Abstract
There are considerable variations in the anatomy of the human ophthalmic artery (OphA), such as anomalous origins of the OphA and anastomoses between the OphA and the adjacent arteries. These anatomical variations seem to attribute to complex embryology of the OphA. In human embryos and fetuses, primitive dorsal and ventral ophthalmic arteries (PDOphA and PVOphA) form the ocular branches, and the supraorbital division of the stapedial artery forms the orbital branches of the OphA, and then numerous anastomoses between the internal carotid artery (ICA) and the external carotid artery (ECA) systems emerge in connection with the OphA. These developmental processes can produce anatomical variations of the OphA, and we should notice these variations for neurosurgical and neurointerventional procedures.
Keywords: ophthalmic artery, anatomy, embryology, stapedial artery, primitive maxillary artery
Introduction
The ophthalmic artery (OphA) consists of ocular and orbital branches. The ocular branches contribute to the blood supply of the optic apparatus, namely, the optic nerve and the retina, and the orbital branches supply the optic adnexae, such as extraocular muscles, lacrimal glands, and eyelids.1)
Embryologically, the ocular branches appear at the optic vesicle stage, and basically develop while optic cup and optic stalk are formed, and then, the orbital branches are formed and annexed with the ocular branches.2–5) Consequently, the anatomy of the OphA is highly complex. We can encounter various anomalous origins of the OphA, and the anastomoses between the internal carotid artery (ICA) and external carotid artery (ECA) systems via the OphA.1,4–9)
These variations could carry a potential risk of procedural complications of craniotomy or endovascular treatment, such as obliteration of the middle meningeal artery (MMA) supplying the OphA during skull base surgery, and embolic material migration during embolization of dural arteriovenous shunt, tumor, or epistaxis.10,11) Therefore, anatomical knowledge of the OphA is essential in neurosurgical and neurointerventional practice. The aim of this article is to elucidate the anatomical variation of the OphA from the embryological viewpoint.
Embryology and Anatomy of the OphA
Development of the OphA (Fig. 1)
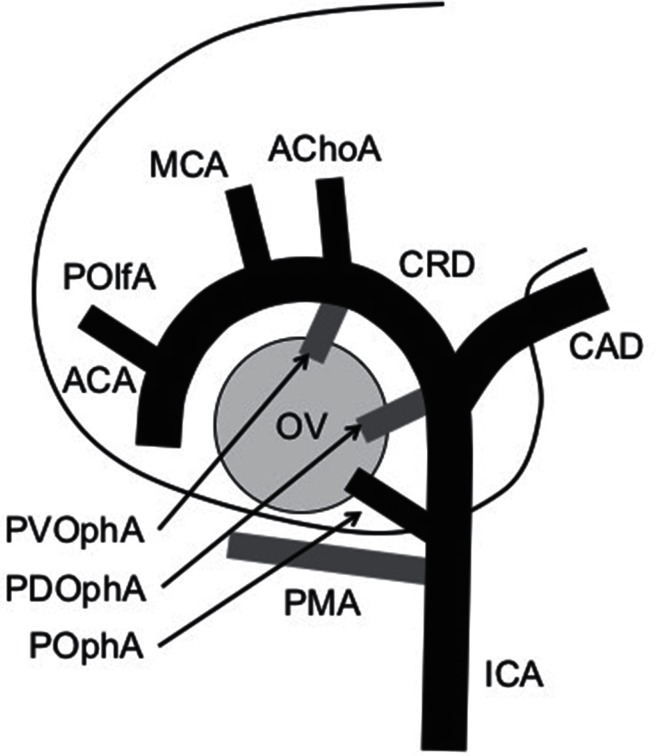
Fig. 1.
Schematic diagram of the embryonic arteries around the forebrain. ICA: internal carotid artery, CRD: cranial division, CAD: caudal division, AChoA: anterior choroidal artery, MCA: middle cerebral artery, ACA: anterior cerebral artery, POlfA: primitive olfactory artery, PMA: primitive maxillary artery, POphA: permanent stem of ophthalmic artery, PDOphA: primitive dorsal ophthalmic artery, PVOphA: primitive ventral ophthalmic artery, OV: optic vesicle. Grey lines show temporary arteries. Modified from Fig. 12, Padget DH, 1948.2)
Padget’s description of the development of the cranial arteries, published in 1948, provides us tremendous information on the embryology of the OphA.2)
In 4–5 mm (crown–rump length) embryo, the blood supply to the optic vesicle is highly plexiform from the primitive maxillary artery (PMA) and the primitive ICA extending cranially to the dorsal aspect of the vesicle. The PMA originates from the future cavernous segment of the ICA. At 5–6 mm stage, the optic cup and the optic stalk begin to form and the primitive dorsal OphA (PDOphA) emerges. The PDOphA originates from the junction of the cranial and caudal division of the primitive ICA, namely, at the level of the origin of the future posterior communicating artery. At 7–12 mm stage, the PDOphA supplies capillary plexus to the optic cup, and the primitive ventral OphA (PVOphA) emerges opposite the anterior choroidal artery. The blood supply to the optic tissue is still plexiform at this stage. In 12–14 mm embryo, the PDOphA has two optic branches, a common temporal ciliary artery (future lateral posterior ciliary artery) and a hyaloid artery (future central retinal artery), and the PVOphA supplies the common nasal ciliary artery (future medial posterior ciliary artery).
At 16–18 mm stage, the stem of the OphA has reached at its adult point, and the PVOphA and the PDOphA become the branches of the permanent stem of the OphA. At the same stage, the stapedial artery from the future petrous segment of the ICA gives rise to the maxillomandibular division and supraorbital division. At 20–24 mm stage, the supraorbital division forms an anastomosis with the OphA, and the optic nerve is surrounded by an arterial ring at the origin of the three primary optic branches from the PDOphA and the PVOphA. At 40 mm stage, the OphA have attained approximate adult configuration.
Course of the OphA
The OphA is the first major branch of the ICA. The usual origin of the OphA is just above the distal dural ring. The course of the OphA can be divided into three parts: intracranial, intracanalicular, and intraorbital sections. The OphA usually runs inferolateral to the optic nerve in the optic canal. About 3% of the artery enters the orbit from the cranial cavity in a separate bony canal called duplicate optic canal.1,6,9)
Intraorbital course can be divided into three segments. In the first segment, the OphA runs forward inferolateral to the optic nerve, and then changes the direction at the junction of the first and second segments. The second segment crosses over to go medial to the optic nerve in 83% and crosses under the optic nerve in 17%. In the third segment, the OphA runs forward medial to the optic nerve and close to the medial wall of the orbit, and terminates at the superomedial angle of the orbital opening.1,7,9)
Branches of the OphA
The branches of the OphA have no fixed pattern because of significant interindividual variations, and it should be referred to as a usual pattern.1,8) The ocular branches include the central retinal artery, the lateral posterior ciliary artery, and the medial posterior ciliary artery. The origin of these branches is adjacent to the junction between the first and second segments of the OphA, therefore, there is no ocular branch distal to the second segment.1,8,9) Since the central retinal artery originates close to the origin of the ciliary arteries, “choroidal brush” depicted by the ciliary arteries is accepted as a landmark of the central retinal artery. The orbital and extra-orbital branches include the lacrimal artery, muscular arteries, the posterior and anterior ethmoidal arteries, the supraorbital artery, the medial palpebral artery, the dorsal nasal artery, and the frontal artery.
There are potential anastomoses between the distal branches of the OphA and the branches of the maxillary artery. The infraorbital artery runs along the inferior wall of the orbit and may anastomose to the medial and lateral muscular branches of the OphA. The anterior deep temporal artery may anastomose to the lacrimal artery. The septal branch of the sphenopalatine artery may anastomose to the anterior and posterior ethmoidal artery. As cutaneous collaterals, there are potential anastomoses to the OphA from the superficial temporal artery, the transverse facial artery, and the facial artery.4,11)
Origin of the OphA
Various anomalous origins of the OphA have been reported in the literature. The MMA origin is the most common variant (Fig. 2).10,12–16) The OphA originates from the MMA to reach the orbit via the superior orbital fissure (SOF) or a foramen in the greater wing of the sphenoid bone. This variant may have a small normal OphA from the ICA, representing double origins from the ECA and ICA. In Hayreh’s investigation of 170 specimens, four specimens (2.4%) had double origin from the MMA and ICA, and two (1.2%) demonstrated the OphA completely supplied by the MMA.1)
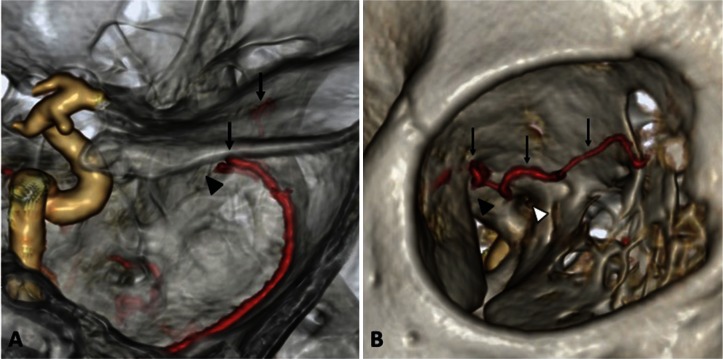
Fig. 2.
The right ophthalmic artery supplied solely by the middle meningeal artery. CT angiography of the orbital region from a superiolateral view (A) and an anterolateral view (B) shows the ophthalmic artery (arrows), the superior orbital fissure (black arrowheads), and the optic canal (white arrowhead). There is no artery passing through the optic canal.
The second most common variant is the OphA arising from the cavernous segment of the ICA (so-called persistent PDOphA), to reach the orbit through the SOF.12,17–21) Uchino et al. evaluated the prevalence of OphA variations on MRA of 826 patients (1,652 OphAs). They found 24 (1.45%) cases of OphAs arising from the MMA, and 7 (0.42%) cases of OphAs arising from the cavernous segment of the ICA.12)
Double origins of the OphA may be observed mostly from the ICA and the MMA,12,14,16) and rarely from the cavernous and supraclinoid segments of the ICA.17–22) The following extremely rare variations of the origin of the OphA have been reported in the literatures: the anterior cerebral artery (ACA),21,23,24) the posterior communicating artery,25) the ICA bifurcation,26) the basilar artery,27–29) the marginal tentorial artery.30) The MMA arising from the OphA31–33) and the infraoptic course of the ACA34–36) also have been reported.
Anastomotic Branches between the MMA and the OphA
The anastomotic branches between the anterior division of the MMA and the lacrimal artery have been well described in the literature.37–39) However, the nomenclature of these branches is highly complicated (Fig. 3). The anastomotic branches from the MMA enter into the orbit either through the SOF or a special foramen in the greater wing of the sphenoid bone, which has been known as the foramen meningo-orbitale,38) Hyrtl’s canal,4) and the cranio-orbital foramen.37,39)
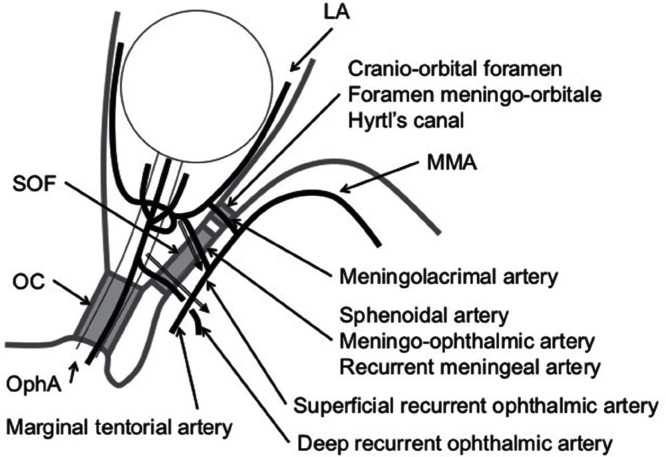
Fig. 3.
Schematic diagram of the anastomosis between the MMA and the OphA, and the recurrent branches to the cavernous sinus. OphA: ophthalmic artery, MMA: middle meningeal artery, LA: lacrimal artery, OC: optic canal, SOF: superior orbital fissure. Note that various names may be used to identify the same artery.
The anastomotic branch passing through this foramen is called the meningolacrimal artery. In the meningolacrimal variant, the anastomosis between the MMA the OphA across the SOF is called the meningo-OphA. In the lacrimal variant (the lacrimal artery arises from the OphA), the branch through the SOF is the recurrent meningeal artery or the sphenoidal artery.5) Double connection between the MMA and the lacrimal artery has also been demonstrated.40)
Regarding the special foramen of the greater wing of the sphenoid bone, Georgiou and Cassell demonstrated that this foramen was observed in about half and represented the remnant of an embryonic conduit for the supraorbital division of the stapedial artery entering to the orbit.38) In 20–24 mm embryo, when the supraorbital division of the stapedial artery enters the orbital cavity, the greater wing of the sphenoid bone has not ossified.41,42) Therefore, the position of the foramen of the greater wing will vary according to the position of the supraorbital division of the stapedial artery at the time of ossification.
Recurrent Cavernous Branches of the OphA and the Inferolateral Trunk
Two recurrent branches of the OphA have been described. The deep recurrent OphA originates from the first segment of the intraorbital OphA and courses caudally through the tendon of Zinn and the medial part of the SOF to anastomose with the anteromedial branch of the inferolateral trunk. The superficial recurrent OphA arises from the intraorbital OphA and the lacrimal artery, passes through the most lateral part of the SOF to reach the dural roof of the cavernous sinus, and sometimes continues as the marginal tentorial artery (Fig. 4).43)
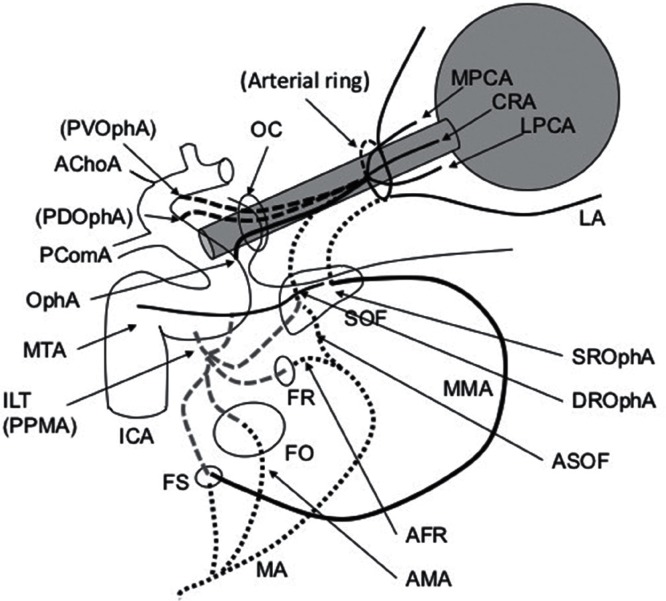
Fig. 4.
Schematic diagram of the adult usual configuration of the ophthalmic artery and maxillary artery and potential anastomoses. ICA: internal carotid artery, ILT: inferolateral trunk, PPMA: persistent primitive maxillary artery, PComA: posterior communicating artery, AChoA: anterior choroidal artery, OphA: ophthalmic artery, CRA: central retinal artery, LPCA: lateral posterior ciliary artery, MPCA: medial posterior ciliary artery, LA: lacrimal artery, PVOphA: primitive ventral ophthalmic artery, PDOphA: primitive dorsal ophthalmic artery, MA: maxillary artery, MMA: middle meningeal artery, AMA: accessory meningeal artery, AFR: artery of the foramen rotundum, ASOF: artery of the superior orbital fissure, OC: optic canal, SOF: superior orbital fissure, FR: foramen rotundum, FO: foramen ovale, FS: foramen spinosum.
The inferolateral trunk divides into four branches: superior, anteromedial, anterolateral, and posterior branches. The superior branch anastomoses with the marginal tentorial artery and the meningohypophyseal trunk. The anteromedial branch anastomoses with deep recurrent OphA. The anterolateral branch anastomoses with artery of foramen rotundum. The posterior branch anastomoses with the accessory meningeal artery.44) Kiyosue et al. Identified an undescribed branch originating from the distal segment of the maxillary artery anastomosing with the anteromedial branch of the inferolateral trunk and designated as the artery of SOF.45) The artery of SOF can also connect to the OphA through the deep recurrent OphA from the first segment of the intraorbital OphA.
Discussion
According to Padget, the origin of the PDOphA is not in the cavernous segment but in the supraclinoid segment of the ICA and the PDOphA is not the orbital branch but the ocular branch. In the literature, however, the anomalous OphA that originates from the cavernous segment of the ICA and enters into the orbit via the SOF is widely misinterpreted as the persistent PDOphA. Recently, some authors have pointed out this wrong nomenclature.5,46,47)
Gregg et al. very recently described that an OphA arising from the cavernous segment of the ICA derives from the PMA, and an OphA arising from the ACA and an infraoptic course ACA represent the partial persistence of a primitive olfactory artery.47) In 4–5 mm human embryo, the ocular vascular supply derives from the cranial division of the ICA that surrounds the caudal aspect of the optic vesicle and in the olfactory region. Therefore, the primitive olfactory artery can also be involved in OphA origin variants, the ACA origin OphA and the infraoptic course ACA, through its close relationship with the periocular capillary plexus.
In the dog, the OphA composes of the internal OphA, which is smaller and arises from the anterior cerebral artery, and the external OphA, which arises from the maxillary artery and frequently forms a common trunk with the anastomotic artery.48) The rare OphA arising from the ACA in human, seem to be the homologue of the internal OphA in the dogs. The dog has abundant anastomoses between the ICA and ECA systems. The anastomotic artery, supplying from the maxillary artery to the cavernous segment of the ICA, is the largest anastomosis. The connection to the external OphA from the maxillary artery and the ICA in the dog seems to be homologous the anastomotic branches to the OphA from the MMA or the inferolateral trunk through the SOF in human.
De La Torre and Netsky examined skull base arteries including the OphAs in human fetus and evaluated the similarities of the anatomy of man and the dog.49) They found two branches, a small medial branch and a large lateral branch, in the cavernous segment of the ICA in all 6 fetuses from 5 to 9 months of fetal age. Both are derived from the PMA. The small medial branch is the inferior hypophyseal artery, and the large lateral branch supplies the adjacent dura and the orbit and designated as the persistent PMA. The persistent PMA is the homologue of the proximal part of the anastomotic branch in the dog, and it must be the inferolateral trunk in the adult man.
According to Padget’s observation of 22 embryos from 4 to 40 mm (from 3.5 to 7 weeks), the PMA is mostly a temporal artery.2) Although the PMA plays the role of blood supply to the optic region in the earliest embryo, as the primitive OphA develops, the PMA dwindles in size, and the only remnant becomes the inferior hypophyseal artery. The discrepancy of the findings between early and late fetal age remains uncertain. Although further research is warranted to corroborate the fate of the primitive arteries, it seems that the anatomical variation of the OphA attributes to the partial persistence of abundant anastomoses between the stapedial artery and the PMA during early embryogenesis.
The vascular system develops by two process, vasculogenesis and angiogenesis. Vasculogenesis is the de novo blood vessel formation by endothelial precursor cells called angioblast. Vascular endothelial growth factor (VEGF) stimulates the proliferation and migration of endothelial cells. Angiogenesis is the complex remodeling process consisting of migration, sprouting, and pruning, which forms a functional vascular system from the primary vascular network.50)
Recent investigations demonstrated that the central and peripheral nervous systems influence the blood vessel patterning from the primary capillary plexus.51–53) Development of the retinal vasculature is preceded by an invasion of migrating astrocytes, which emerge from the optic nerve head and spread radically over the retina.51) Sensory neurons or Schwann cells can induce arterial marker expression in embryonic endothelial cells, and VEGF is essential for the formation of arteries.52)
In the development of the OphA, the PDOphA, which emerges concurrently with the formation of the optic cup and the optic stalk at 5–6 mm stage, should be the branch of the optic tissue, whereas the supraorbital division of the stapedial artery should be the orbital branches, which develops together with the peripheral branches of the ophthalmic division of the trigeminal nerve at 16–18 mm stage.
Conclusion
In the early embryo, the developing optic tissue is supplied by the primary capillary plexus from the primitive ICA and its branch, the PMA. Then, the PDOphA arising from the ICA in the level of the posterior communicating artery and the PVOphA arising in the level of the anterior choroidal artery forms the ocular arteries. The supraorbital division of the stapedial artery enters the orbit through the SOF, forms an arterial ring encircling the optic nerve with the ocular arteries, and become the orbital branches accompanying the ophthalmic division of the trigeminal nerve. The supraorbital division of the stapedial artery is the source of the anastomosis between the MMA and the OphA or the lacrimal artery. There are also potential anastomoses between the maxillary artery and the cavernous segment of the ICA. The persistent PMA seems to be the principal anastomosis, connecting the cavernous segment of the ICA and the maxillary artery, and must be the source of the OphA arising from the cavernous segment of the ICA.
Acknowledgments
The author would like to express my special thanks to Dr. Masaki Komiyama, Department of Neuro-Intervention, Osaka City General Hospital, and Dr. Hiro Kiyosue, Department of Radiology, Oita University Faculty of Medicine, for their insightful suggestions and comments.
Footnotes
Conflicts of Interest Disclosure
The author has no conflicts of interest with regard to this manuscript. The author has registered online Self-reported COI Disclosure Statement Forms.
References
- 1). Hayreh SS: Orbital vascular anatomy. Eye 20: 1130– 1144, 2006. [DOI] [PubMed] [Google Scholar]
- 2). Padget DH: The development of the cranial arteries in the human embryo. Contrib Embryol 32: 205– 261, 1948. [Google Scholar]
- 3). Vignaud J, Hasso AN, Lasjaunias P, Clay C: Orbital vascular anatomy and embryology. Radiology 111: 617– 626, 1974. [DOI] [PubMed] [Google Scholar]
- 4). Lasjaunias P, Berenstein A, ter Brugge K: Surgical neuroangiography Vol. 1. Clinical vascular anatomy and variations. Berlin, Springer-Verlag, 2001, pp. 480–96 [Google Scholar]
- 5). Gailloud P, Gregg L, San Millan Ruiz D: Developmental anatomy, angiography, and clinical implications of orbital arterial variations involving the stapedial artery. Neuroimaging Clin N Am 19: 169– 179, 2009. [DOI] [PubMed] [Google Scholar]
- 6). Hayreh SS, Dass R: The ophthalmic artery: I: origin and intra-cranial and intra-canalicular course. Br J Ophthal 46: 65– 98, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Hayreh SS, Dass R: The ophthalmic artery: II: intra-orbital course. Br J Ophthal 46: 165– 185, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Hayreh SS, Dass R: The ophthalmic artery: III: branches. Br J Ophthal 46: 212– 247, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Perrini P, Cardia A, Fraser K, Lanzino G: A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg 106: 142– 150, 2007. [DOI] [PubMed] [Google Scholar]
- 10). Hayashi N, Kubo M, Tsuboi Y, Nishimura S, Nishijima M, Ahmed Abdel-Aal M, Endo S: Impact of anomalous origin of the ophthalmic artery from the middle meningeal artery on selection of surgical approach to skull base meningioma. Surg Neurol 68: 568– 71, 2007. [DOI] [PubMed] [Google Scholar]
- 11). Geibprasert S., Pongpech S, Armstrong D, Krings T: Dangerous external and internal anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. Am J Neuroradiol 30: 1459– 1468, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Uchino A, Saito N, Takahashi M, Kozawa E, Mizukoshi W, Nakajima R, Okano N: Persistent dorsal ophthalmic artery and ophthalmic artery arising from the middle meningeal artery diagnosed by MR angiography at 3 T. Surg Radiol Anat 35: 775– 782, 2013. [DOI] [PubMed] [Google Scholar]
- 13). Watanabe A, Hirano K, Ishii R: Dural caroticocavernous fistula with both ophthalmic arteries arising from middle meningeal arteries. Neuroradiology 38: 806– 808, 1996. [DOI] [PubMed] [Google Scholar]
- 14). Morandi X, Le Bourdon E, Darnault P, Brassier G, Duval JM: Unusual origin of the ophthalmic artery and occlusion of the central retinal artery. Surg Radiol Anat 20: 69– 71, 1998. [DOI] [PubMed] [Google Scholar]
- 15). Liu Q, Rhoton AL: Middle meningeal origin of the ophthalmic artery. Neurosurgery 49: 401– 407, 2001. [DOI] [PubMed] [Google Scholar]
- 16). Tsutsumi S, Yasutomo Y, Tabuchi T, Ito M: Visualization of the ophthalmic artery by phase-contrast magnetic resonance angiography: a pilot study. Surg Radiol Anat 34: 833– 838, 2012. [DOI] [PubMed] [Google Scholar]
- 17). Ogawa T, Miyauchi T, Kato T, Tamakawa Y: Internal carotid origin of double ophthalmic arteries. Neuroradiology 32: 508– 510, 1990. [DOI] [PubMed] [Google Scholar]
- 18). Kam CK, Alvarez H, Lasjaunias P: Double internal carotid origin of the ophthalmic artery with ruptured aneurysm of the posterior communicating artery. Interv Neuroradiol 9: 383– 388, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Tanaka M: Primitive dorsal ophthalmic artery associated with paraclinoid internal carotid artery aneurysm. JNET 3: 39– 41, 2009. [Google Scholar]
- 20). Namba K, Nemoto S: Double ophthalmic artery visualized with new technology. Neuroradiol J 26: 371– 372, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Indo M, Oya S, Tanaka M, Matsui T: High incidence of ICA anterior wall aneurysms in patients with an anomalous origin of the ophthalmic artery: possible relevance to the pathogenesis of aneurysm formation. J Neurosurg 120: 93– 98, 2014. [DOI] [PubMed] [Google Scholar]
- 22). Uchino A, Saito N, Kurita H, Ishihara S: Double ophthalmic arteries arising from the internal carotid artery. Surg Radiol Anat 35: 173– 175, 2013. [DOI] [PubMed] [Google Scholar]
- 23). Honma Y, Ogawa T, Nagao S: Angiographically occult anomalous ophthalmic artery arising from the anterior cerebral artery. Acta Neurochir (Wien) 139: 480– 481, 1997. [DOI] [PubMed] [Google Scholar]
- 24). Li Y, Horiuchi T, Yako T, Ishizaka S, Hongo K: Anomalous origin of the ophthalmic artery from the anterior cerebral artery. Neurol Med Cir (Tokyo) 51: 579– 581, 2011. [DOI] [PubMed] [Google Scholar]
- 25). Naeini RM, De J, Satow T, Benndorf G: Unilateral agenesis of internal carotid artery with ophthalmic artery arising from posterior communicating artery. Am J Roentgenol 184: 571– 573, 2005. [DOI] [PubMed] [Google Scholar]
- 26). Hamada J, Kitamura I, Kurino M, Sueyoshi N, Uemura S, Ushio Y: Abnormal origin of bilateral ophthalmic arteries: case report. J Neurosurg 74: 287– 289, 1991. [DOI] [PubMed] [Google Scholar]
- 27). Schumacher M, Wakhloo AK: An orbital arteriovenous malformation in a patient with origin of the ophthalmic artery from the basilar artery. Am J Neuroradiol 15: 550– 553, 1994. [PMC free article] [PubMed] [Google Scholar]
- 28). Sade B, Tampieri D, Mohr G: Ophthalmic artery originating from basilar artery: a rare variant. Am J Neuroradiol 25: 1730– 1731, 2004. [PMC free article] [PubMed] [Google Scholar]
- 29). Rivera R, Choi IS, Sordo JG, Giacaman P, Badilla L, Bravo E, Echeverria D: Unusual origin of the left ophthalmic artery from the basilar trunk. Surg Radiol Anat 37: 399– 401, 2015. [DOI] [PubMed] [Google Scholar]
- 30). Tonetti DA, Jadhav AP, Ducruet AF: A rare marginal tentorial artery to ophthalmic artery anastomosis. J Clin Neurosci 22: 773– 774, 2015. [DOI] [PubMed] [Google Scholar]
- 31). McLennan JE, Rosenbaum AE, Haughton VM: Internal carotid origins of the middle meningeal artery: the ophthalmic-middle meningeal and stapedial-middle meningeal arteries. Neuroradiology 7: 265– 275, 1974. [DOI] [PubMed] [Google Scholar]
- 32). Maiuri F, Donzelli R, de Divitiis O, Fusco M, Briganti F: Anomalous meningeal branches of the ophthalmic artery feeding meningiomas of the brain convexity. Surg Radiol Anat 20: 279– 284, 1998. [DOI] [PubMed] [Google Scholar]
- 33). Kimball D, Kimball H, Tubbs RS, Loukas M: Variant middle meningeal artery origin from the ophthalmic artery: a case report. Surg Radiol Anat 37: 105– 108, 2015. [DOI] [PubMed] [Google Scholar]
- 34). Spinnato S, Pasqualin A, Chioffi F, Da Pian R: Infraoptic course of the anterior cerebral artery associated with an anterior communicating artery aneurysm: anatomic case report and embryological considerations. Neurosurgery 44: 1315– 1319, 1999. [PubMed] [Google Scholar]
- 35). Peltier J, Fichten A, Havet E, Page C, Foulon P, Laude M, Le Gars D: The infra-optic course of the anterior cerebral arteries: an anatomic case report. Surg Radiol Anat 29: 389– 392, 2007. [DOI] [PubMed] [Google Scholar]
- 36). Akiyama Y, Okada T, Hayashi N, Yokoi T: Infraoptic course of the anterior cerebral artery originating from the extradural internal carotid artery associated with contralateral internal carotid artery agenesis and multiple intracerebral aneurysms. Neurol Med Chir (Tokyo) 50: 984– 987, 2010. [DOI] [PubMed] [Google Scholar]
- 37). Diamond MK: Homologies of the meningeal–orbital arteries of humans: a reappraisal. J Anat 178: 223– 241, 1991. [PMC free article] [PubMed] [Google Scholar]
- 38). Georgiou C, Cassell MD: The foramen meningo-orbitale and its relationship to the development of the ophthalmic artery. J Anat 180: 119– 125, 1992. [PMC free article] [PubMed] [Google Scholar]
- 39). Erturk M, Kayalioglu G, Govsa F, Varol T, Ozgur T: The cranio-orbital foramen, the groove on the lateral wall of the human orbit, and the orbital branch of the middle meningeal artery. Clin Anat 18: 10– 14, 2005. [DOI] [PubMed] [Google Scholar]
- 40). Lasjaunias P, Vignaud J, Hasso AN: Maxillary artery blood supply to the orbit: normal and pathological aspects. Neuroradiology 9: 87– 97, 1975. [Google Scholar]
- 41). Laine FJ, Nadal L, Braun IF: CT and MR imaging of the central skull base part 1: technique, embryologic development, and anatomy. Radiographics 10: 591– 602, 1990. [DOI] [PubMed] [Google Scholar]
- 42). Nemzek WR, Brodie HA, Hecht ST, Chong BW, Babcook CJ, Seibert JA: MR, CT, and plain film imaging of the developing skull base in fetal specimens. AJNR Am J Neuroradiol 21: 1699– 1706, 2000. [PMC free article] [PubMed] [Google Scholar]
- 43). Lasjaunias P, Brismar J, Moret J, Theron J: Recurrent cavernous branches of the ophthalmic artery. Acta Radiol Diagn (Stockh) 19: 553– 560, 1978. [DOI] [PubMed] [Google Scholar]
- 44). Lasjaunias P, Moret J, Mink J: The anatomy of the inferolateral trunk (ILT) of the internal carotid artery. Neuroradiology 13: 215– 220, 1977. [DOI] [PubMed] [Google Scholar]
- 45). Kiyosue H, Tanoue S, Hongo N, Sagara Y, Mori H: Artery of the superior orbital fissure: an undescribed branch from the pterygopalatine segment of the maxillary artery to the orbital apex connecting with the anteromedial branch of the inferolateral trunk. Am J Neuroradiol 36: 1741– 1747, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Komiyama M: Embryology of the ophthalmic artery: a revived concept. Interv Neuroradiol 15: 363– 368, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Gregg L, San Millan D, Orru E, Tamargo RJ, Gailloud P: Ventral and dorsal persistent primitive ophthalmic arteries. Neurosurgery, 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48). Jewell PA: The anastomoses between internal and external carotid circulations in the dog. J Anat 86: 83– 94, 1952. [PMC free article] [PubMed] [Google Scholar]
- 49). De La Torre E, Netsky MG: Study of persistent primitive maxillary artery in human fetus: some homologies of cranial arteries in man and dog. Am J Anat 106: 185– 195, 1960. [Google Scholar]
- 50). Coultas L, Chawengsaksophak K, Rossant J: Endothelial cells and VEGF in vascular development. Nature 438: 937– 945, 2005. [DOI] [PubMed] [Google Scholar]
- 51). James JM, Mukouyama YS: Neuronal action on the developing blood vessel pattern. Semin Cell Dev Biol 22: 1019– 1027, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Fruttiger M: Development of the retinal vasculature. Angiogenesis 10: 77– 88, 2007. [DOI] [PubMed] [Google Scholar]
- 53). Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ: Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109: 693– 705, 2002. [DOI] [PubMed] [Google Scholar]