a stroke and initiation of the PBMT may be an important factor. There are reports in the literature that neuroprotection must be administered as soon as possible after a stroke [70], [71]. Furthermore, stroke trials in particular should adhere to the RIGOR (rigorous research) guidelines and STAIR (stroke therapy academic industry roundtable) criteria [72]. Other contributory causes to the failure of NEST-3 may have been included the decision to use only one single tPBM treatment, instead of a series of treatments. Moreover, the optimum brain areas to be treated in acute stroke remain to be determined. It is possible that certain areas of the brain that have sustained ischemic damage should be preferentially illuminated and not others.
4.3. Chronic stroke
Somewhat surprisingly, there have not as yet been many trials of PBM for rehabilitation of stroke patients with only the occasional report to date. Naeser reported in an abstract the use of tPBM to treat chronic aphasia in post-stroke patients [73]. Boonswang et al. [74] reported a single patient case in which PBM was used in conjunction with physical therapy to rehabilitate chronic stroke damage. However the findings that PBM can stimulate synaptogenesis in mice with TBI, does suggest that tPBM may have particular benefits in rehabilitation of stroke patients. Norman Doidge, in Toronto, Canada has described the use of PBM as a component of a neuroplasticity approach to rehabilitate chronic stroke patients [75].
5. PBM for traumatic brain injury (TBI)
5.1. Mouse and rat models
There have been a number of studies looking at the effects of PBM in animal models of TBI. Oron's group was the first [76] to demonstrate that a single exposure of the mouse head to a NIR laser (808 nm) a few hours after creation of a TBI lesion could improve neurological performance and reduce the size of the brain lesion. A weight-drop device was used to induce a closed-head injury in the mice. An 808 nm diode laser with two energy densities (1.2–2.4 J/cm2 over 2 min of irradiation with 10 and 20 mW/cm2) was delivered to the head 4 h after TBI was induced. Neurobehavioral function was assessed by the neurological severity score (NSS). There were no significant difference in NSS between the power densities (10 vs 20 mW/cm2) or significant differentiation between the control and laser treated group at early time points (24 and 48 h) post TBI. However, there was a significant improvement (27% lower NSS score) in the PBM group at times of 5 days to 4 weeks. The laser treated group also showed a smaller loss of cortical tissue than the sham group [76].
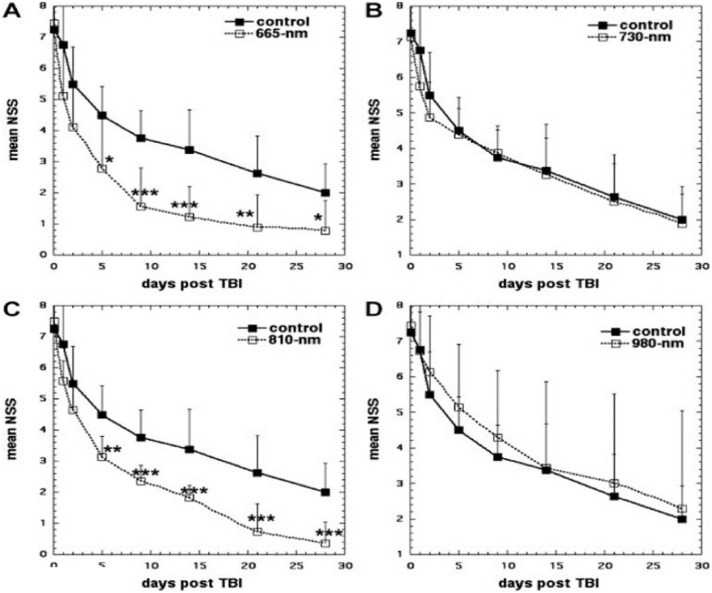
Hamblin's laboratory then went on (in a series of papers [76]) to show that 810 nm laser (and 660 nm laser) could benefit experimental TBI both in a closed head weight drop model [77], and also in controlled cortical impact model in mice [25]. Wu et al. [77] explored the effect that varying the laser wavelengths of LLLT had on closed-head TBI in mice. Mice were randomly assigned to LLLT treated group or to sham group as a control. Closed-head injury (CHI) was induced via a weight drop apparatus. To analyze the severity of the TBI, the neurological severity score (NSS) was measured and recorded. The injured mice were then treated with varying wavelengths of laser (665, 730, 810 or 980 nm) at an energy level of 36 J/cm2 at 4 h directed onto the scalp. The 665 nm and 810 nm groups showed significant improvement in NSS when compared to the control group at day 5 to day 28. Results are shown in Fig. 3. Conversely, the 730 and 980 nm groups did not show a significant improvement in NSS and these wavelengths did not produce similar beneficial effects as in the 665 nm and 810 nm LLLT groups [77]. The tissue chromophore cytochrome c oxidase (CCO) is proposed to be responsible for the underlying mechanism that produces the many PBM effects that are the byproduct of LLLT. COO has absorption bands around 665 nm and 810 nm while it has low absorption bands at the wavelength of 730 nm [78]. It should be noted that this particular study found that the 980 nm did not produce the same positive effects as the 665 nm and 810 nm wavelengths did; nevertheless previous studies did find that the 980 nm wavelength was an active one for LLLT. Wu et al. proposed that these dissimilar results may be due to the variance in the energy level, irradiance, etc. between the other studies and this particular study [77].
Fig. 3.
tPBM for TBI in a mouse model. Mice received a closed head injury and 4 hours later a single exposure of the head to one of four different lasers (36 J/cm2 delivered at 150 mW/cm2 over 4 min with spot size 1-cm diameter) [77]. A, 665 nm; B, 730 nm; C, 810 nm; D, 980 nm.
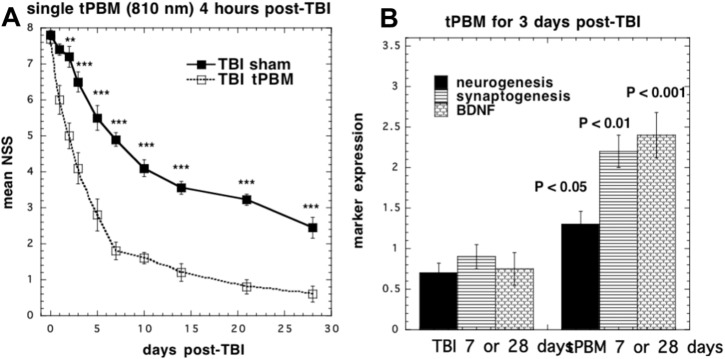
Ando et al. [25] used the 810 nm wavelength laser parameters from the previous study and varied the pulse modes of the laser in a mouse model of TBI. These modes consisted of either pulsed wave at 10 Hz or at 100 Hz (50% duty cycle) or continuous wave laser. For the mice, TBI was induced with a controlled cortical impact device via open craniotomy. A single treatment with an 810 nm Ga-Al-As diode laser with a power density of 50 mW/m2 and an energy density of 36 J/cm2 was given via tLLLT to the closed head in mice for a duration of 12 min at 4 h post CCI. At 48 h to 28 days post TBI, all laser treated groups had significant decreases in the measured neurological severity score (NSS) when compared to the control (Fig. 4A). Although all laser treated groups had similar NSS improvement rates up to day 7, the PW 10 Hz group began to show greater improvement beyond this point as seen in Fig. 4. At day 28, the forced swim test for depression and anxiety was used and showed a significant decrease in the immobility time for the PW 10 Hz group. In the tail suspension test which measures depression and anxiety, there was also a significant decrease in the immobility time at day 28, and this time also at day 1, in the PW 10 Hz group.
Fig. 4.
tPBM for controlled cortical impact TBI in a mouse model. (A) Mice received a single exposure (810 nm laser, 36 J/cm2 delivered at 50 mW/cm2 over 12 min) [121]. (B) Mice received 3 daily exposures starting 4 h post-TBI and were sacrificed after 7 or 28 days. BDNF and neurogenesis (BrdU) were increased at 7 days [81], while synaptogenesis was increased at 28 days [80].
Studies using immunofluorescence of mouse brains showed that tPBM increased neuroprogenitor cells in the dentate gyrus (DG) and subventricular zone at 7 days after the treatment [79]. The neurotrophin called brain derived neurotrophic factor (BDNF) was also increased in the DG and SVZ at 7 days , while the marker (synapsin-1) for synaptogenesis and neuroplasticity was increased in the cortex at 28 days but not in the DG, SVZ or at 7 days [80] (Fig. 4B). Learning and memory as measured by the Morris water maze was also improved by tPBM [81]. Whalen's laboratory [82] and Whelan's laboratory [83] also successfully demonstrated therapeutic benefits of tPBM for TBI in mice and rats respectively.
Zhang et al. [84] showed that secondary brain injury occurred to a worse degree in mice that had been genetically engineered to lack “Immediate Early Response” gene X-1 (IEX-1) when exposed to a gentle head impact (this injury is thought to closely resemble mild TBI in humans). Exposing IEX-1 knockout mice to LLLT 4 h post injury, suppressed proinflammatory cytokine expression of interleukin (IL)-Iβ and IL-6, but upregulated TNF-α. The lack of IEX-1 decreased ATP production, but exposing the injured brain to LLLT elevated ATP production back to near normal levels.
Dong et al. [85] even further improved the beneficial effects of PBM on TBI in mice, by combining the treatment with metabolic substrates such as pyruvate and/or lactate. The goal was to even further improve mitochondrial function. This combinatorial treatment was able to reverse memory and learning deficits in TBI mice back to normal levels, as well as leaving the hippocampal region completely protected from tissue loss; a stark contrast to that found in control TBI mice that exhibited severe tissue loss from secondary brain injury.
5.2. TBI in humans
Margaret Naeser and collaborators have tested PBM in human subjects who had suffered TBI in the past [86]. Many sufferers from severe or even moderate TBI, have very long lasting and even life-changing sequelae (headaches, cognitive impairment, and difficulty sleeping) that prevent them working or living any kind or normal life. These individuals may have been high achievers before the accident that caused damage to their brain [87]. Initially Naeser published a report [88] describing two cases she treated with PBM applied to the forehead twice a week. A 500 mW continuous wave LED source (mixture of 660 nm red and 830 nm NIR LEDs) with a power density of 22.2 mW/cm2 (area of 22.48 cm2), was applied to the forehead for a typical duration of 10 min (13.3 J/cm2). In the first case study the patient reported that she could concentrate on tasks for a longer period of time (the time able to work at a computer increased from 30 min to 3 h). She had a better ability to remember what she read, decreased sensitivity when receiving haircuts in the spots where LLLT was applied, and improved mathematical skills after undergoing LLLT. The second patient had statistically significant improvements compared to prior neuropsychological tests after 9 months of treatment. The patient had a 2 standard deviation (SD) increase on tests of inhibition and inhibition accuracy (9th percentile to 63rd percentile on the Stroop test for executive function and a 1 SD increase on the Wechsler Memory scale test for the logical memory test (83rd percentile to 99th percentile) [89].
Naeser et al. then went on to report a case series of a further eleven patients [90]. This was an open protocol study that examined whether scalp application of red and near infrared (NIR) light could improve cognition in patients with chronic, mild traumatic brain injury (mTBI). This study had 11 participants ranging in age from 26 to 62 (6 males, 5 females) who suffered from persistent cognitive dysfunction after mTBI. The participants' injuries were caused by motor vehicle accidents, sports related events and for one participant, an improvised explosive device (IED) blast. tLLLT consisted of 18 sessions (Monday, Wednesday, and Friday for 6 weeks) and commenced anywhere from 10 months to 8 years post-TBI. A total of 11 LED clusters (5.25 cm in diameter, 500 mW, 22.2 mW/cm2, 13 J/cm2) were applied for about 10 min per session (5 or 6 LED placements per set, Set A and then Set B, in each session). Neuropsychological testing was performed pre-LED application and 1 week, 1 month and 2 months after the final treatment. Naeser and colleagues found that there was a significant positive linear trend observed for the Stroop Test for executive function, in trial 2 inhibition (p = 0.004); Stroop, trial 4 inhibition switching (p = 0.003); California Verbal Learning Test (CVLT)-II, total trials 1–5 (p = 0.003); CVLT-II, long delay free recall (p = 0.006). Improved sleep and fewer post-traumatic stress disorder (PTSD) symptoms, if present beforehand, were observed after treatment. Participants and family members also reported better social function and a better ability to perform interpersonal and occupational activities. Although these results were significant, further placebo-controlled studies will be needed to ensure the reliability of this these data [90].
Henderson and Morries [91] used a high-power NIR laser (10–15 W at 810 and 980 nm) applied to the head to treat a patient with moderate TBI. The patient received 20 NIR applications over a 2-month period. They carried out anatomical magnetic resonance imaging (MRI) and perfusion single-photon emission computed tomography (SPECT). The patient showed decreased depression, anxiety, headache, and insomnia, whereas cognition and quality of life improved, accompanied by changes in the SPECT imaging.
6. PBM for Alzheimer's disease (AD)
6.1. Animal models
There was a convincing study [92] carried out in an AβPP transgenic mouse of AD. tPBM (810 nm laser) was administered at different doses 3 times/week for 6 months starting at 3 months of age. The numbers of Aβ plaques were significantly reduced in the brain with administration of tPBM in a dose-dependent fashion. tPBM mitigated the behavioral effects seen with advanced amyloid deposition and reduced the expression of inflammatory markers in the transgenic mice. In addition, TLT showed an increase in ATP levels, mitochondrial function, and c-fos expression suggesting that there was an overall improvement in neurological function.
6.2. Humans
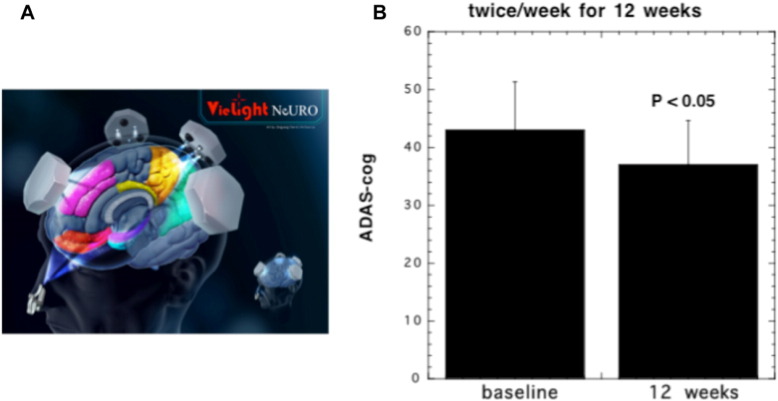
There has been a group of investigators in Northern England who have used a helmet built with 1072 nm LEDs to treat AD, but somewhat surprisingly no peer-reviewed publications have described this approach [93]. However a small pilot study (19 patients) that took the form of a randomized placebo-controlled trial investigated the effect of the Vielight Neuro system (see Fig. 5A) (a combination of tPBM and intranasal PBM) on patients with dementia and mild cognitive impairment [94]. This was a controlled single blind pilot study in humans to investigate the effects of PBM on memory and cognition. 19 participants with impaired memory/cognition were randomized into active and sham treatments over 12 weeks with a 4-week no-treatment follow-up period. They were assessed with MMSE and ADAS-cog scales. The protocol involved in-clinic use of a combined transcranial-intranasal PBM device; and at-home use of an intranasal-only PBM device and participants/ caregivers noted daily experiences in a journal. Active participants with moderate to severe impairment (MMSE scores 5–24) showed significant improvements (5-points MMSE score) after 12 weeks. There was also a significant improvement in ADAS-cog scores (see Fig. 5B). They also reported better sleep, fewer angry outbursts and decreased anxiety and wandering. Declines were noted during the 4-week no-treatment follow-up period. Participants with mild impairment to normal (MMSE scores of 25 to 30) in both the active and sham sub-groups showed improvements. No related adverse events were reported.
Fig. 5.
tPBM for Alzheimer's disease. (A) Nineteen patients were randomized to receive real or sham tPBM (810 nm LED, 24.6 J/cm2 at 41 mW/cm2). (B) Significant decline in ADAS-cog (improved cognitive performance) in real but not sham (unpublished data).
An interesting paper from Russia [95] described the use of intravascular PBM to treat 89 patients with AD who received PBM (46 patients) or standard treatment with memantine and rivastigmine (43 patients). The PBM consisted of threading a fiber-optic through a cathéter in the fémoral artery and advancing it to the distal site of the anterior and middle cerebral arteries and delivering 20 mW of red laser for 20–40 min. The PBM group had improvement in cerebral microcirculation leading to permanent (from 1 to 7 years) reduction in dementia and cognitive recovery.
7. Parkinson's disease
The majority of studies on PBM for Parkinson's disease have been in animal models and have come from the laboratory of John Mitrofanis in Australia [96]. Two basic models of Parkinson's disease were used. The first employed administration of the small molecule (MPTP or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) to mice [97]. MPTP was discovered as an impurity in an illegal recreational drug to cause Parkinson's like symptoms (loss of substantia nigra cells) in young people who had taken this drug [98]. Mice were treated with tPBM (670-nm LED, 40 mW/cm2, 3.6 J/cm2) 15 min after each MPTP injection repeated 4 times over 30 h. There were significantly more (35%–45%) dopaminergic cells in the brains of the tPBM treated mice [97]. A subsequent study showed similar results in a chronic mouse model of MPTP-induced Parkinson's disease [99]. They repeated their studies in another mouse model of Parkinson's disease, the tau transgenic mouse strain (K3) that has a progressive degeneration of dopaminergic cells in the substantia nigra pars compacta (SNc) [100]. They went on to test a surgically implanted intracranial fiber designed to deliver either 670 nm LED (0.16 mW) or 670 nm laser (67 mW) into the lateral ventricle of the brain in MPTP-treated mice [101]. Both low power LED and high power laser were effective in preserving SNc cells, but the laser was considered to be unsuitable for long-term use (6 days) due to excessive heat production. As mentioned above, these authors also reported a protective effect of abscopal light exposure (head shielded) in this mouse model [43]. Recently this group has tested their implanted fiber approach in a model of Parkinson's disease in adult Macaque monkeys treated with MPTP [102]. Clinical evaluation of Parkinson's symptoms (posture, general activity, bradykinesia, and facial expression) in the monkeys were improved at low doses of light (24 J or 35 J) compared to high doses (125 J) [103].
The only clinical report of PBM for Parkinson's disease in humans was an abstract presented in 2010 [104]. Eight patients between 18 and 80 years with late stage PD participated in a non-controlled, non-randomized study. Participants received tPBM treatments of the head designed to deliver light to the brain stem, bilateral occipital, parietal, temporal and frontal lobes, and treatment along the sagittal suture. A Visual Analog Scale (VAS), was used to record the severity of their symptoms of balance, gait, freezing, cognitive function, rolling in bed, and difficulties with speech pre-procedure and at study endpoint with 10 being most severe and 0 as no symptom. Compared with baseline, all participants demonstrated a numerical improvement in the VAS from baseline to study endpoint. A statistically significant reduction in VAS rating for gait and cognitive function was observed with average mean change of —1.87 (p < 0.05) for gait and a mean reduction of —2.22 (p < 0.05) for cognitive function. Further, freezing and difficulty with speech ratings were significantly lower (mean reduction of 1.28 (p < 0.05) for freezing and 2.22 (p < 0.05) for difficulty with speech).
8. PBM for psychiatric disorders
8.1. Animal models
A common and well-accepted animal model of depression is called “chronic mild stress” [105]. After exposure to a series of chronic unpredictable mild stressors, animals develop symptoms seen in human depression, such as anhedonia (loss of the capacity to experience pleasure, a core symptom of major depressive disorder), weight loss or slower weight gain, decrease in locomotor activity, and sleep disorders [106]. Wu et al. used Wistar rats to show that after 5 weeks of chronic stress, application of tPBM 3 times a week for 3 weeks (810 nm laser, 100 Hz with 20% duty cycle, 120 J/cm2) gave significant improvement in the forced swimming test (FST) [107]. In a similar study Salehpour et al. [108] compared the effects of two different lasers (630 m nm at 89 mW/cm2, and 810 nm at 562 mW/cm2, both pulsed at 10 Hz, 50% duty cycle). The 810 nm laser proved better than the 630 nm laser in the FST, in the elevated plus maze and also reduced blood cortisol levels.
8.2. Depression and anxiety
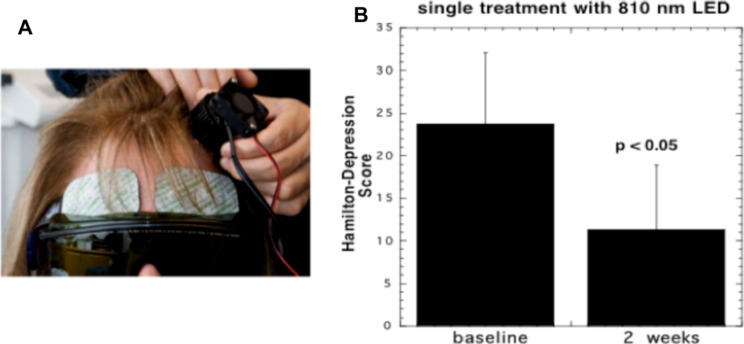
The first clinical study in depression and anxiety was published by Schiffer et al. in 2009 [109]. They used a fairly small area 1 W 810 nm LED array (see Fig. 6A) applied to the forehead in patients with major depression and anxiety. They found improvements in the Hamilton depression rating scale (HAM-D) (see Fig. 6B), and the Hamilton anxiety rating scale (HAM-A), 2 weeks after a single treatment. They also found increases in frontal pole regional cerebral blood flow (rCBF) during the light delivery using a commercial NIR spectroscopy device. Cassano and co-workers [110] used tPBM with an 810 nm laser (700 mW/cm2 and a fluence of 84 J/cm2 delivered per session for 6 sessions in patients with major depression. Baseline mean HAM-D17 scores decreased from 19.8 ± 4.4 (SD) to 13 ± 5.35 (SD) after treatment (p = 0.004).
Fig. 6.
tPBM for major depression and anxiety in humans. (A) Ten patients received a single exposure to the forehead (810 LED, 60 J/cm2 delivered at 250 mW/cm2). (B) Mean Hamilton score for depression at baseline and at two weeks post-treatment [109].
9. Cognitive enhancement
From what we have seen above, it need come as no surprise, to learn that there are several reports about cognitive enhancement in normal people or healthy animals using PBM. The first report was in middle aged (12 months) CD1 female mice [111]. Exposure of the mice to 1072 nm LED arrays led to improved performance in a 3D maze compared to sham treated age-matched controls. Francisco Gonzalez-Lima at the University of Texas Austin, has worked in this area for some time [112]. Working in rats they showed that transcranial PBM (9 mW/cm2 with 660 nm LED array) induced a dose-dependent increase in oxygen consumption of 5% after 1 J/cm2 and 16% after 5 J/cm2 [113]. They also found that tPBM reduced fear renewal and prevented the reemergence of extinguished conditioned fear responses [113]. In normal human volunteers they used transcranial PBM (1064 nm laser, 60 J/cm2 at 250 mW/cm2) delivered to the forehead in a placebo-controlled, randomized study, to influence cognitive tasks related to the prefrontal cortex, including a psychomotor vigilance task (PVT), a delayed match-to-sample (DMS) memory task, and the positive and negative affect schedule (PANAS-X) to show improved mood [16]. Subsequent studies in normal humans showed that tPBM with 1064 nm laser could improve performance in the Wisconsin Card Sorting Task (considered the gold standard test for executive function) [114]. They also showed that tPBM to the right forehead (but not the left forehead) had better effects on improving attention bias modification (ABM) in humans with depression [115].
A study by Salgado et al. used transcranial LED PBM on cerebral blood flow in healthy elderly women analyzed by transcranial Doppler ultrasound (TCD) of the right and left middle cerebral artery and basilar artery. Twenty-five non-institutionalized elderly women (mean age 72 years old), with cognitive status > 24, were assessed using TCD before and after transcranial LED therapy. tPBM (627 nm, 70 mW/cm2, 10 J/cm2) was performed at four points of the frontal and parietal region for 30 s each twice a week for 4 weeks. There was a significant increase in the systolic and diastolic velocity of the left middle cerebral artery (25 and 30%, respectively) and the basilar artery (up to 17 and 25%), as well as a decrease in the pulsatility index and resistance index values of the three cerebral arteries analyzed [116].
10. Conclusion
Many investigators believe that PBM for brain disorders will become one of the most important medical applications of light therapy in the coming years and decades. Despite the efforts of “Big Pharma”, prescription drugs for psychiatric disorders are not generally regarded very highly (either by the medical profession or by the public), and many of these drugs perform little better than placebos in different trials, and moreover can also have major side-effects [117]. Moreover it is well accepted that with the overall aging of the general population, together with ever lengthening life spans, that dementia, Alzheimer's, and Parkinson's diseases will become a global health problem [118], [119]. Even after many years of research, no drug has yet been developed to benefit these neurodegenerative disorders. A similar state of play exists with drugs for stroke (with the exception of clot-busting enzymes) and TBI. New indications for tPBM such as global ischemia (brain damage after a heart attack), post-operative cognitive dysfunction [120], and neurodevelopmental disorders such as autism spectrum disorder may well emerge. Table 2 shows the wide range of brain disorders and diseases that may eventually be treated by some kind of tPBM, whether that be an office/clinic based procedure or a home-use based device. If inexpensive LED helmets can be developed and successfully marketed as home use devices, then we are potentially in a position to benefit large numbers of patients (to say nothing of healthy individuals). Certainly the advent of the Internet has made it much easier for knowledge about this kind of home treatment to spread (almost by word of mouth so to speak).
Table 2.
List of brain disorders that may in principle be treated by tPBM.
| Type of brain disorder | |||
|---|---|---|---|
| Traumatic | Neurodegenerative | Psychiatric | Neurodevelopmental |
| Acute stroke | Alzheimer's disease | Depression (major, bipolar, suicidal ideation) | Autism (autism spectrum disorder) |
| Chronic stroke | Parkinson's disease | Psychosis (schizophrenia) | Attention deficit hyperactivity disorder (ADHD) |
| Acute TBI | Other dementias (vascular, Lewy bodies, frontotemporal) | Post traumatic stress disorder (PTSD) | |
| Chronic TBI | Chronic traumatic encephalopathy | Addiction | |
| Global ischemia | Amyotrophic lateral sclerosis (Lou Gehrig's disease) | Insomnia | |
| Coma (vegetative state) | Primary progressive aphasia | ||
| Birth trauma (neonatal stroke) | Prion diseases (Creutzfeldt-Jakob) | ||
| “Chemo-brain” | Huntington's disease | ||
Conflict of interest statement
The author declares no conflict of interest.
Transparency document
Transparency document.
Acknowledgments
MRH was supported by the US NIH grants R01AI050875 and R21AI121700, the Air Force Office of Scientific Research grant FA9550-13-1-0068, the US Army Medical Research Acquisition Activity grant W81XWH-09-1-0514, and by the US Army Medical Research and Materiel Command grant W81XWH-13-2-0067.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- 1.McGuff P.E., Deterling R.A., Jr., Gottlieb L.S. Tumoricidal effect of laser energy on experimental and human malignant tumors. N. Engl. J. Med. 1965;273:490–492. doi: 10.1056/NEJM196508262730906. [DOI] [PubMed] [Google Scholar]
- 2.Maiman T.H. Stimulated optical radiation in ruby. Nature. 1960;187:493–494. [Google Scholar]
- 3.Mester E., Ludány G., Sellyei M., Szende B., Total G.J. The simulating effect of low power laser rays on biological systems. Laser Rev. 1968;1:3. [Google Scholar]
- 4.Mester E., Szende B., Gartner P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. (Berl) 1968;9:621–626. [PubMed] [Google Scholar]
- 5.Mester E., Mester A.F., Mester A. The biomedical effects of laser application. Lasers Surg. Med. 1985;5:31–39. doi: 10.1002/lsm.1900050105. [DOI] [PubMed] [Google Scholar]
- 6.Mester E., Nagylucskay S., Doklen A., Tisza S. Laser stimulation of wound healing. Acta Chir. Acad. Sci. Hung. 1976;17:49–55. [PubMed] [Google Scholar]
- 7.Mester E., Spiry T., Szende B. Effect of laser rays on wound healing. Bull. Soc. Int. Chir. 1973;32:169–173. [PubMed] [Google Scholar]
- 8.Chung H., Dai T., Sharma S.K., Huang Y.Y., Carroll J.D., Hamblin M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anders J.J., Lanzafame R.J., Arany P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015;33:183–184. doi: 10.1089/pho.2015.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Freitas L.F., Hamblin M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016;22:7000417. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane N. Cell biology: power games. Nature. 2006;443:901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 12.Waypa G.B., Smith K.A., Schumacker P.T. O2 sensing, mitochondria and ROS signaling: the fog is lifting. Mol. Asp. Med. 2016;47-48:76–89. doi: 10.1016/j.mam.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y., Vanhoutte P.M., Leung S.W. Vascular nitric oxide: beyond eNOS. J. Pharmacol. Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante M., Petrini M., Trentini P., Perfetti G., Spoto G. Effect of low-level laser therapy after extraction of impacted lower third molars. Lasers Med. Sci. 2013;28:845–849. doi: 10.1007/s10103-012-1174-4. [DOI] [PubMed] [Google Scholar]
- 15.Skopin M.D., Molitor S.C. Effects of near-infrared laser exposure in a cellular model of wound healing. Photodermatol. Photoimmunol. Photomed. 2009;25:75–80. doi: 10.1111/j.1600-0781.2009.00406.x. [DOI] [PubMed] [Google Scholar]
- 16.Barrett D.W., Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13–23. doi: 10.1016/j.neuroscience.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Dougal G., Lee S.Y. Evaluation of the efficacy of low-level light therapy using 1072 nm infrared light for the treatment of herpes simplex labialis. Clin. Exp. Dermatol. 2013;38:713–718. doi: 10.1111/ced.12069. [DOI] [PubMed] [Google Scholar]
- 18.Vatansever F., Hamblin M.R. Far infrared radiation (FIR): its biological effects and medical applications. Photonics Lasers Med. 2012;4:255–266. doi: 10.1515/plm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palazzo E., Rossi F., de Novellis V., Maione S. Endogenous modulators of TRP channels. Curr. Top. Med. Chem. 2013;13:398–407. doi: 10.2174/1568026611313030014. [DOI] [PubMed] [Google Scholar]
- 20.Planells-Cases R., Valente P., Ferrer-Montiel A., Qin F., Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: implications for therapeutic intervention. Adv. Exp. Med. Biol. 2011;704:491–515. doi: 10.1007/978-94-007-0265-3_27. [DOI] [PubMed] [Google Scholar]
- 21.Caterina M.J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 22.Cassano P., Petrie S.R., Hamblin M.R., Henderson T.A., Iosifescu D.V. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics. 2016;3:031404. doi: 10.1117/1.NPh.3.3.031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morries L.D., Cassano P., Henderson T.A. Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat. 2015;11:2159–2175. doi: 10.2147/NDT.S65809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian F., Hase S.N., Gonzalez-Lima F., Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg. Med. 2016;48:343–349. doi: 10.1002/lsm.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ando T., Xuan W., Xu T., Dai T., Sharma S.K., Kharkwal G.B., Huang Y.Y., Wu Q., Whalen M.J., Sato S., Obara M., Hamblin M.R. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabrese E.J. Hormesis and medicine. Br. J. Clin. Pharmacol. 2008;66:594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luckey T.D. Nurture with ionizing radiation: a provocative hypothesis. Nutr. Cancer. 1999;34:1–11. doi: 10.1207/S15327914NC340101. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y.Y., Chen A.C., Carroll J.D., Hamblin M.R. Biphasic dose response in low level light therapy. Dose-Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y.Y., Sharma S.K., Carroll J.D., Hamblin M.R. Biphasic dose response in low level light therapy - an update. Dose-Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S., Zhou F., Wei Y., Chen W.R., Chen Q., Xing D. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid. Redox Signal. 2014;20:733–746. doi: 10.1089/ars.2013.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hode L. The importance of the coherency. Photomed. Laser Surg. 2005;23:431–434. doi: 10.1089/pho.2005.23.431. [DOI] [PubMed] [Google Scholar]
- 32.Cui X., Bray S., Bryant D.M., Glover G.H., Reiss A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haeussinger F.B., Heinzel S., Hahn T., Schecklmann M., Ehlis A.C., Fallgatter A.J. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strangman G.E., Zhang Q., Li Z. Scalp and skull influence on near infrared photon propagation in the Colin27 brain template. NeuroImage. 2014;85(Pt 1):136–149. doi: 10.1016/j.neuroimage.2013.04.090. [DOI] [PubMed] [Google Scholar]
- 35.Okada E., Delpy D.T. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl. Opt. 2003;42:2915–2922. doi: 10.1364/ao.42.002915. [DOI] [PubMed] [Google Scholar]
- 36.Jagdeo J.R., Adams L.E., Brody N.I., Siegel D.M. Transcranial red and near infrared light transmission in a cadaveric model. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tedford C.E., DeLapp S., Jacques S., Anders J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg. Med. 2015;47:312–322. doi: 10.1002/lsm.22343. [DOI] [PubMed] [Google Scholar]
- 38.Lapchak P.A., Boitano P.D., Butte P.V., Fisher D.J., Holscher T., Ley E.J., Nuno M., Voie A.H., Rajput P.S. Transcranial near-infrared laser transmission (NILT) profiles (800 nm): systematic comparison in four common research species. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitzschke A., Lovisa B., Seydoux O., Zellweger M., Pfleiderer M., Tardy Y., Wagnieres G. Red and NIR light dosimetry in the human deep brain. Phys. Med. Biol. 2015;60:2921–2937. doi: 10.1088/0031-9155/60/7/2921. [DOI] [PubMed] [Google Scholar]
- 40.Pitzschke A., Lovisa B., Seydoux O., Haenggi M., Oertel M.F., Zellweger M., Tardy Y., Wagnieres G. Optical properties of rabbit brain in the red and near-infrared: changes observed under in vivo, postmortem, frozen, and formalin-fixated conditions. J. Biomed. Opt. 2015;20:25006. doi: 10.1117/1.JBO.20.2.025006. [DOI] [PubMed] [Google Scholar]
- 41.Yaroslavsky A.N., Schulze P.C., Yaroslavsky I.V., Schober R., Ulrich F., Schwarzmaier H.J. Optical properties of selected native and coagulated human brain tissues in vitro in the visible and near infrared spectral range. Phys. Med. Biol. 2002;47:2059–2073. doi: 10.1088/0031-9155/47/12/305. [DOI] [PubMed] [Google Scholar]
- 42.Henderson T.A., Morries L.D. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 2015;11:2191–2208. doi: 10.2147/NDT.S78182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnstone D.M., el Massri N., Moro C., Spana S., Wang X.S., Torres N., Chabrol C., De Jaeger X., Reinhart F., Purushothuman S., Benabid A.L., Stone J., Mitrofanis J. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism - an abscopal neuroprotective effect. Neuroscience. 2014;274:93–101. doi: 10.1016/j.neuroscience.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Johnstone D.M., Mitrofanis J., Stone J. Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light. Neural Regen. Res. 2015;10:349–351. doi: 10.4103/1673-5374.153673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farfara D., Tuby H., Trudler D., Doron-Mandel E., Maltz L., Vassar R.J., Frenkel D., Oron U. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer's disease. J. Mol. Neurosci. 2015;55:430–436. doi: 10.1007/s12031-014-0354-z. [DOI] [PubMed] [Google Scholar]
- 46.Oron A., Oron U. Low-level laser therapy to the bone marrow ameliorates neurodegenerative disease progression in a mouse model of Alzheimer's disease: a minireview. Photomed. Laser Surg. 2016 doi: 10.1089/pho.2015.4072. [DOI] [PubMed] [Google Scholar]
- 47.Iwashita T., Tada T., Zhan H., Tanaka Y., Hongo K. Harvesting blood stem cells from cranial bone at craniotomy–a preliminary study. J. Neuro-Oncol. 2003;64:265–270. doi: 10.1023/a:1025684903137. [DOI] [PubMed] [Google Scholar]
- 48.Quah-Smith I., Williams M.A., Lundeberg T., Suo C., Sachdev P. Differential brain effects of laser and needle acupuncture at LR8 using functional MRI. Acupunct. Med. 2013;31:282–289. doi: 10.1136/acupmed-2012-010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quah-Smith I., Sachdev P.S., Wen W., Chen X., Williams M.A. The brain effects of laser acupuncture in healthy individuals: an FMRI investigation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutalangka C., Wattanathorn J., Muchimapura S., Thukham-Mee W., Wannanon P., Tong-un T. Laser acupuncture improves memory impairment in an animal model of Alzheimer's disease. J. Acupunct. Meridian Stud. 2013;6:247–251. doi: 10.1016/j.jams.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Khongrum J., Wattanathorn J. Laser acupuncture improves behavioral disorders and brain oxidative stress status in the valproic acid rat model of autism. J. Acupunct. Meridian Stud. 2015;8:183–191. doi: 10.1016/j.jams.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Quah-Smith I., Suo C., Williams M.A., Sachdev P.S. The antidepressant effect of laser acupuncture: a comparison of the resting brain's default mode network in healthy and depressed subjects during functional magnetic resonance imaging. Med. Acupunct. 2013;25:124–133. doi: 10.1089/acu.2012.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henderson T.A. Multi-watt near-infrared light therapy as a neuroregenerative treatment for traumatic brain injury. Neural Regen. Res. 2016;11:563–565. doi: 10.4103/1673-5374.180737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hacke W., Schellinger P.D., Albers G.W., Bornstein N.M., Dahlof B.L., Fulton R., Kasner S.E., Shuaib A., Richieri S.P., Dilly S.G., Zivin J., Lees K.R., Committees N. Investigators, transcranial laser therapy in acute stroke treatment: results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke. 2014;45:3187–3193. doi: 10.1161/STROKEAHA.114.005795. [DOI] [PubMed] [Google Scholar]
- 55.Albin R.L., Miller R.A. Mini-review: retarding aging in murine genetic models of neurodegeneration. Neurobiol. Dis. 2016;85:73–80. doi: 10.1016/j.nbd.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bey A.L., Jiang Y.H. Overview of mouse models of autism spectrum disorders. Curr. Protoc. Pharmacol. 2014;66 doi: 10.1002/0471141755.ph0566s66. (5 66 61–26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung M.C., Lo S.C., Siu F.K., So K.F. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg. Med. 2002;31:283–288. doi: 10.1002/lsm.10096. [DOI] [PubMed] [Google Scholar]
- 58.Peplow P.V. Neuroimmunomodulatory effects of transcranial laser therapy combined with intravenous tPA administration for acute cerebral ischemic injury. Neural Regen. Res. 2015;10:1186–1190. doi: 10.4103/1673-5374.162687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oron A., Oron U., Chen J., Eilam A., Zhang C., Sadeh M., Lampl Y., Streeter J., DeTaboada L., Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37:2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L., Chen J., Li Y., Zhang Z.G., Chopp M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J. Neurol. Sci. 2000;174:141–146. doi: 10.1016/s0022-510x(00)00268-9. [DOI] [PubMed] [Google Scholar]
- 61.Meyer D.M., Chen Y., Zivin J.A. Dose-finding study of phototherapy on stroke outcome in a rabbit model of ischemic stroke. Neurosci. Lett. 2016;630:254–258. doi: 10.1016/j.neulet.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 62.Lapchak P.A., Salgado K.F., Chao C.H., Zivin J.A. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148:907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Detaboada L., Ilic S., Leichliter-Martha S., Oron U., Oron A., Streeter J. Transcranial application of low-energy laser irradiation improves neurological deficits in rats following acute stroke. Lasers Surg. Med. 2006;38:70–73. doi: 10.1002/lsm.20256. [DOI] [PubMed] [Google Scholar]
- 64.Lapchak P.A., Wei J., Zivin J.A. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- 65.Lampl Y., Zivin J.A., Fisher M., Lew R., Welin L., Dahlof B., Borenstein P., Andersson B., Perez J., Caparo C., Ilic S., Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 66.Huisa B.N., Stemer A.B., Walker M.G., Rapp K., Meyer B.C., Zivin J.A. Nest, investigators, transcranial laser therapy for acute ischemic stroke: a pooled analysis of NEST-1 and NEST-2. Int. J. Stroke. 2013;8:315–320. doi: 10.1111/j.1747-4949.2011.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zivin J.A., Sehra R., Shoshoo A., Albers G.W., Bornstein N.M., Dahlof B., Kasner S.E., Howard G., Shuaib A., Streeter J., Richieri S.P., Hacke W., N.-. investigators NeuroThera(R) Efficacy and Safety Trial-3 (NEST-3): a double-blind, randomized, sham-controlled, parallel group, multicenter, pivotal study to assess the safety and efficacy of transcranial laser therapy with the NeuroThera(R) laser system for the treatment of acute ischemic stroke within 24 h of stroke onset. Int. J. Stroke. 2014;9:950–955. doi: 10.1111/j.1747-4949.2012.00896.x. [DOI] [PubMed] [Google Scholar]
- 68.Zivin J.A., Albers G.W., Bornstein N., Chippendale T., Dahlof B., Devlin T., Fisher M., Hacke W., Holt W., Ilic S., Kasner S., Lew R., Nash M., Perez J., Rymer M., Schellinger P., Schneider D., Schwab S., Veltkamp R., Walker M., Streeter J., NeuroThera E., Safety Trial I. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40:1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- 69.Lapchak P.A., Boitano P.D. Transcranial near-infrared laser therapy for stroke: how to recover from futility in the NEST-3 clinical trial. Acta Neurochir. Suppl. 2016;121:7–12. doi: 10.1007/978-3-319-18497-5_2. [DOI] [PubMed] [Google Scholar]
- 70.Lapchak P.A. Fast neuroprotection (fast-NPRX) for acute ischemic stroke victims: the time for treatment is now. Transl. Stroke. Res. 2013;4:704–709. doi: 10.1007/s12975-013-0303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapchak P.A. Recommendations and practices to optimize stroke therapy: developing effective translational research programs. Stroke. 2013;44:841–843. doi: 10.1161/STROKEAHA.112.680439. [DOI] [PubMed] [Google Scholar]
- 72.Lapchak P.A., Zhang J.H., Noble-Haeusslein L.J. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl. Stroke. Res. 2013;4:279–285. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naeser M., Ho M., Martin P.E., Treglia E.M., Krengel M., Hamblin M.R., Baker E.H. Improved language after scalp application of red/near-infrared light-emitting diodes: pilot study supporting a new, noninvasive treatment for chronic aphasia. Procedia. Soc. Behav. Sci. 2012;61:138–139. [Google Scholar]
- 74.Boonswang N.A., Chicchi M., Lukachek A., Curtiss D. A new treatment protocol using photobiomodulation and muscle/bone/joint recovery techniques having a dramatic effect on a stroke patient's recovery: a new weapon for clinicians. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.08.2011.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doidge N. Viking Press; New York, NY: 2015. The Brain's Way of Healing: Remarkable Discoveries and Recoveries from the Frontiers of Neuroplasticity. [Google Scholar]
- 76.Oron A., Oron U., Streeter J., de Taboada L., Alexandrovich A., Trembovler V., Shohami E. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J. Neurotrauma. 2007;24:651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- 77.Wu Q., Xuan W., Ando T., Xu T., Huang L., Huang Y.Y., Dai T., Dhital S., Sharma S.K., Whalen M.J., Hamblin M.R. Low-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengths. Lasers Surg. Med. 2012;44:218–226. doi: 10.1002/lsm.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karu T.I., Pyatibrat L.V., Afanasyeva N.I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005;36:307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- 79.Xuan W., Vatansever F., Huang L., Wu Q., Xuan Y., Dai T., Ando T., Xu T., Huang Y.Y., Hamblin M.R. Transcranial low-level laser therapy improves neurological performance in traumatic brain injury in mice: effect of treatment repetition regimen. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xuan W., Agrawal T., Huang L., Gupta G.K., Hamblin M.R. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics. 2015;8:502–511. doi: 10.1002/jbio.201400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xuan W., Vatansever F., Huang L., Hamblin M.R. Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J. Biomed. Opt. 2014;19:108003. doi: 10.1117/1.JBO.19.10.108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khuman J., Zhang J., Park J., Carroll J.D., Donahue C., Whalen M.J. Low-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in mice. J. Neurotrauma. 2012;29:408–417. doi: 10.1089/neu.2010.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quirk B.J., Torbey M., Buchmann E., Verma S., Whelan H.T. Near-infrared photobiomodulation in an animal model of traumatic brain injury: improvements at the behavioral and biochemical levels. Photomed. Laser Surg. 2012;30:523–529. doi: 10.1089/pho.2012.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Q., Zhou C., Hamblin M.R., Wu M.X. Low-level laser therapy effectively prevents secondary brain injury induced by immediate early responsive gene X-1 deficiency. J. Cereb. Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong T., Zhang Q., Hamblin M.R., Wu M.X. Low-level light in combination with metabolic modulators for effective therapy of injured brain. J. Cereb. Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naeser M.A., Hamblin M.R. Traumatic brain injury: a major medical problem that could be treated using transcranial, red/near-infrared LED photobiomodulation. Photomed. Laser Surg. 2015 doi: 10.1089/pho.2015.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McClure J. The role of causal attributions in public misconceptions about brain injury. Rehabil. Psychol. 2011;56:85–93. doi: 10.1037/a0023354. [DOI] [PubMed] [Google Scholar]
- 88.Naeser M.A., Saltmarche A., Krengel M.H., Hamblin M.R., Knight J.A. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed. Laser Surg. 2011;29:351–358. doi: 10.1089/pho.2010.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naeser M.A., Martin P.I., Lundgren K., Klein R., Kaplan J., Treglia E., Ho M., Nicholas M., Alonso M., Pascual-Leone A. Improved language in a chronic nonfluent aphasia patient after treatment with CPAP and TMS. Cogn. Behav. Neurol. 2010;23:29–38. doi: 10.1097/WNN.0b013e3181bf2d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naeser M.A., Zafonte R., Krengel M.H., Martin P.I., Frazier J., Hamblin M.R., Knight J.A., Meehan W.P., III, Baker E.H. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J. Neurotrauma. 2014;31:1008–1017. doi: 10.1089/neu.2013.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henderson T.A., Morries L.D. SPECT perfusion imaging demonstrates improvement of traumatic brain injury with transcranial near-infrared laser phototherapy. Adv. Mind Body Med. 2015;29:27–33. [PubMed] [Google Scholar]
- 92.De Taboada L., Yu J., El-Amouri S., Gattoni-Celli S., Richieri S., McCarthy T., Streeter J., Kindy M.S. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J. Alzheimers Dis. 2011;23:521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 93.https://www.sciencebasedmedicine.org/science-by-press-release-a-helmet-to-fight-alzheimers-disease/ (in)
- 94.Saltmarche A.E., Naeser M.A., Ho K.F., Hamblin M.R., Lim L. Alzheimer's Association International Conference, Toronto, Canada. 2016. Significant Improvement in Cognition after Transcranial and Intranasal Photobiomodulation: A Controlled, Single-Blind Pilot Study in Participants with Dementia (Abstract) [Google Scholar]
- 95.Maksimovich I.V. Dementia and cognitive impairment reduction after laser transcatheter treatment of Alzheimer's disease. World J. Neurosci. 2015;5 [Google Scholar]
- 96.Johnstone D.M., Moro C., Stone J., Benabid A.L., Mitrofanis J. Turning on lights to stop neurodegeneration: the potential of near infrared light therapy in Alzheimer's and Parkinson's disease. Front. Neurosci. 2015;9:500. doi: 10.3389/fnins.2015.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shaw V.E., Spana S., Ashkan K., Benabid A.L., Stone J., Baker G.E., Mitrofanis J. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 2010;518:25–40. doi: 10.1002/cne.22207. [DOI] [PubMed] [Google Scholar]
- 98.Barcia C. Who else was intoxicated with MPTP in Santa Clara? Parkinsonism Relat. Disord. 2012;18:1005–1006. doi: 10.1016/j.parkreldis.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 99.Peoples C., Spana S., Ashkan K., Benabid A.L., Stone J., Baker G.E., Mitrofanis J. Photobiomodulation enhances nigral dopaminergic cell survival in a chronic MPTP mouse model of Parkinson's disease. Parkinsonism Relat. Disord. 2012;18:469–476. doi: 10.1016/j.parkreldis.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Purushothuman S., Nandasena C., Johnstone D.M., Stone J., Mitrofanis J. The impact of near-infrared light on dopaminergic cell survival in a transgenic mouse model of parkinsonism. Brain Res. 2013;1535:61–70. doi: 10.1016/j.brainres.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 101.Moro C., Massri N.E., Torres N., Ratel D., De Jaeger X., Chabrol C., Perraut F., Bourgerette A., Berger M., Purushothuman S., Johnstone D., Stone J., Mitrofanis J., Benabid A.L. Photobiomodulation inside the brain: a novel method of applying near-infrared light intracranially and its impact on dopaminergic cell survival in MPTP-treated mice. J. Neurosurg. 2014;120:670–683. doi: 10.3171/2013.9.JNS13423. [DOI] [PubMed] [Google Scholar]
- 102.El Massri N., Moro C., Torres N., Darlot F., Agay D., Chabrol C., Johnstone D.M., Stone J., Benabid A.L., Mitrofanis J. Near-infrared light treatment reduces astrogliosis in MPTP-treated monkeys. Exp. Brain Res. 2016 doi: 10.1007/s00221-016-4720-7. [DOI] [PubMed] [Google Scholar]
- 103.Moro C., Massri N.E., Darlot F., Torres N., Chabrol C., Agay D., Auboiroux V., Johnstone D.M., Stone J., Mitrofanis J., Benabid A.L. Effects of a higher dose of near-infrared light on clinical signs and neuroprotection in a monkey model of Parkinson's disease. Brain Res. 2016 doi: 10.1016/j.brainres.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 104.Maloney R., Shanks S., Maloney J. The application of low-level laser therapy for the symptomatic care of late stage Parkinson's disease: a non-controlled, non-randomized study (abstract) Lasers Surg. Med. 2010;185 [Google Scholar]
- 105.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 106.Anisman H., Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 107.Wu X., Alberico S.L., Moges H., De Taboada L., Tedford C.E., Anders J.J. Pulsed light irradiation improves behavioral outcome in a rat model of chronic mild stress. Lasers Surg. Med. 2012;44:227–232. doi: 10.1002/lsm.22004. [DOI] [PubMed] [Google Scholar]
- 108.Salehpour F., Rasta S.H., Mohaddes G., Sadigh-Eteghad S., Salarirad S. Therapeutic effects of 10-HzPulsed wave lasers in rat depression model: a comparison between near-infrared and red wavelengths. Lasers Surg. Med. 2016 doi: 10.1002/lsm.22542. [DOI] [PubMed] [Google Scholar]
- 109.Schiffer F., Johnston A.L., Ravichandran C., Polcari A., Teicher M.H., Webb R.H., Hamblin M.R. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav. Brain Funct. 2009;5:46. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassano P., Cusin C., Mischoulon D., Hamblin M.R., De Taboada L., Pisoni A., Chang T., Yeung A., Ionescu D.F., Petrie S.R., Nierenberg A.A., Fava M., Iosifescu D.V. Near-infrared transcranial radiation for major depressive disorder: proof of concept study. Psychiatry J. 2015;2015:352979. doi: 10.1155/2015/352979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Michalikova S., Ennaceur A., van Rensburg R., Chazot P.L. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light. Neurobiol. Learn. Mem. 2008;89:480–488. doi: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 112.Gonzalez-Lima F., Barrett D.W. Augmentation of cognitive brain functions with transcranial lasers. Front. Syst. Neurosci. 2014;8:36. doi: 10.3389/fnsys.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rojas J.C., Bruchey A.K., Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. J. Alzheimers Dis. 2012;32:741–752. doi: 10.3233/JAD-2012-120817. [DOI] [PubMed] [Google Scholar]
- 114.Blanco N.J., Maddox W.T., Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J. Neuropsychol. 2015 doi: 10.1111/jnp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Disner S.G., Beevers C.G., Gonzalez-Lima F. Transcranial laser stimulation as neuroenhancement for attention bias modification in adults with elevated depression symptoms. Brain Stimul. 2016 doi: 10.1016/j.brs.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salgado A.S., Zangaro R.A., Parreira R.B. Kerppers, II, the effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers Med. Sci. 2015;30:339–346. doi: 10.1007/s10103-014-1669-2. [DOI] [PubMed] [Google Scholar]
- 117.Kalmar S. The importance of neuropsychopharmacology in the development of psychiatry. Neuropsychopharmacol. Hung. 2014;16:149–156. [PubMed] [Google Scholar]
- 118.Sindi S., Mangialasche F., Kivipelto M. Advances in the prevention of Alzheimer's disease. F1000Prime Rep. 2015;7:50. doi: 10.12703/P7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bellou V., Belbasis L., Tzoulaki I., Evangelou E., Ioannidis J.P. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat. Disord. 2016;23:1–9. doi: 10.1016/j.parkreldis.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 120.Liebert A.D., Chow R.T., Bicknell B.T., Varigos E. Neuroprotective effects against POCD by photobiomodulation: evidence from assembly/disassembly of the cytoskeleton. J. Exp. Neurosci. 2016;10:1–19. doi: 10.4137/JEN.S33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ando T., Xuan W., Xu T., Dai T., Sharma S.K., Kharkwal G.B., Huang Y.Y., Wu Q., Whalen M.J., Sato S., Obara M., Hamblin M.R. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One. 2011;6:e26212–e26220. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.