Abstract
There is a long history of surgical treatment for Parkinson disease (PD). After pioneering trials and errors, the current primary surgical treatment for PD is deep brain stimulation (DBS). DBS is a promising treatment option for patients with medically refractory PD. However, there are still many problems and controversies associated with DBS. In this review, we discuss current issues in DBS for PD, including patient selection, clinical outcomes, complications, target selection, long-term outcomes, management of axial symptoms, timing of surgery, surgical procedures, cost-effectiveness, and new technology.
Keywords: Parkinson disease, deep brain stimulation
Introduction
There is a long history of surgical treatment for Parkinson disease (PD).1,2) James Parkinson published “An essay on the shaking palsy” in 1817. However, the etiology and cure for this intractable disease long remained unknown. The concept of the extrapyramidal tract was proposed in the 1920s and direct surgery on the basal ganglia was attempted. In 1947, Spiegel and Wycis developed a stereotactic frame for humans, enabling less invasive surgery on the extrapyramidal tract.3,4) Stereotactic pallidotomy or thalamotomy was subsequently developed for the treatment of PD. However, the use of surgical treatment rapidly declined after the introduction of levodopa in 1969. Dopamine replacement therapy became a mainstay of the treatment of PD. However, some patients suffered from motor complications of dopaminergic medication such as fluctuation or dyskinesia. In 1992, Laitinen revived pallidotomy for patients with motor complications from levodopa.5)
A noteworthy event clarified the pathophysiology of PD. An American student used a synthetic narcotic drug contaminated with 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine (MPTP) and developed parkinsonism shortly thereafter. Langston demonstrated that MPTP was a neurotoxin causing PD in 1983.6) Consequently, an animal model for PD using MPTP was developed and the pathophysiology of PD was clarified. In 1989, Albin et al. demonstrated the functional anatomy of the basal ganglia related to the pathophysiology of movement disorders.7) Shortly after, Bergman et al. demonstrated that motor symptoms of MPTP-treated monkeys were dramatically improved by lesioning of the subthalamic nucleus (STN).8) In 1993, Benabid and colleagues developed deep brain stimulation (DBS) of the STN and achieved great success.9)
After pioneering trials and errors, the current primary surgical treatment for PD is DBS. To date, more than 100,000 patients worldwide have undergone DBS. DBS is a promising treatment option for patients with medically refractory PD. However, there are still many problems and controversies associated with DBS. In this review, we discuss current issues in DBS for PD including patient selection, clinical outcomes, complications, target selection, long-term outcomes, management of axial symptoms, timing of surgery, surgical procedures, and new technology.
Patient Selection
There is no radical cure for PD. Therefore, all treatments for PD are symptomatic. First-line treatment for PD is medical. In considering indications for DBS,10,11) a correct diagnosis of idiopathic PD is essential. An initial good response to levodopa is a good indicator of a correct diagnosis of PD. Atypical parkinsonism or secondary PD are not the indications for DBS because of the poor response to surgery.12) The most appropriate surgical candidate for DBS is a patient who suffers from the motor complications of dopaminergic medications such as fluctuation and dyskinesia. A patient who suffers from disabling tremor despite optimal medical treatment is also a good candidate for DBS. Furthermore, the potential candidate should have no dementia or active psychiatric issues. Ideally, the patient should also be young (i.e., <70 years of age), although carefully-selected older patients can also respond favorably.13,14) Some experts recommend excluding patients on the basis of a mini-mental state examination cutoff score of 23 or 24.10)
As described below, STN DBS can reduce the dose of antiparkinsonian dopaminergic medication with improved motor function. Therefore, it is indicated for patients suffering from medication-induced psychotic symptoms such as hallucinations and delusions. There is some evidence to support this concept.15,16)
EARLYSTIMULUS is an online tool developed to support decision making on indications for DBS. This tool can be freely accessed at www.earlystimulus.com. It is based on the expert opinion o-g 82 international DBS neurologists and neurosurgeons.17) In this tool, eight variables (age, PD duration, off-motor symptoms, dyskinesia, tremor, levodopa-unresponsive gait and balance abnormality, and non-motor side effects of medication) are assessed for patients who meet five absolute criteria. It is specially designed to guide general neurologists in identifying appropriate referrals to a DBS surgical center.
Clinical Outcomes
The theoretical target of DBS based on the pathophysiology of PD is the STN or the globus pallidus internus (GPi). An early comparative study revealed the superiority of STN DBS in improvement of motor scores in the medication-off period and reduction of dopaminergic medication.18) Consequently, the STN has long been the most common target of DBS for PD.
STN DBS results in a significant reduction in the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score in the medication-off state but does not alter the score in the medication-on state. STN DBS effectively improves levodopa-responsive symptoms of PD and significantly reduces dyskinesia, motor fluctuation, and the dose of dopaminergic medication. Several controlled randomized studies demonstrated that STN DBS yielded better outcomes in motor function and quality of life (QOL) than medical treatment alone for patients with advanced PD.19–21)
According to a meta-analysis of early outcomes, STN DBS improves UPDRS III motor scores in the medication-off state by 52% and UPDRS II activities of daily living (ADL) score by 50%. STN DBS also reduces dyskinesia by 69%, the daily-off period by 68%, and the dose of dopaminergic medication by 56%. Average improvement in quality of life (QOL) using PDQ-39 is 35%.22) These numbers seem to be benchmarks when STN DBS is introduced. Thus, STN DBS provides a second honeymoon period for patients suffering from the motor complications of dopaminergic medication.23)
There are several reports concerning the effect of DBS for postural abnormality in PD.24–26) Postural abnormality such as camptocormia or Pisa syndrome could be corrected by DBS in some patient although the long-term benefit is limited. Early introduction of DBS after onset of postural abnormality seems to be beneficial.
The effects of DBS on non-motor symptoms have been investigated.27–29) Some studies demonstrated that STN DBS improves gastrointestinal and urinary autonomic function. Arai et al. demonstrated that STN DBS improved gastric emptying by altering the neural system that controls gastrointestinal function.30) STN DBS also improves urinary tract symptoms in PD such as hyper-reflexic bladder by modulating cortical control of the bladder.31–34)
STN DBS improves insomnia by increasing total sleep time and decreasing wakefulness after sleep onset.35–38) Sleep architecture seems to be restored. However, STN DBS does not alleviate REM-sleep behavior disorders.
STN DBS also improves PD-related pain, especially levodopa responsive pain.39–42) However, vigilance is needed, because newly developed back pain or deterioration of preexisting back pain sometimes occurs after successful STN DBS, especially in patients who have some lumbar spine pathology.40,43)
The effect of DBS on impulse control disorders (ICD) and dopamine dysregulation syndrome (DDS) is controversial.44–48) Preexisting ICD or DDS is improved by significant reduction of dopaminergic medication in some patients; however, newly developed ICD may occur after STN DBS. Frank et al. introduced the concept that STN DBS directly induces impulsive behavior independent of medication.49)
Complications
A significant incidence of adverse effects associated with DBS in PD has been reported.50) Most are mild and transient, but serious morbidity is also reported. According to a large study (1183 patients),51) the mortality rate during the first 30 postoperative days after stereotactic surgery is 0.4% and the permanent surgical morbidity rate is 1%. The morbidity is mainly caused by intracerebral hemorrhage (ICH) (2.2%). An analysis of adverse events in published data revealed that common surgery-related complications included 2.0% with symptomatic ICH and 2.0% with infections in 928 STN DBS cases.52) Permanent stimulation or disease progression-related adverse events included 12.8% with dysarthria, 11.3% with apraxia of eyelid opening, 5.8% with cognitive decline, 4.7% with disabling dyskinesia, and 4.3% with depression in 256 STN DBS cases.52)
Regarding verbal problem after STN DBS, Tsuboi et al. classified speech disorders after STN DBS into four types.53) They demonstrated that stuttering and breathy voice are due to aging or disease progression, but strained voice and spastic dysarthria are corticobulbar side effects.
The neuropsychological aspects of STN DBS have recently attracted considerable attention and numerous studies concerning neuropsychological outcome after STN DBS have been performed.54,55) Mood changes including hypomania or depression are common adverse effects in patients treated with STN DBS.56) Most are usually transient in the immediate postoperative period. The spread of stimulation to the limbic STN seems to be a cause of altered mood states.57) On the other hand, depression or apathy occurring several months after surgery often coincides with excessive reduction of dopaminergic medication, and is generally alleviated by increasing the dose.58) Severe depression after successful STN DBS has even been reported to lead to suicide; therefore, great care should be taken with regard to the patient’s mental state. Suicide is the most important factor in mortality in the first year following STN DBS.59)
There are many studies on cognitive outcomes after STN DBS. Most concluded that STN DBS is relatively safe from a cognitive perspective despite mild cognitive morbidity.54,60,61) A meta-analysis of cognitive sequelae by Parsons et al. revealed small but significant declines in executive function and verbal learning and memory, and moderate declines in both semantic and phonemic verbal fluency after STN DBS.60) A randomized controlled study by Witt et al. demonstrated that STN DBS did not reduce overall cognition, but resulted in a selective decrease in frontal cognitive function.61) These changes did not affect improvement in QOL.
Several factors are considered to contribute to cognitive changes after STN DBS. As the STN has widespread connections with basal ganglia and the prefrontal cortex,57,62) the direct effect of stimulation may contribute to cognitive changes. Furthermore, the impact of surgical intervention or drastic postoperative reduction of dopaminergic medication may cause cognitive decline.63) Lead trajectory through the caudate nuclei also may affect cognitive decline.64)
Target Selection
As an early non-randomized comparative study demonstrated the superiority of STN DBS compared with GPi DBS,18) STN DBS has been widely performed as the primary surgical procedure. However, GPi DBS was reevaluated in a recent randomized comparative study, which revealed that GPi DBS yielded improvement in motor function comparable to STN DBS, with less psychiatric or cognitive problems after surgery.65) However, another randomized controlled study still demonstrated the preferability of STN DBS.66) Recent meta-analyses concluded that both STN and GPi DBS have similar effects on motor function and ADL.67,68)
Current consensus is that STN and GPi DBS equally improve motor function in the medication-off period and dyskinesia. Only STN DBS can reduce the dose of dopaminergic medication. GPi DBS directly controls dyskinesia, while STN DBS improves dyskinesia by the reduction of dopaminergic medication. Besides, additional stimulation of the subthalamic fiber tract above the STN is effective in controlling dyskinesia in some patients.69) In STN DBS, the risk of neuropsychological complications seems to be high compared with GPi DBS. Therefore, selection of the DBS target for PD should be considered for each patient based on the characteristics of each target.
Thus, the STN should be chosen for patients who need the reduction of antiparkinsonian medication (e.g., patients taking too much medication or suffering from the side effects). In contrast, GPi should be chosen in patients suffering from severe dyskinesia or dystonia despite low-dose medications, and in patients with a high risk of neuropsychological or psychiatric complications (e.g., older patients or patients with mild cognitive decline).
In the long-term follow-up after STN DBS, some patients suffer from medication or stimulation-induced intractable dyskinesia. There are several reports concerning additional GPi DBS for intractable dyskinesia after successful STN DBS.70–72) This strategy could provide a third honeymoon period in the long-term treatment of PD.
Long-term Outcomes
There are many studies of long-term (more than 5 years) outcomes of STN DBS.73) In most studies, STN DBS improved motor function and ADL in the medication-off period, dyskinesia, and fluctuation, and decreased the dose of dopaminergic medication. These effects were mostly preserved even for 5 years after surgery. Moreover, improvements in cardinal motor symptoms such as tremor, rigidity, and bradykinesia are well-maintained 5 years after surgery. However, axial symptoms affecting speech, gait, and postural instability progressively worsened. These symptoms are refractory to both medication and DBS. The symptoms of gait disturbance or postural instability seem to be mediated by non-dopaminergic mechanisms. STN DBS substantially improves only the dopamine-mediated motor symptoms. Therefore, the aggravation of axial symptoms reflects the progression of PD itself. Persistent adverse effects in long-term follow-up after STN DBS include apraxia of eyelid opening, weight gain, psychiatric disorders, depression, dysarthria, dyskinesias, and apathy.
There have been a few reports on long-term outcomes of STN DBS of greater than 5 years.74–77) According to these reports, not only axial motor symptoms, but also cognitive decline affect worsening of ADL.
Regarding long-term outcome of GPi DBS, a few studies demonstrated improvements in motor and ADL scores, and dyskinesia were also controlled longer with GPi DBS.78,79) The dose of dopaminergic medication was unchanged or gradually increased. A meta-regression analysis revealed that long-term postural stability and gait outcome in the medication-on period was better with GPi DBS than STN DBS.80)
It is controversial whether STN DBS contributes to improvements in the survival of patients with PD. Ngoga et al. demonstrated that patients undergoing STN DBS have significantly longer survival than those who are managed only by medication. STN DBS markedly reduces the death rate related to respiratory complications, such as pneumonia.81) However, Lilleeng et al. have demonstrated no significant difference in long-term mortality between an STN DBS group and a control group.82)
Management of Axial Symptoms
There are many types of motor symptoms in PD. As noted, cardinal motor symptoms such as tremor, rigidity, and bradykinesia are treatable with dopaminergic medication and DBS. However, axial symptoms such as freezing of gait, postural instability, swallowing disturbance, and speech problem are difficult to treat.
Several strategies have been attempted for axial motor symptoms.83) Animal experiments suggest that the pedunculopontine nucleus (PPN) is a locomotion center controlling initiation and modulation of gait.84,85) Patients with PD have significant loss of PPN neurons. Therefore, PPN is considered as a therapeutic target for gait disturbance in PD. Stefani et al. applied both STN and PPN DBS in six patients with PD and demonstrated the beneficial effects of PPN DBS for gait.86) However, other studies showed interindividual variability in gait outcome, with insufficient evidence for PPN DBS.87–89) A surgical procedure that includes targeting and physiological refinement of PPN is not established.
Moreau et al. demonstrated that low frequency (60 Hz) with high-voltage stimulation was effective for gait disturbances that developed after STN DBS.90) Xie et al. showed that 60-Hz stimulation improved swallowing function as well as freezing of gait.91) However, the reported effects of low-frequency stimulation are variable. Some studies demonstrated that low-frequency stimulation had transient or no effect on gait.92,93)
Chastan et al. showed that bilateral stimulation of the substantia nigra pars reticulata (SNr) improved axial symptoms of gait and balance disorders in patients who underwent STN DBS.94) A recent randomized controlled trial revealed that the combined stimulation of the STN and SNr safely improved freezing of gait but not balance impairment.95) Thus, intentional placement of DBS leads into the SNr could be preparation for future deterioration of axial symptoms.
Fuentes et al. showed that spinal cord stimulation (SCS) improved locomotion in an animal model of PD.96) However, this effect of SCS on gait was not seen in subsequent treatment of two patients with PD.97) Agari et al. reported beneficial effects of SCS on axial symptoms such as posture, postural stability, and gait.98)
As for medication, beneficial effects of amantadine99) and the anti-cholinergic trihexyphenidyl100) on axial symptoms after STN DBS have been reported.
Timing of Surgery
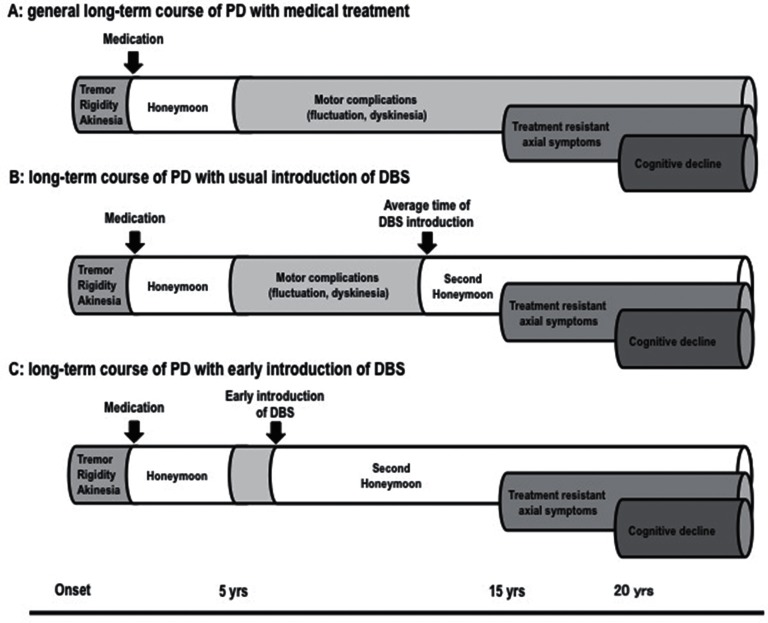
The timing of DBS surgery is one of current interests. The general course of PD with medical treatment is shown in Fig. 1A. After onset, patients remain well with only medication for several years (honeymoon period). However, most patients subsequently suffer from motor complications of dopaminergic medication such as fluctuation and dyskinesia. In the advanced stage, treatment-resistant axial symptoms and cognitive decline appear. Until now, DBS has been considered as a last resort after medical treatment, and was usually introduced in the late phase of motor complications (Fig. 1B). Patients could achieve a second honeymoon period after DBS, but treatment-resistant axial symptoms appeared in several years. Currently, early introduction of STN DBS is recommended based on new evidence (EARLYSTIM study).101,102) This study demonstrated that STN DBS improved QOL and motor function not only in advanced PD, but also in PD with early motor complications. In this case, the second honeymoon period will be longer (Fig. 1C). However, there is criticism that very few patients would meet the EARLYSTIM criteria.103) Mestre et al. emphasize that the most relevant issue is not when but on whom to operate, and that early is not always better.104)
Fig. 1.
Timing of DBS in long-term course of PD with medical treatment.
Surgical Procedure
The surgical procedure for STN DBS varies among centers.105) There is some controversy about surgical aspects of DBS. Currently, DBS leads are implanted into the target area stereotactically under magnetic resonance imaging (MRI) guidance with physiological refinement by microelectrode recording (MER) under local anesthesia in most centers.
As recent progress in MRI technology has enabled direct visualization of the STN or GPi, some groups avoid using MER for placement of the DBS lead.106,107) They insist that MER may increase the risk of ICH. The combination of MER and hypertension will definitely increase the incidence of bleeding.108) However, physiological refinement by MER is the gold standard for identifying the STN and its borders. The optimal region for STN stimulation might be missed due to individual anatomical variations or intraoperative brain shift. In our own series, about 20% of cases required two or more trajectories to obtain sufficient activity of STN by MER.109)
Recently, multiple simultaneous MER using a multiple electrode holder is employed in some centers. It is controversial whether single tract or multiple tracts MER is more advantageous. Temel et al. compared single with multiple tract MER.110) There were no significant differences in the STN length between single and multiple tracts MER. They demonstrated that multiple MER resulted in better motor outcome but deterioration in neuropsychological function. More extensive microlesions caused by the microelectrodes could be a possible explanation for the deterioration.
There is also controversy concerning the use of local or general anesthesia during DBS-lead placement.106) Local anesthesia enables more correct assessment of effects and side effects of stimulation during surgery. On the other hand, general anesthesia can reduce stress and pain from disease severity or anxiety.
As patients with advanced PD generally suffer from bilateral motor symptoms, bilateral implantation of DBS is required. Some centers prefer staged unilateral implantation rather than simultaneous bilateral implantation to reduce postoperative complications, but most studies showed no significant difference in effectiveness of DBS.111–113) However, simultaneous bilateral implantation may be liable to postoperative neuropsychological complications.112)
Cost-effectiveness of DBS
From the standpoint of health economics, several studies analyzed cost-effectiveness of DBS in patients with PD.114–116) Table 1 shows the incremental cost-effectiveness ratio (ICER) calculated in terms of cost per quality-adjusted life year (QALY) in these studies. Although health care systems are different among countries, these studies conclude that DBS is a cost-effective intervention for advanced PD. Costs of DBS are mainly driven by the cost of initial surgery and battery exchange. Therefore, the use of current rechargeable battery will further increase the cost-effectiveness of DBS.
Table 1.
Summary of studies concerning cost-effectiveness of DBS for PD
| Author, year | Country | Incremental cost effectiveness ratio |
|---|---|---|
| Tomaszewski, 2001114) | USA | US$49,194 per QALY |
| Valldeoriola, 2007115) | Spain | €34,389 per QALY |
| Eggington, 2014116) | UK | £20,678 per QALY |
QALY: quality-adjusted life year.
New Technology
DBS devices had long been provided only by Medtronic Inc. for long time. Recently, Boston Scientific and St. Jude Medical also entered the DBS market. Each company developed a unique product concerning the electrode arrangement, stimulation setup, battery character, etc. This competition may result in the development of better products.
A conventional implantable pulse generator (IPG) has a primary cell battery, and IPG replacement is necessary for every 4–5 years. Currently, a rechargeable battery is used in selected patients.117,118) VerciseTM rechargeable IPG (Boston Scientific) is especially superior in terms of long battery life, wireless recharge, and Zero VoltTM technology, which avoid failure by battery charge depletion.119)
Performing MRI in patients with a conventional IPG is not officially permitted. Currently, only the Medtronic IPG (Activa) allows MRI under specific conditions of use.120)
In programing of stimulation parameters, the amplitude setup is changing from constant voltage mode to constant current mode. The effect of stimulation is dependent on strength of current. However, stimulation current fluctuates with tissue impedance change after surgery in conventional constant voltage IPG.121) More stable stimulation effect is expected using constant current IPG.122,123)
Several methods of current steering to modify the stimulation field have been developed in new devices. The multiple independent current control (MICC) technology of Boston Scientific IPG (VerciseTM) provides independent current settings in eight contacts in one lead.119,124) The interleaving mode of the Medtronic IPG allows two independent settings with different pulse width and amplitude values in one lead. These technologies enable generation of precise control to refine the size and shape of the stimulation field.125) They are useful in creating stimulation fields to minimize the side effects of stimulation while maintaining the beneficial effects. In addition, they are applied in situations in which stimulation at different contacts was beneficial for controlling specific symptoms with different stimulation amplitudes.126,127)
A directional lead has been developed as a strategy to avoid the side effects of stimulation. A multiple-contacts lead enables two dimensional electric field shaping. In STN or GPi DBS, if the lead is not implanted in the precise position, side effects caused by current spreading to the internal capsule may occur with a conventional lead. This side effect seems to be controlled using a directional lead. Several manufacturers are developing unique directional leads.128,129)
Currently, pathological beta-oscillation recorded from the STN in local field potential recording is the most noteworthy phenomenon in the context of PD.130) The concept of the adaptive DBS is based on a closed-loop model.131) In adaptive DBS, stimulation is applied only when pathological beta-oscillation is detected. In clinical use, unilateral and bilateral adaptive DBS were more efficient than conventional continuous DBS.132,133)
Conclusion
More than 20 years have passed since DBS was introduced in the treatment of PD. Currently, DBS is the most promising surgical treatment option for patients with medically refractory PD. DBS is also used for other movement disorders and neuropsychiatric diseases. DBS has evolved along with the development of surgical procedures and device technology. In the future, alternative surgical therapies such as gene therapy,134) neural transplantation using induced pluripotent stem cells,135) and optogenetics136) are also anticipated.
Footnotes
Conflicts of Interest Disclosure
The Department of Research and Therapeutics for Movement Disorders, Juntendo University Graduate School of Medicine is an endowment department supported with an unrestricted grant from Medtronic, Boston Scientific, Kyowa Hakko Kirin Co, Boehringer Ingelheim, and Kissei Pharmaceutical. Professor Nobutaka Hattori is a representative of this department. All other authors have no conflicts of interest with regard to the manuscript.
References
- 1). Miocinovic S, Somayajula S, Chitnis S, Vitek JL: History, applications, and mechanisms of deep brain stimulation. JAMA Neurol 70: 163– 171, 2013. [DOI] [PubMed] [Google Scholar]
- 2). Danish SF, Baltuch GH: History of deep brain stimulation. in Baltuch GH, Matthew BS. (eds): Deep brain stimulation for Parkinson’s disease. New York, Informa Healthcare, 2007, pp 1–15 [Google Scholar]
- 3). Gildenberg PL: Spiegel and Wycis: the early years. Stereotact Funct Neurosurg 77: 11– 16, 2001. [DOI] [PubMed] [Google Scholar]
- 4). Laitinen LV: Personal memories of the history of stereotactic neurosurgery. Neurosurgery 55: 1420– 1428; discussion 1428–1429, 2004. [DOI] [PubMed] [Google Scholar]
- 5). Laitinen LV, Bergenheim AT, Hariz MI: Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg 76: 53– 61, 1992. [DOI] [PubMed] [Google Scholar]
- 6). Langston JW, Ballard P, Tetrud JW, Irwin I: Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219: 979– 980, 1983. [DOI] [PubMed] [Google Scholar]
- 7). Albin RL, Young AB, Penney JB: The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366– 375, 1989. [DOI] [PubMed] [Google Scholar]
- 8). Bergman H, Wichmann T, DeLong MR: Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249: 1436– 1438, 1990. [DOI] [PubMed] [Google Scholar]
- 9). Pollak P, Benabid AL, Gross C, Gao DM, Laurent A, Benazzouz A, Hoffmann D, Gentil M, Perret J: Effects of the stimulation of the subthalamic nucleus in Parkinson disease. Rev Neurol (Paris) 149: 175–176, 1993. [PubMed] [Google Scholar]
- 10). Lang AE, Houeto JL, Krack P, Kubu C, Lyons KE, Moro E, Ondo W, Pahwa R, Poewe W, Tröster AI, Uitti R, Voon V: Deep brain stimulation: preoperative issues. Mov Disord 21: S171– S96, 2006. [DOI] [PubMed] [Google Scholar]
- 11). Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, Pahwa R, Henderson JM, Hariz MI, Bakay RA, Rezai A, Marks WJ, Jr, Moro E, Vitek JL, Weaver FM, Gross RE, DeLong MR: Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 68: 165– 171, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Shih LC, Tarsy D: Deep brain stimulation for the treatment of atypical parkinsonism. Mov Disord 22: 2149– 2155, 2007. [DOI] [PubMed] [Google Scholar]
- 13). DeLong MR, Huang KT, Gallis J, Lokhnygina Y, Parente B, Hickey P, Turner DA, Lad SP: Effect of advancing age on outcomes of deep brain stimulation for Parkinson disease. JAMA Neurol 71: 1290– 1295, 2014. [DOI] [PubMed] [Google Scholar]
- 14). Parent B, Awan N, Berman SB, Suski V, Moore R, Crammond D, Kondziolka D: The relevance of age and disease duration for intervention with subthalamic nucleus deep brain stimulation surgery in Parkinson disease. J Neurosurg 114: 927– 931, 2011. [DOI] [PubMed] [Google Scholar]
- 15). Umemura A, Oka Y, Okita K, Matsukawa N, Yamada K: Subthalamic nucleus stimulation for Parkinson disease with severe medication-induced hallucinations or delusions. J Neurosurg 14: 1701– 1705, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Yoshida F, Miyagi Y, Kishimoto J, Morioka T, Murakami N, Hashiguchi K, Samura K, Sakae N, Yamasaki R, Kawaguchi M, Sasaki T: Subthalamic nucleus stimulation does not cause deterioration of preexisting hallucinations in Parkinson’s disease patients. Stereotact Funct Neurosurg 87: 45– 49, 2009. [DOI] [PubMed] [Google Scholar]
- 17). Moro E, Schüpbach M, Wächter T, Allert N, Eleopra R, Honey CR, Rueda M, Schiess MC, Shimo Y, Valkovic P, Whone A, Stoevelaar H: Referring Parkinson’s disease patients for deep brain stimulation: a RAND/UCLA appropriateness study. J Neurol 263: 112– 119, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Deep-Brain Stimulation for Parkinson’s Disease Study Group : Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med 345: 956– 963, 2001. [DOI] [PubMed] [Google Scholar]
- 19). Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, Daniels C, Deutschländer A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, German Parkinson Study Group , Neurostimulation Section: a randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355: 896– 908, 2006. [DOI] [PubMed] [Google Scholar]
- 20). Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, CSP 468 Study Group : Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301: 63– 73, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, Patel S, Wheatley K, PD SURG Collaborative Group Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 9: 581– 591, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, Lang AE, Deuschl G: Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord 21: S290– S304, 2006. [DOI] [PubMed] [Google Scholar]
- 23). Simonin C, Tir M, Devos D, Kreisler A, Dujardin K, Salleron J, Delval A, Blond S, Defebvre L, Destée A, Krystkowiak P: Reduced levodopa-induced complications after 5 years of subthalamic stimulation in Parkinson’s disease: a second honeymoon. J Neurol 256: 1736– 1741, 2009. [DOI] [PubMed] [Google Scholar]
- 24). Umemura A, Oka Y, Ohkita K, Yamawaki T, Yamada K: Effect of subthalamic deep brain stimulation on postural abnormality in Parkinson disease. J Neurosurg 112: 1283– 1288, 2010. [DOI] [PubMed] [Google Scholar]
- 25). Schulz-Schaeffer WJ, Margraf NG, Munser S, Wrede A, Buhmann C, Deuschl G, Oehlwein C: Effect of neurostimulation on camptocormia in Parkinson’s disease depends on symptom duration. Mov Disord 30: 368– 372, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Chieng LO, Madhavan K, Wang MY: Deep brain stimulation as a treatment for Parkinson’s disease related camptocormia. J Clin Neurosci 22: 1555– 1561, 2015. [DOI] [PubMed] [Google Scholar]
- 27). Fasano A, Daniele A, Albanese A: Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol 11: 429– 442, 2012. [DOI] [PubMed] [Google Scholar]
- 28). Kim HJ, Jeon BS, Paek SH: Nonmotor symptoms and subthalamic deep brain stimulation in Parkinson’s disease. J Mov Disord 8: 83– 91, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Dafsari HS, Reddy P, Herchenbach C, Wawro S, Petry-Schmelzer JN, Visser-Vandewalle V, Rizos A, Silverdale M, Ashkan K, Samuel M, Evans J, Huber CA, Fink GR, Antonini A, Chaudhuri KR, Martinez-Martin P, Timmermann L, IPMDS Non-Motor Symptoms Study Group : Beneficial effects of bilateral subthalamic stimulation on non-motor symptoms in Parkinson’s disease. Brain Stimul 9: 78– 85, 2016. [DOI] [PubMed] [Google Scholar]
- 30). Arai E, Arai M, Uchiyama T, Higuchi Y, Aoyagi K, Yamanaka Y, Yamamoto T, Nagano O, Shiina A, Maruoka D, Matsumura T, Nakagawa T, Katsuno T, Imazeki F, Saeki N, Kuwabara S, Yokosuka O: Subthalamic deep brain stimulation can improve gastric emptying in Parkinson’s disease. Brain 135: 1478– 1485, 2012. [DOI] [PubMed] [Google Scholar]
- 31). Finazzi-Agrò E, Peppe A, D’Amico A, Petta F, Mazzone P, Stanzione P, Micali F, Caltagirone C: Effects of subthalamic nucleus stimulation on urodynamic findings in patients with Parkinson’s disease. J Urol 169: 1388– 1391, 2003. [DOI] [PubMed] [Google Scholar]
- 32). Winge K, Nielsen KK, Stimpel H, Lokkegaard A, Jensen SR, Werdelin L: Lower urinary tract symptoms and bladder control in advanced Parkinson’s disease: effects of deep brain stimulation in the subthalamic nucleus. Mov Disord 22: 220– 225, 2007. [DOI] [PubMed] [Google Scholar]
- 33). Herzog J, Weiss PH, Assmus A, Wefer B, Seif C, Braun PM, Herzog H, Volkmann J, Deuschl G, Fink GR: Subthalamic stimulation modulates cortical control of urinary bladder in Parkinson’s disease. Brain 129: 3366– 3375, 2006. [DOI] [PubMed] [Google Scholar]
- 34). Herzog J, Weiss PH, Assmus A, Wefer B, Seif C, Braun PM, Pinsker MO, Herzog H, Volkmann J, Deuschl G, Fink GR: Improved sensory gating of urinary bladder afferents in Parkinson’s disease following subthalamic stimulation. Brain 131: 132– 145, 2008. [DOI] [PubMed] [Google Scholar]
- 35). Arnulf I, Bejjani BP, Garma L, Bonnet AM, Houeto JL, Damier P, Derenne JP, Agid Y: Improvement of sleep architecture in PD with subthalamic nucleus stimulation. Neurology 55: 1732– 1734, 2000. [DOI] [PubMed] [Google Scholar]
- 36). Iranzo A, Valldeoriola F, Santamaría J, Tolosa E, Rumià J: Sleep symptoms and polysomnographic architecture in advanced Parkinson’s disease after chronic bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatr 72: 661– 664, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Cicolin A, Lopiano L, Zibetti M, Torre E, Tavella A, Guastamacchia G, Terreni A, Makrydakis G, Fattori E, Lanotte MM, Bergamasco B, Mutani R: Effects of deep brain stimulation of the subthalamic nucleus on sleep architecture in parkinsonian patients. Sleep Med 5: 207– 210, 2004. [DOI] [PubMed] [Google Scholar]
- 38). Nishida N, Murakami T, Kadoh K, Tohge R, Yamanegi M, Saiki H, Ueda K, Matsumoto S, Ishikawa M, Takahashi JA, Toda H. Subthalamic nucleus deep brain stimulation restores normal rapid eye movement sleep in Parkinson’s disease. Mov Disord 26: 2418– 2422, 2011. [DOI] [PubMed] [Google Scholar]
- 39). Kim HJ, Paek SH, Kim JY, Lee JY, Lim YH, Kim MR, Kim DG, Jeon BS: Chronic subthalamic deep brain stimulation improves pain in Parkinson disease. J Neurol 255: 1889– 1894, 2008. [DOI] [PubMed] [Google Scholar]
- 40). Oshima H, Katayama Y, Morishita T, Sumi K, Otaka T, Kobayashi K, Suzuki Y, Fukaya C, Yamamoto T: Subthalamic nucleus stimulation for attenuation of pain related to Parkinson disease. J Neurosurg 116: 99– 106, 2012. [DOI] [PubMed] [Google Scholar]
- 41). Sürücü O, Baumann-Vogel H, Uhl M, Imbach LL, Baumann CR: Subthalamic deep brain stimulation versus best medical therapy for l-dopa responsive pain in Parkinson’s disease. Pain 154: 1477– 1479, 2013. [DOI] [PubMed] [Google Scholar]
- 42). Cury RG, Galhardoni R, Fonoff ET, Dos Santos Ghilardi MG, Fonoff F, Arnaut D, Myczkowski ML, Marcolin MA, Bor-Seng-Shu E, Barbosa ER, Teixeira MJ, Ciampi de Andrade D: Effects of deep brain stimulation on pain and other nonmotor symptoms in Parkinson disease. Neurology 83: 1403– 1409, 2014. [DOI] [PubMed] [Google Scholar]
- 43). Umemura A, Oka Y, Okura A, Okita K, Yamada K. Newly developed back pain after subthalamic nucleus stimulation in Parkinson’s disease. Acta Neurochir (Wien) 153: 1591–1592, 2011. [DOI] [PubMed] [Google Scholar]
- 44). Broen M, Duits A, Visser-Vandewalle V, Temel Y, Winogrodzka A: Impulse control and related disorders in Parkinson’s disease patients treated with bilateral subthalamic nucleus stimulation: a review. Parkinsonism Relat Disord 17: 413– 417, 2011. [DOI] [PubMed] [Google Scholar]
- 45). Lhommée E, Klinger H, Thobois S, Schmitt E, Ardouin C, Bichon A, Kistner A, Fraix V, Xie J, Aya Kombo M, Chabardès S, Seigneuret E, Benabid AL, Mertens P, Polo G, Carnicella S, Quesada JL, Bosson JL, Broussolle E, Pollak P, Krack P: Subthalamic stimulation in Parkinson’s disease: restoring the balance of motivated behaviours. Brain 135: 1463– 1477, 2012. [DOI] [PubMed] [Google Scholar]
- 46). Moum SJ, Price CC, Limotai N, Oyama G, Ward H, Jacobson C, Foote KD, Okun MS: Effects of STN and GPi deep brain stimulation on impulse control disorders and dopamine dysregulation syndrome. PLoS One 7: e29768, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Eusebio A, Witjas T, Cohen J, Fluchère F, Jouve E, Régis J, Azulay JP: Subthalamic nucleus stimulation and compulsive use of dopaminergic medication in Parkinson’s disease. J Neurol Neurosurg Psychiatr 84: 868– 874, 2013. [DOI] [PubMed] [Google Scholar]
- 48). Amami P, Dekker I, Piacentini S, Ferré F, Romito LM, Franzini A, Foncke EM, Albanese A: Impulse control behaviours in patients with Parkinson’s disease after subthalamic deep brain stimulation: de novo cases and 3-year follow-up. J Neurol Neurosurg Psychiatr 86: 562– 564, 2015. [DOI] [PubMed] [Google Scholar]
- 49). Frank MJ, Samanta J, Moustafa AA, Sherman SJ: Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318: 1309– 1312, 2007. [DOI] [PubMed] [Google Scholar]
- 50). Umemura A, Oka Y, Yamamoto K, Okita K, Matsukawa N, Yamada K: Complications of subthalamic nucleus stimulation in Parkinson’s disease. Neurol Med Chir (Tokyo) 51: 749–755, 2011. [DOI] [PubMed] [Google Scholar]
- 51). Voges J, Hilker R, Bötzel K, Kiening KL, Kloss M, Kupsch A, Schnitzler A, Schneider GH, Steude U, Deuschl G, Pinsker MO: Thirty days complication rate following surgery performed for deep-brain-stimulation. Mov Disord 22: 1486– 1489, 2007. [DOI] [PubMed] [Google Scholar]
- 52). Videnovic A, Metman LV: Deep brain stimulation for Parkinson’s disease: prevalence of adverse events and need for standardized reporting. Mov Disord 23: 343– 349, 2008. [DOI] [PubMed] [Google Scholar]
- 53). Tsuboi T, Watanabe H, Tanaka Y, Ohdake R, Yoneyama N, Hara K, Nakamura R, Watanabe H, Senda J, Atsuta N, Ito M, Hirayama M, Yamamoto M, Fujimoto Y, Kajita Y, Wakabayashi T, Sobue G: Distinct phenotypes of speech and voice disorders in Parkinson’s disease after subthalamic nucleus deep brain stimulation. J Neurol Neurosurg Psychiatr 86: 856– 864, 2015. [DOI] [PubMed] [Google Scholar]
- 54). Voon V, Kubu C, Krack P, Houeto JL, Tröster AI: Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord 21: S305– S27, 2006. [DOI] [PubMed] [Google Scholar]
- 55). Castrioto A, Lhommée E, Moro E, Krack P: Mood and behavioural effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol 13: 287– 305, 2014. [DOI] [PubMed] [Google Scholar]
- 56). Takeshita S, Kurisu K, Trop L, Arita K, Akimitsu T, Verhoeff NP: Effect of subthalamic stimulation on mood state in Parkinson’s disease: evaluation of previous facts and problems. Neurosurg Rev 28: 179– 186; discussion 187, 2005. [DOI] [PubMed] [Google Scholar]
- 57). Benarroch EE: Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology 70: 1991– 1995, 2008. [DOI] [PubMed] [Google Scholar]
- 58). Czernecki V, Schüpbach M, Yaici S, Lévy R, Bardinet E, Yelnik J, Dubois B, Agid Y: Apathy following subthalamic stimulation in Parkinson disease: a dopamine responsive symptom. Mov Disord 23: 964– 969, 2008. [DOI] [PubMed] [Google Scholar]
- 59). Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schüpbach M, D’Ambrosia J, Thobois S, Tamma F, Herzog J, Speelman JD, Samanta J, Kubu C, Rossignol H, Poon YY, Saint-Cyr JA, Ardouin C, Moro E: A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain 131: 2720– 2728, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Parsons TD, Rogers SA, Braaten AJ, Woods SP, Tröster AI: Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a meta-analysis. Lancet Neurol 5: 578– 588, 2006. [DOI] [PubMed] [Google Scholar]
- 61). Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, Krause M, Tronnier V, Kloss M, Schnitzler A, Wojtecki L, Bötzel K, Danek A, Hilker R, Sturm V, Kupsch A, Karner E, Deuschl G: Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol 7: 605– 614, 2008. [DOI] [PubMed] [Google Scholar]
- 62). Volkmann J, Daniels C, Witt K: Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat Rev Neurol 6: 487– 498, 2010. [DOI] [PubMed] [Google Scholar]
- 63). Yamanaka T, Ishii F, Umemura A, Miyata M, Horiba M, Oka Y, Yamada K, Okita K, Matsukawa N, Ojika K: Temporary deterioration of executive function after subthalamic deep brain stimulation in Parkinson’s disease. Clin Neurol Neurosurg 114: 347– 351, 2012. [DOI] [PubMed] [Google Scholar]
- 64). Witt K, Granert O, Daniels C, Volkmann J, Falk D, van Eimeren T, Deuschl G. Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: results from a randomized trial. Brain 136: 2109– 2119, 2013. [DOI] [PubMed] [Google Scholar]
- 65). Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ, CSP 468 Study Group : Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 362: 2077– 2091, 2010. [DOI] [PubMed] [Google Scholar]
- 66). Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF, Mink MS, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RM: Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 12: 37– 44, 2013. [DOI] [PubMed] [Google Scholar]
- 67). Sako W, Miyazaki Y, Izumi Y, Kaji R: Which target is best for patients with Parkinson’s disease? A meta-analysis of pallidal and subthalamic stimulation. J Neurol Neurosurg Psychiatr 85: 982– 986, 2014. [DOI] [PubMed] [Google Scholar]
- 68). Liu Y, Li W, Tan C, Liu X, Wang X, Gui Y, Qin L, Deng F, Hu C, Chen L. Meta-analysis comparing deep brain stimulation of the globus pallidus and subthalamic nucleus to treat advanced Parkinson disease. J Neurosurg 121: 709– 18, 2014. [DOI] [PubMed] [Google Scholar]
- 69). Herzog J, Pinsker M, Wasner M, Steigerwald F, Wailke S, Deuschl G, Volkmann J: Stimulation of subthalamic fibre tracts reduces dyskinesias in STN-DBS. Mov Disord 15: 679– 684, 2007. [DOI] [PubMed] [Google Scholar]
- 70). Allert N, Schnitzler A, Sturm V, Maarouf M: Failure of long-term subthalamic nucleus stimulation corrected by additional pallidal stimulation in a patient with Parkinson’s disease. J Neurol 259: 1244– 1246, 2012. [DOI] [PubMed] [Google Scholar]
- 71). Minafra B, Fasano A, Pozzi NG, Zangaglia R, Servello D, Pacchetti C: Eight-years failure of subthalamic stimulation rescued by globus pallidus implant. Brain Stimul 7: 179– 181, 2014. [DOI] [PubMed] [Google Scholar]
- 72). Cook RJ, Jones L, Fracchia G, Anderson N, Miu J, Meagher LJ, Silburn PA, Silberstein P: Globus pallidus internus deep brain stimulation as rescue therapy for refractory dyskinesias following effective subthalamic nucleus stimulation. Stereotact Funct Neurosurg 93: 25– 29, 2015. [DOI] [PubMed] [Google Scholar]
- 73). Romito LM, Albanese A: Dopaminergic therapy and subthalamic stimulation in Parkinson’s disease: a review of 5-year reports. J Neurol 257: S298– S304, 2010. [DOI] [PubMed] [Google Scholar]
- 74). Fasano A, Romito LM, Daniele A, Piano C, Zinno M, Bentivoglio AR, Albanese A: Motor and cognitive outcome in patients with Parkinson’s disease 8 years after subthalamic implants. Brain 133: 2664– 2676, 2010. [DOI] [PubMed] [Google Scholar]
- 75). Zibetti M, Merola A, Rizzi L, Ricchi V, Angrisano S, Azzaro C, Artusi CA, Arduino N, Marchisio A, Lanotte M, Rizzone M, Lopiano L: Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson’s disease. Mov Disord 26: 2327– 2334, 2011. [DOI] [PubMed] [Google Scholar]
- 76). Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E: Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol 68: 1550– 1556, 2011. [DOI] [PubMed] [Google Scholar]
- 77). Bang Henriksen M, Johnsen EL, Sunde N, Vase A, Gjelstrup MC, Østergaard K: Surviving 10 years with deep brain stimulation for Parkinson’s disease: a follow-up of 79 patients. Eur J Neurol 23: 53– 61, 2016. [DOI] [PubMed] [Google Scholar]
- 78). Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP, Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, Xie J, Benabid AL, Lozano AM, Saint-Cyr J, Romito L, Contarino MF, Scerrati M, Fraix V, Van Blercom N: Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain 128: 2240– 2249, 2005. [DOI] [PubMed] [Google Scholar]
- 79). Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, Kulisevsky J, Obeso JA, Albanese A, Hariz MI, Quinn NP, Speelman JD, Benabid AL, Fraix V, Mendes A, Welter ML, Houeto JL, Cornu P, Dormont D, Tornqvist AL, Ekberg R, Schnitzler A, Timmermann L, Wojtecki L, Gironell A, Rodriguez-Oroz MC, Guridi J, Bentivoglio AR, Contarino MF, Romito L, Scerrati M, Janssens M, Lang AE: Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord 25: 578– 586, 2010. [DOI] [PubMed] [Google Scholar]
- 80). St George RJ, Nutt JG, Burchiel KJ, Horak FB: A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology 75: 1292– 1299, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81). Ngoga D, Mitchell R, Kausar J, Hodson J, Harries A, Pall H. Deep brain stimulation improves survival in severe Parkinson’s disease. J Neurol Neurosurg Psychiatr 85: 17– 22, 2014. [DOI] [PubMed] [Google Scholar]
- 82). Lilleeng B, Brønnick K, Toft M, Dietrichs E, Larsen JP: Progression and survival in Parkinson’s disease with subthalamic nucleus stimulation. Acta Neurol Scand 130: 292– 298, 2014. [DOI] [PubMed] [Google Scholar]
- 83). Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR: Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol 11: 98– 110, 2015. [DOI] [PubMed] [Google Scholar]
- 84). Pahapill PA, Lozano AM: The pedunculopontine nucleus and Parkinson’s disease. Brain 123: 1767– 1783, 2000. [DOI] [PubMed] [Google Scholar]
- 85). Karachi C, Grabli D, Bernard FA, Tandé D, Wattiez N, Belaid H, Bardinet E, Prigent A, Nothacker HP, Hunot S, Hartmann A, Lehéricy S, Hirsch EC, François C: Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 120: 2745– 2754, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86). Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P: Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 130: 1596– 1607, 2007. [DOI] [PubMed] [Google Scholar]
- 87). Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, Henry-Lagrange C, Seigneuret E, Piallat B, Krack P, Le Bas JF, Benabid AL, Chabardès S, Pollak P: Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain 133: 205– 214, 2010. [DOI] [PubMed] [Google Scholar]
- 88). Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM: Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain 133: 215– 224, 2010. [DOI] [PubMed] [Google Scholar]
- 89). Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, Silburn PA: Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 69: 1248– 1253; discussion 1254, 2011. [DOI] [PubMed] [Google Scholar]
- 90). Moreau C, Defebvre L, Destée A, Bleuse S, Clement F, Blatt JL, Krystkowiak P, Devos D: STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 71: 80– 84, 2008. [DOI] [PubMed] [Google Scholar]
- 91). Xie T, Vigil J, MacCracken E, Gasparaitis A, Young J, Kang W, Bernard J, Warnke P, Kang UJ: Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology 84: 415– 420, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Sidiropoulos C, Walsh R, Meaney C, Poon YY, Fallis M, Moro E: Low-frequency subthalamic nucleus deep brain stimulation for axial symptoms in advanced Parkinson’s disease. J Neurol 260: 2306– 2311, 2013. [DOI] [PubMed] [Google Scholar]
- 93). Ricchi V, Zibetti M, Angrisano S, Merola A, Arduino N, Artusi CA, Rizzone M, Lopiano L, Lanotte M: Transient effects of 80 Hz stimulation on gait in STN DBS treated PD patients: a 15 months follow-up study. Brain Stimul 5: 388– 392, 2012. [DOI] [PubMed] [Google Scholar]
- 94). Chastan N, Westby GW, Yelnik J, Bardinet E, Do MC, Agid Y, Welter ML: Effects of nigral stimulation on locomotion and postural stability in patients with Parkinson’s disease. Brain 132: 172– 184, 2009. [DOI] [PubMed] [Google Scholar]
- 95). Weiss D, Walach M, Meisner C, Fritz M, Scholten M, Breit S, Plewnia C, Bender B, Gharabaghi A, Wächter T, Krüger R: Nigral stimulation for resistant axial motor impairment in Parkinson’s disease? A randomized controlled trial. Brain 136: 2098– 2108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96). Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA: Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science 323: 1578– 1582, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97). Thevathasan W, Mazzone P, Jha A, Djamshidian A, Dileone M, Di Lazzaro V, Brown P: Spinal cord stimulation failed to relieve akinesia or restore locomotion in Parkinson disease. Neurology 74: 1325– 1327, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98). Agari T, Date I: Spinal cord stimulation for the treatment of abnormal posture and gait disorder in patients with Parkinson’s disease. Neurol Med Chir (Tokyo) 52: 470–474, 2012. [DOI] [PubMed] [Google Scholar]
- 99). Chan HF, Kukkle PL, Merello M, Lim SY, Poon YY, Moro E: Amantadine improves gait in PD patients with STN stimulation. Parkinsonism Relat Disord 19: 316– 319, 2013. [DOI] [PubMed] [Google Scholar]
- 100). Baba Y, Higuchi MA, Abe H, Fukuyama K, Onozawa R, Uehara Y, Inoue T, Yamada T: Anti-cholinergics for axial symptoms in Parkinson’s disease after subthalamic stimulation. Clin Neurol Neurosurg 114: 1308– 1311, 2012. [DOI] [PubMed] [Google Scholar]
- 101). Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Hälbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltête D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Krüger R, Pinsker MO, Amtage F, Régis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, EARLYSTIM Study Group : Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 368: 610– 622, 2013. [DOI] [PubMed] [Google Scholar]
- 102). Schüpbach WM, Rau J, Houeto JL, Krack P, Schnitzler A, Schade-Brittinger C, Timmermann L, Deuschl G: Myths and facts about the EARLYSTIM study. Mov Disord 29: 1742– 1750, 2014. [DOI] [PubMed] [Google Scholar]
- 103). Sprenger FS, Seppi K, Wolf E, Poewe W: Relevance of EARLYSTIM in a tertiary movement disorders center. Mov Disord 29: 1220– 1221, 2014. [DOI] [PubMed] [Google Scholar]
- 104). Mestre TA, Espay AJ, Marras C, Eckman MH, Pollak P, Lang AE: Subthalamic nucleus-deep brain stimulation for early motor complications in Parkinson’s disease-the EARLYSTIM trial: early is not always better. Mov Disord 29: 1751– 1756, 2014. [DOI] [PubMed] [Google Scholar]
- 105). Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL: Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson’s disease and tremor. Mov Disord 21: S259– S283, 2006. [DOI] [PubMed] [Google Scholar]
- 106). Nakajima T, Zrinzo L, Foltynie T, Olmos IA, Taylor C, Hariz MI, Limousin P: MRI-guided subthalamic nucleus deep brain stimulation without microelectrode recording: can we dispense with surgery under local anaesthesia? Stereotact Funct Neurosurg 89: 318–325, 2011. [DOI] [PubMed] [Google Scholar]
- 107). Aviles-Olmos I, Kefalopoulou Z, Tripoliti E, Candelario J, Akram H, Martinez-Torres I, Jahanshahi M, Foltynie T, Hariz M, Zrinzo L, Limousin P: Long-term outcome of subthalamic nucleus deep brain stimulation for Parkinson’s disease using an MRI-guided and MRI-verified approach. J Neurol Neurosurg Psychiatr 85: 1419– 1425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108). Zrinzo L, Foltynie T, Limousin P, Hariz MI: Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg 116: 84– 94, 2012. [DOI] [PubMed] [Google Scholar]
- 109). Umemura A, Oka Y, Yamada K, Oyama G, Shimo Y, Hattori N: Validity of single tract microelectrode recording in subthalamic nucleus stimulation. Neurol Med Chir (Tokyo) 53: 821–827, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110). Temel Y, Wilbrink P, Duits A, Boon P, Tromp S, Ackermans L, van Kranen-Mastenbroek V, Weber W, Visser-Vandewalle V: Single electrode and multiple electrode guided electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. Neurosurgery 61: 346– 355; discussion 355–357, 2007. [DOI] [PubMed] [Google Scholar]
- 111). Rothlind JC, Cockshott RW, Starr PA, Marks WJ, Jr: Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. J Int Neuropsychol Soc 13: 68– 79, 2007. [DOI] [PubMed] [Google Scholar]
- 112). Tanei T, Kajita Y, Kaneoke Y, Takebayashi S, Nakatsubo D, Wakabayashi T: Staged bilateral deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Acta Neurochir (Wien) 151: 589–594, 2009. [DOI] [PubMed] [Google Scholar]
- 113). Petraglia FW, 3rd, Farber SH, Han JL, Verla T, Gallis J, Lokhnygina Y, Parente B, Hickey P, Turner DA, Lad SP: Comparison of bilateral vs. staged unilateral deep brain stimulation (DBS) in Parkinson’s disease in patients under 70 years of age. Neuromodulation 19: 31– 37, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114). Tomaszewski KJ, Holloway RG: Deep brain stimulation in the treatment of Parkinson’s disease: a cost-effectiveness analysis. Neurology 57: 663– 671, 2001. [DOI] [PubMed] [Google Scholar]
- 115). Valldeoriola F, Morsi O, Tolosa E, Rumià J, Martí MJ, Martínez-Martín P: Prospective comparative study on cost-effectiveness of subthalamic stimulation and best medical treatment in advanced Parkinson’s disease. Mov Disord 22: 2183– 2191, 2007. [DOI] [PubMed] [Google Scholar]
- 116). Eggington S, Valldeoriola F, Chaudhuri KR, Ashkan K, Annoni E, Deuschl G: The cost-effectiveness of deep brain stimulation in combination with best medical therapy, versus best medical therapy alone, in advanced Parkinson’s disease. J Neurol 261: 106– 116, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117). Timmermann L, Schüpbach M, Hertel F, Wolf E, Eleopra R, Franzini A, Servello D, Skogseid IM, Rumia J, Aliaga AS, Barbe MT, Pauls KA, Lin JP, Moro E, Lloyd A, Maarouf M: A new rechargeable device for deep brain stimulation: a prospective patient satisfaction survey. Eur Neurol 69: 193– 199, 2013. [DOI] [PubMed] [Google Scholar]
- 118). Waln O, Jimenez-Shahed J: Rechargeable deep brain stimulation implantable pulse generators in movement disorders: patient satisfaction and conversion parameters. Neuromodulation 17: 425– 430, 2014. [DOI] [PubMed] [Google Scholar]
- 119). Timmermann L, Jain R, Chen L, Maarouf M, Barbe MT, Allert N, Brücke T, Kaiser I, Beirer S, Sejio F, Suarez E, Lozano B, Haegelen C, Vérin M, Porta M, Servello D, Gill S, Whone A, Van Dyck N, Alesch F: Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson’s disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. Lancet Neurol 14: 693– 701, 2015. [DOI] [PubMed] [Google Scholar]
- 120). http://professional.medtronic.com/pt/neuro/dbs-md/ind/mri-guidelines/
- 121). Bronstein JM, Tagliati M, McIntyre C, Chen R, Cheung T, Hargreaves EL, Israel Z, Moffitt M, Montgomery EB, Stypulkowski P, Shils J, Denison T, Vitek J, Volkman J, Wertheimer J, Okun MS: The rationale driving the evolution of deep brain stimulation to constant-current devices. Neuromodulation 18: 85– 88; discussion 88–89, 2015. [DOI] [PubMed] [Google Scholar]
- 122). Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, Alterman R, Jankovic J, Simpson R, Junn F, Verhagen L, Arle JE, Ford B, Goodman RR, Stewart RM, Horn S, Baltuch GH, Kopell BH, Marshall F, Peichel D, Pahwa R, Lyons KE, Tröster AI, Vitek JL, Tagliati M, SJM DBS Study Group : Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol 11: 140– 149, 2012. [DOI] [PubMed] [Google Scholar]
- 123). Ramirez de Noriega F, Eitan R, Marmor O, Lavi A, Linetzky E, Bergman H, Israel Z: Constant current versus constant voltage subthalamic nucleus deep brain stimulation in Parkinson’s disease. Stereotact Funct Neurosurg 93: 114– 121, 2015. [DOI] [PubMed] [Google Scholar]
- 124). Barbe MT, Maarouf M, Alesch F, Timmermann L: Multiple source current steering: a novel deep brain stimulation concept for customized programming in a Parkinson’s disease patient. Parkinsonism Relat Disord 20: 471– 473, 2014. [DOI] [PubMed] [Google Scholar]
- 125). Miocinovic S, Khemani P, Whiddon R, Zeilman P, Martinez-Ramirez D, Okun MS, Chitnis S: Outcomes, management, and potential mechanisms of interleaving deep brain stimulation settings. Parkinsonism Relat Disord 20: 1434– 1437, 2014. [DOI] [PubMed] [Google Scholar]
- 126). Wojtecki L, Vesper J, Schnitzler A: Interleaving programming of subthalamic deep brain stimulation to reduce side effects with good motor outcome in a patient with Parkinson’s disease. Parkinsonism Relat Disord 17: 293– 294, 2011. [DOI] [PubMed] [Google Scholar]
- 127). Weiss D, Breit S, Wächter T, Plewnia C, Gharabaghi A, Krüger R: Combined stimulation of the substantia nigra pars reticulata and the subthalamic nucleus is effective in hypokinetic gait disturbance in Parkinson’s disease. J Neurol 258: 1183– 1185, 2011. [DOI] [PubMed] [Google Scholar]
- 128). Pollo C, Kaelin-Lang A, Oertel MF, Stieglitz L, Taub E, Fuhr P, Lozano AM, Raabe A, Schüpbach M: Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain 137: 2015– 2026, 2014. [DOI] [PubMed] [Google Scholar]
- 129). Contarino MF, Bour LJ, Verhagen R, Lourens MA, de Bie RM, van den Munckhof P, Schuurman PR: Directional steering: a novel approach to deep brain stimulation. Neurology 83: 1163– 1169, 2014. [DOI] [PubMed] [Google Scholar]
- 130). Hammond C, Bergman H, Brown P: Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 30: 357– 364, 2007. [DOI] [PubMed] [Google Scholar]
- 131). Priori A, Foffani G, Rossi L, Marceglia S: Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol 245: 77– 86, 2013. [DOI] [PubMed] [Google Scholar]
- 132). Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, FitzGerald J, Green AL, Aziz TZ, Brown P: Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 74: 449– 457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133). Little S, Beudel M, Zrinzo L, Foltynie T, Limousin P, Hariz M, Neal S, Cheeran B, Cagnan H, Gratwicke J, Aziz TZ, Pogosyan A, Brown P: Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatr 2015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134). Coune PG, Schneider BL, Aebischer P: Parkinson’s disease: gene therapies. Cold Spring Harb Perspect Med 2: a009431, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135). Ross CA, Akimov SS: Human-induced pluripotent stem cells: potential for neurodegenerative diseases. Hum Mol Genet 23: R17– R26, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136). Kalanithi PS, Henderson JM: Optogenetic neuromodulation. Int Rev Neurobiol 107: 185– 205, 2012. [DOI] [PubMed] [Google Scholar]