Abstract
Epilepsy surgery is aimed to remove the brain tissues that are indispensable for generating patient’s epileptic seizures. There are two purposes in the pre-operative evaluation: localization of the epileptogenic zone and localization of function. Surgery is planned to remove possible epileptogenic zone while preserving functional area. Since no single diagnostic modality is superior to others in identifying and localizing the epileptogenic zone, multiple non-invasive evaluations are performed to estimate the location of the epileptogenic zone after concordance between evaluations. Essential components of non-invasive pre-surgical evaluation of epilepsy include detailed clinical history, long-term video-electroencephalography monitoring, epilepsy-protocol magnetic resonance imaging (MRI), and neuropsychological testing. However, a significant portion of drug-resistant epilepsy is associated with no or subtle MRI lesions or with ambiguous electro-clinical signs. Additional evaluations including fluoro-deoxy glucose positron emission tomography (FDG-PET), magnetoencephalography and ictal single photon emission computed tomography can play critical roles in planning surgery. FDG-PET should be registered on three-dimensional MRI for better detection of focal cortical dysplasia. All diagnostic tools are complementary to each other in defining the epileptogenic zone, so that it is always important to reassess the data based on other results to pick up or confirm subtle abnormalities.
Keywords: epilepsy surgery, evaluation, electroencephalography, magnetic resonance imaging, semiology
Introduction
Epilepsy surgery is indicated for patients with ‘drug-resistant’ epilepsy. Current practical definition of drug-resistance is defined as failure of seizure control after adequate medical therapy with two or more appropriate anti-epileptic drugs.1,2) The goal of epilepsy surgery is the improvement of patient’s quality of life by seizure control with or without continuing anti-epileptic medications. Albeit not the goal of treatment, it is always kept in mind that surgical treatment is an only strategy that can remove the cause of, or ‘cure,’ epilepsy. Epilepsy surgery can be indicated earlier when drug-resistance is highly expected such as in the mesial temporal lobe epilepsy with hippocampal sclerosis.3) or when adverse effect of poor seizure control is expected on patient’s development in young children.2)
Surgical treatment is planned after comprehensive evaluation of the patient’s epilepsy. In the first part of this review, we introduce a concept of epileptogenic zone and how epilepsy surgery is considered on multiple evaluations. Then, several recent topics on non-invasive evaluations are reviewed, although cyclopedic coverage is beyond the scope of this review.
Epileptogenic Zone and Concept of Pre-surgical Evaluation in Epilepsy
Complete removal of epileptogenic zone is aimed in epilepsy surgery. The concept, epileptogenic zone, is defined as the area of cortex that is indispensable for the generation of epileptic seizures.4) This concept is built on the notion that no single diagnostic modality is currently available to identify the accurate brain area generating patient’s seizures. For example, cavernous malformation certainly causes patient’s epilepsy, but the brain region responsible for generating seizures usually exists in the surrounding brain, where enduring hyperexcitability was acquired by degeneration and hemosiderin deposition induced by the malformation. It is currently hard to know pre-operatively to what extent, the surrounding brain should be removed to achieve seizure control.5,6) This is also the case in other etiologies, including focal cortical dysplasia, brain tumors, stroke, and traumatic brain injury, although intrinsic epileptogenicity is proved in certain lesions with neuronal components, such as focal cortical dysplasia, ganglioglioma, and cortical tubers.7,8) Therefore, the epileptogenic zone is a theoretical concept.
Epileptogenic zone is estimated after results of multiple evaluations. A variety of diagnostic tools define different cortical zones of epileptic abnormality (Table 1). These cortical zones can overlap with high concordance or can be discordant each other, because each diagnostic method has their own sensitivity and specificity for defining the location and extent of epileptogenic zone. When the concordance is high, estimated location and the extent of epileptogenic zone becomes accurate and confident. Currently, the presence of a structural epileptogenic lesion in MRI is most reliable and accurate information for epileptogenic zone. Complete removal of MRI-visible epileptogenic lesion is associated with seizure freedom after epilepsy surgery.9)
Table 1.
Non-invasive pre-surgical evaluations for epilepsy
| Evaluations | Cortical zones of epileptic abnormalitya |
| Detailed clinical history* | — |
| Video-EEG monitoring* | Seizure onset zone, Symptomatogenic zone, Irritative zone |
| MRI* | Epileptongenic lesion |
| Neuropsychological evaluation* | Functional deficit zone |
| FDG-PET | Epileptogenic lesionb , Functional deficit zone |
| Magnetoencephalography (MEG) | Irritative zone |
| Iomazenil-SPECT | Epileptogenic lesionb , Functional deficit zone |
| Ictal ECD-SPECT | Seizure onset zone |
| Functional MRI / Functional MEG | Eloquent cortex |
Essential evaluations.
Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain 124:1683–700, 2001.
MRI-negative epileptogenic lesion could be detected as functionally impaired region.
Information obtained by a single evaluation can be fragmented and spatially not clear, but highly specific for laterality or possible location of the epileptogenic zone. For example, the presence of head version to the right side immediately before secondary generalized tonic-clonic seizure strongly indicates seizure originating in the left hemisphere. Similarly, the focal tonic-clonic seizure in the left leg strongly indicates the epileptogenic zone nearby the right primary motor cortex of the leg level. These are highly diagnostic even in the absence of clear MRI lesion and clear EEG (electroenphalography) substrates.10) All diagnostic results are complementary to each other, and are interpreted on their own sensitivity and specificity.
Non-invasive Pre-surgical Evaluation of Epilepsy
There are two purposes in the pre-operative evaluation: localization of the epileptogenic zone and localization of function. Surgery is planned to remove possible epileptogenic zone while preserving functional area. Non-invasive evaluations of drug-resistant epilepsy are listed in Table 1. Detailed history taking, structural MRI, long-term video-EEG monitoring, and neuropsychological testing are considered as essential for pre-surgical evaluation in clinical practice. Optional evaluations can be performed on necessity. Because the evaluation is aimed to make surgical decision, no routine sets exist and evaluations can be tailored to the patient’s needs. For example, anterior temporal lobectomy can be indicated for a patient with mesial temporal lobe epilepsy only based on structural MRI, routine EEG, and a detailed clinical history, after confirming the presence of unilateral hippocampal atrophy and typical history for mesial temporal lobe epilepsy ‘syndrome,’11) On the other hand, optional evaluations such as MEG and ictal SPECT can be critical for surgical decision making in patients with ‘MRI-negative’ focal epilepsy.
Long-term Video-EEG Monitoring (LTVEEG) with Scalp Electrodes
The purpose of LTVEEG is 1) to rule out non-epileptic seizures and 2) to analyze ictal semiology / EEG for localization of epilepsy. Even if a patient has established diagnosis of epilepsy, the presence of concomitant non-epileptic seizures should be carefully ruled out. A significant proportion of medical intractability is caused by misdiagnosis of non-epileptic seizures.2) Moreover, 10–30% of patients with drug-resistant seizures have both epileptic and non-epileptic seizures. Although epilepsy can be affirmed by clear history of witnessed seizures and interictal epileptic EEG, further documentation of the patient’s habitual seizures with LTVEEG is routinely recommended.
The sensitivity and specificity of localizing/lateralizing features in seizure semiology have been reported.12,13) Localizing/lateralizing features are often only supportive for other evaluations, and lack of those features does not mean non-localizable or non-lateralizable epilepsy. However, seizure semiology can be highly diagnostic when the epileptic lesion is absent, very small, or deeply seated so that minimum EEG changes are associated.10) Important seizures for such situations include supplementary motor area seizures, focal tonic-clonic seizures and focal somato-sensory auras.
Semiological features are well documented in mesial temporal lobe epilepsy and seizures near the primary functional areas, such as the primary motor, somato-sensory, and visual cortices. In contrast, localizing semiological features are not well understood in frontal and parietal association areas, and several limbic and paralimbic regions such as cingulum, orbito-frontal and insula cortices. Recent studies with stereo-tactic intracranial EEG are revealing electro-clinical correlation of seizures originating in those areas.14) In frontal lobe seizures, those originating in the anterior lateral and medial prefrontal regions are characterized by integrated gestural motor behaviors with distal stereotypy, and those originating in the ventromedial prefrontal cortex are characterized by ‘fearful’ facial expression and behaviors. Importantly, the medial prefrontal seizures are typically not associated with elementary motor signs and occasionally characterized by hyperkinetic behaviors.15) Seizures generated by insular lesions usually mimic temporal or frontal lobe epilepsy, characterized by a variety of symptoms. Specific signs of insular seizures include autonomic, viscero- and somato-sensory symptoms, a sensation of laryngeal constriction, and paresthesia,16,17) Seizures arising from the posterior cingulate gyrus are electro-clinically similar to temporal lobe epilepsy. Those arising from the anterior cingulate gyrus are characterized by hyperkinetic behaviors with the presence of fear or laughter.18)
Epilepsy Protocol MRI
Patients should be evaluated with epilepsy protocol MRI: sets of MRI sequences specialized for detecting epileptogenic lesions. High-quality MRI is mandatory for the evaluation of epilepsy. Non-expert reading of standard MRI fails to detect 61% of epileptogenic lesions.19) Recommended sets of sequences were proposed as ‘essential 6’ (Table 2).20) The combination of FLAIR, T2/STIR, and hemosiderin/calcification sensitive sequences such as susceptibility weighted imaging can detect nearly all epileptogenic lesions. Three-dimensional T1-weighted images are suited for detecting migration disorders such as periventricular heterotopia, as well as for anatomical co-registration with other images including PET and SPECT. All T2 and FLAIR images should be angulated perpendicular to the hippocampal axis.
Table 2.
Essential six sequences of magnetic resonance imaging for epilepsy patients (Wellmer J, et al. Epilepsia 54(11): 1977–87, 2013)
| Sequence | Slice thickness | Cut-plane orientation |
|---|---|---|
| T1 / MPRAGE | 1 mm isotropic | 3-dimensional |
| T2 / STIR | <3 mm | axial* |
| T2 / STIR | <3 mm | coronal* |
| FLAIR | <3 mm | axial* |
| FLAIR | <3 mm | coronal* |
| T2* / SWI | <3 mm | axial* |
should be acquired angulated to hippocampal axis. MPRAGE: magnetization prepared rapid acquisition gradient echo, STIR: short-tau inversion recovery, FLAIR: fluid attenuated inversion recovery, SWI: susceptibility-weighted imaging.
The epilepsy protocol MRI is especially aimed to detect hippocampal sclerosis and focal cortical dysplasia, which are the most prevalent etiology of drug-resistant epilepsy. Detection of hippocampal sclerosis is important because it can be associated with neocortical epileptogenic lesion (dual pathology). In the dual pathology, resection of both sclerotic hippocampus and neocortical lesion is required for maximum chance of seizure freedom.21) Hippocampal sclerosis is characterized by volume loss, increased T2 signal, and loss of the internal structures of the hippocampus.22,23) However, 5–10% of pathological hippocampal sclerosis shows no obvious atrophy on MRI,24) volumetric analysis and T2 relaxometry can enhance the detection of mild form or bilateral hippocampal abnormality.25) Additionally, quantitative analysis of glucose metabolism and white matter signal changes is helpful in identifying anterior temporal lobe abnormality associated with mesial temporal epileptogenicity.26)
Focal cortical dysplasia often shows only subtle abnormalities in MRI. Volume acquisition of T1-weighted, T2-weighted, and FLAIR images can be added to identify and confirm abnormally-thickened cortex, gray-white matter blurring, or trans-mantle signs. The volume acquisition enables us to review suspected abnormality in any slice angulation. Volume-rendered brain imaging is useful to identify abnormal sulci. Voxel-based morphometry and sulcal pattern analysis are developed for objective and automated detection of focal cortical dysplasia.27–29)
Recently, small middle-fossa encephalocele is recognized as a cause of ‘non-lesional’ TLE.30–32) Thin-sliced examination of the middle fossa would be recommended for patients with MRI-negative temporal lobe epilepsy, not to overlook such under-recognized etiology.
FDG-PET
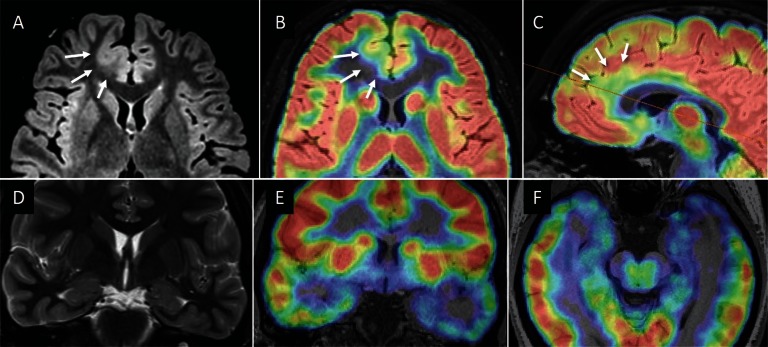
Epileptic abnormality usually presents glucose hypometabolism interictally. FDG-PET is indicated especially when no structural lesions are identified on MRI. The PET imaging should be registered on three-dimensional MRI (fusion image) for accurate interpretation (Fig. 1A–C), because co-registration of FDG-PET and MRI is known to improve detection of focal cortical dysplasia.33) This method especially enhances detection of MRI-negative type I cortical dysplasia, and complete removal of PET-positive lesion is associated with excellent seizure outcome.
Fig. 1.
Co-registration of FDG-PET and MRI. (A) Axial FLAIR image in a 19-year-old man with the right medial frontal focal cortical dysplasia (FCD). Abnormally thickened cortex is associated with blurred gray-white matter junction (arrows). (B, C) Co-registered FDG-PET images show focal glucose hypometabolism in the lesion (arrows). Co-registration of FDG-PET and MRI improves detection and identification of FCD. The patient became seizure free after lesionectomy. Histo-pathological diagnosis was FCD type 2b. (D) Coronal T2 weighted image shows no remarkable abnormalities in a 27-year-old woman with the left temporal lobe epilepsy. (E, F) Co-registered FDG-PET showed glucose hypometabolism in the left anterior temporal lobe. The patient underwent left anterior temporal lobectomy, followed by seizure freedom. Histo-pathologically no specific abnormality was identified in the hippocampus and temporal neocortex.
FDG-PET is also useful in identification of surgically treatable MRI-negative temporal lobe epilepsy (TLE) (Fig. 1D–F). Anterior temporal glucose hypometabolism is recently recognized as a typical pattern in non-lesional mesial TLE and denoted as ‘MRI-negative PET-positive TLE’.34,35) The surgical outcome of MRI-negative PET-positive TLE is comparable with mesial TLE with hippocampal sclerosis, although dichotic response was pointed out in its post-operative seizure outcome, i.e. the patients were divided into those with class I outcome and those with class III or IV outcome.
Magnetoencephalography (MEG) and ictal Single-photon Emission Computed Tomography (SPECT)
MEG and ictal SPECT are important localization tools for epileptogenic zone in patients with inconclusive findings in the above evaluations. Generators of interictal spikes are estimated with equivalent current dipole (ECD) modeling in MEG. Registered images of ECDs on the patient’s MRI are called magnetic source imaging (MSI). When interpreting MSI, the following two limitations should be kept in mind. First, due to inherent limitation in MEG and ECD modeling, MSI inevitably has unknown amounts of errors in its spatial accuracy. Second, MSI is usually derived from interictal recording, and interictal epileptic spikes can occur remote from the epileptogenic zone.36)
Nevertheless, MSI can provide crucial information in surgical decision making. Anterior temporal distribution of ECDs is affirmative in the diagnosis of mesial temporal lobe epilepsy.37) In extra-temporal lobe epilepsy, complete removal of ‘clustered’ ECDs is associated with better post-operative seizure outcome.38,39) In contrast, diffusely distributed ECDs are suggestive of diffuse epileptogenic zone. The orientation of ECD provides an important clue for determining the epileptogenic side of opposing cortices in the cerebral sulcus.36) At their peak, epileptic spikes usually generate dipolar current oriented to the basal side of the cortex. For example, in the central sulcus, anteriorly oriented dipoles suggest activation of the anterior, or frontal, bank of the sulcus.
Ictal SPECT visualizes the area of increased cerebral blood flow induced by an epileptic seizure. Although it is only feasible in patients with frequent seizures, Ictal SPECT is a powerful tool for localization of the possible epileptogenic zone. Subtraction ictal SPECT co-registered to MRI (SISCOM) has higher predictive value for epileptogenic zone than side-by-side comparison of ictal and interictal SPECT.40) Yield of ictal SPECT depends on the timing of tracer injection. Delayed injection may only detect secondary hyperperfusion after seizure spread, producing diffuse or non-localized findings. Early tracer injection is an important factor for better localizability.41)
Combination of Multimodal Imaging
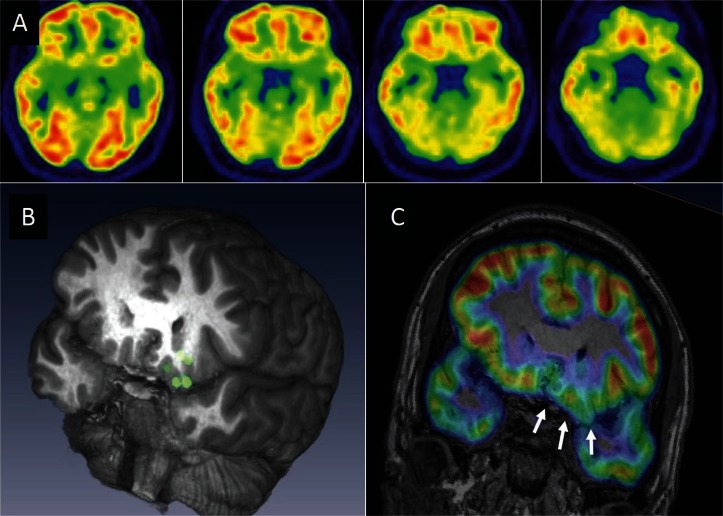
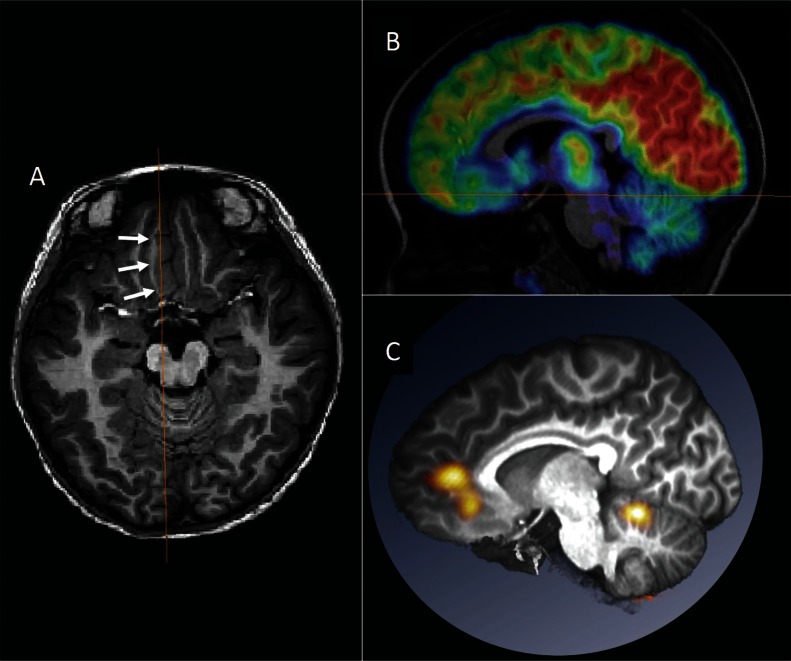
Multimodal evaluations should be reviewed and interpreted together, because epilepsy-related abnormality is occasionally subtle, ambiguous, or uncertain. Localizing information of one modality may enhance detection of or convince the presence of subtle abnormalities in another modality. For example, reviewing the area of MEG dipoles may detect small focal cortical dysplasia that was not previously found by routine MRI (Fig. 2).10) It is recommended that multimodal results are spatially co-registered and reviewed in a common anatomical space, which is useful in planning surgery (Fig. 3). Automated brain extraction, multimodal image registration, and volume-rendered visualization are easily available by free software packages.42,43)
Fig. 2.
Magnetic source imaging enhanced detection of focal glucose hypometabolism caused by focal cortical dysplasia. A 20-year-old man with the left orbito-frontal lobe epilepsy is presented. No remarkable abnormality was noted in the conventional presentation of FDG-PET. (A) Magnetoencephalography recording revealed that equivalent current dipoles (green circles in the 3-dimensional MR imaging) of his interictal spikes were localized in the left orbito-frontal region. (B) Review of the dipole region led us to identify focal glucose hypometabolism in the same region (arrows) in FDG-PET. (C) The patient underwent focal cortical resection of the orbito-frontal lobe after the orbito-frontal seizure onset was confirmed by invasive evaluation with chronically implanted subdural electrodes. Histo-pathological diagnosis of microdysgenesis was established.
Fig. 3.
Multimodal presentation in the anatomical space. A 6-year-old girl with the right medial frontal lobe epilepsy is presented. Abnormally-thickened cortex was noted in the right medial frontal lobe (arrows in A). FDG-PET in the sagittal section revealed the area of glucose hypometabolism corresponding to the lesion (B). Subtraction ictal SPECT co-registered to MRI (SISCOM) images were registered on the patient’s MRI and presented in the same section with the FDG-PET (C). Ictal hyperperfusion was found to occur in the dorsal part of the lesion. Surgery was planned to remove both the lesion and the area of ictal hyperperfusion. Spatial co-registration of multimodal images is useful in surgical planning.
Neuropsychological Testing
Neuropsychological evaluation of patient’s cognitive capabilities is essential before and after epilepsy surgery. Neuropsychological deficits help not only to localize or lateralize epileptogenic zone, but also to estimate post-operative risks of cognitive decline. For example, impairment of verbal memory performance is caused by damage in the hippocampal memory system, thus supportive of the diagnosis of mesial temporal lobe epilepsy, especially of the language-dominant hemisphere.
The risk of post-operative cognitive impairment depends on the ‘functional adequacy of the tissues to be resected’ and ‘reserve capacity’.44) An important principle is that the risk of post-operative cognitive decline is minimum, when surgery is limited to tissues not involved in normal function. For example, removal of non-atrophic hippocampus in the left or language-dominant side carries high risk of post-operative decline in verbal memory. In contrast, removal of atrophic hippocampus carries lower risk of post-operative cognitive decline.45) Better baseline cognitive performance is indicative of both the ‘adequacy of tissues to be resected’ and ‘reserve capacity.’
Conclusion
In pre-surgical evaluation of epilepsy, multiple diagnostic tools are used to estimate the location of epileptogenic zone. Non-invasive evaluation of epilepsy includes detailed history taking, long-term video-EEG recording, epilepsy-protocol MRI, neuropsychological testing, FDG-PET, MEG, and SPECT. It is important to recognize that no single diagnostic modality is superior to others in identifying and localizing the epileptogenic zone, and that all evaluations are complementary to each other. Epilepsy-related abnormality is occasionally subtle, ambiguous, and uncertain. Localizing information of one modality may enhance detection of subtle abnormality in another modality.
Acknowledgment
This work was supported by Grant-in-Aid for Scientific Research Nos. 16K10780 from the Japan Society for the Promotion of Science.
Footnotes
Conflicts of Interest Disclosure
The authors report no conflicts of interest concerning with the materials or methods used in this study or the findings specified in this paper. The authors Masaki Iwasaki, Nobukazu Nakasato, Teiji Tominaga have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1). Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J: Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE Commission on therapeutic strategies. Epilepsia 51: 1069– 1077, 2010. [DOI] [PubMed] [Google Scholar]
- 2). Kwan P, Schachter SC, Brodie MJ: Drug-resistant epilepsy. N Engl J Med 365: 919– 926, 2011. [DOI] [PubMed] [Google Scholar]
- 3). Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G, Fried I, Jacobs M, Vinters HV, Mintzer S, Kieburtz K, Early Randomized Surgical Epilepsy Trial (ERSET) Study Group Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 307: 922– 930, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Rosenow F, Lüders H: Presurgical evaluation of epilepsy. Brain 124: 1683– 1700, 2001. [DOI] [PubMed] [Google Scholar]
- 5). Sevy A, Gavaret M, Trebuchon A, Vaugier L, Wendling F, Carron R, Regis J, Chauvel P, Gonigal AM, Bartolomei F: Beyond the lesion: The epileptogenic networks around cavernous angiomas. Epilepsy Res 108: 701– 708, 2014. [DOI] [PubMed] [Google Scholar]
- 6). Jehi LE, Palmini A, Aryal U, Coras R, Paglioli E: Cerebral cavernous malformations in the setting of focal epilepsies: pathological findings, clinical characteristics, and surgical treatment principles. Acta Neuropathol 128: 55– 65, 2014. [DOI] [PubMed] [Google Scholar]
- 7). Mohamed AR, Bailey CA, Freeman JL, Maixner W, Jackson GD, Harvey AS: Intrinsic epileptogenicity of cortical tubers revealed by intracranial EEG monitoring. Neurology 79: 2249– 2257, 2012. [DOI] [PubMed] [Google Scholar]
- 8). Battaglia G, Colciaghi F, Finardi A, Nobili P: Intrinsic epileptogenicity of dysplastic cortex: Converging data from experimental models and human patients. Epilepsia 54: 33– 36, 2013. [DOI] [PubMed] [Google Scholar]
- 9). Berkovic SF, McIntosh AM, Kalnins RM, Jackson GD, Fabinyi GC, Brazenor GA, Bladin PF, Hopper JL: Preoperative MRI predicts outcome of temporal lobectomy: an actuarial analysis. Neurology 45: 1358– 1363, 1995. [DOI] [PubMed] [Google Scholar]
- 10). Itabashi H, Jin K, Iwasaki M, Okumura E, Kanno A, Kato K, Tominaga T, Kawashima R, Nakasato N: Electro- and magneto-encephalographic spike source localization of small focal cortical dysplasia in the dorsal peri-rolandic region. Clin Neurophysiol 125: 2358– 2363, 2014. [DOI] [PubMed] [Google Scholar]
- 11). Kasradze S, Alkhidze M, Lomidze G, Japaridze G, Tsiskaridze A, Zangaladze A: Perspectives of epilepsy surgery in resource-poor countries: a study in Georgia. Acta Neurochir (Wien) 157: 1533– 1540, 2015. [DOI] [PubMed] [Google Scholar]
- 12). Bleasel A, Kotagal P, Kankirawatana P, Rybicki L: Lateralizing value and semiology of ictal limb posturing and version in temporal lobe and extratemporal epilepsy. Epilepsia 38: 168– 174, 1997. [DOI] [PubMed] [Google Scholar]
- 13). Kellinghaus C, Luders H: The symptomatogenic zone - general principles. In: Luders H, ed. Textbook of Epilepsy Surgery. London: Informa Healthcare; 425–431, 2008. [Google Scholar]
- 14). Bonini F, McGonigal A, Trébuchon A, Gavaret M, Bartolomei F, Giusiano B, Chauvel P: Frontal lobe seizures: from clinical semiology to localization. Epilepsia 55: 264– 277, 2014. [DOI] [PubMed] [Google Scholar]
- 15). Leung H, Schindler K, Clusmann H, Bien CG, Pöpel A, Schramm J, Kwan P, Wong LK, Elger CE: Mesial frontal epilepsy and ictal body turning along the horizontal body axis. Arch Neurol 65: 71– 77, 2008. [DOI] [PubMed] [Google Scholar]
- 16). von Lehe M, Wellmer J, Urbach H, Schramm J, Elger CE, Clusmann H: Insular lesionectomy for refractory epilepsy: management and outcome. Brain 132: 1048– 1056, 2009. [DOI] [PubMed] [Google Scholar]
- 17). Isnard J, Guénot M, Sindou M, Mauguière F: Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia 45: 1079– 1090, 2004. [DOI] [PubMed] [Google Scholar]
- 18). Alkawadri R, So NK, Van Ness PC, Alexopoulos AV: Cingulate epilepsy: report of 3 electroclinical subtypes with surgical outcomes. JAMA Neurol 70: 1– 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernández G, Elger CE: Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry 73: 643– 647, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Wellmer J, Quesada CM, Rothe L, Elger CE, Bien CG, Urbach H: Proposal for a magnetic resonance imaging protocol for the detection of epileptogenic lesions at early outpatient stages. Epilepsia 54: 1977– 1987, 2013. [DOI] [PubMed] [Google Scholar]
- 21). Kim DW, Lee SK, Nam H, Chu K, Chung CK, Lee SY, Choe G, Kim HK: Epilepsy with dual pathology: surgical treatment of cortical dysplasia accompanied by hippocampal sclerosis. Epilepsia 51: 1429– 1435, 2010. [DOI] [PubMed] [Google Scholar]
- 22). Eriksson SH, Thom M, Bartlett PA, Symms MR, McEvoy AW, Sisodiya SM, Duncan JS: PROPELLER MRI visualizes detailed pathology of hippocampal sclerosis. Epilepsia 49: 33– 39, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Iwasaki M, Nakasato N, Suzuki H, Tominaga T: Endfolium sclerosis in temporal lobe epilepsy diagnosed preoperatively by 3-tesla magnetic resonance imaging. J Neurosurg 110: 1124– 1126, 2009. [DOI] [PubMed] [Google Scholar]
- 24). Jackson GD, Kuzniecky RI, Cascino GD: Hippocampal sclerosis without detectable hippocampal atrophy. Neurology 44: 42– 46, 1994. [DOI] [PubMed] [Google Scholar]
- 25). Coan AC, Kubota B, Bergo FPG, Campos BM, Cendes F: 3T MRI Quantification of hippocampal volume and signal in mesial temporal lobe epilepsy improves detection of hippocampal sclerosis. AJNR Am J Neuroradiol 35: 77– 83, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Morimoto E, Okada T, Kanagaki M, Yamamoto A, Fushimi Y, Matsumoto R, Takaya S, Ikeda A, Kunieda T, Kikuchi T, Paul D, Miyamoto S, Takahashi R, Togashi K: Evaluation of focus laterality in temporal lobe epilepsy a quantitative study comparing double inversion-recovery MR imaging at 3T with FDG-PET. Epilepsia 54: 2174– 2183, 2013. [DOI] [PubMed] [Google Scholar]
- 27). Wagner J, Weber B, Urbach H, Elger CE, Huppertz HJ: Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain 134: 2844– 2854, 2011. [DOI] [PubMed] [Google Scholar]
- 28). Wang ZI, Jones SE, Ristic AJ, Wong C, Kakisaka Y, Jin K, Schneider F, Gonzalez-Martinez JA, Mosher JC, Nair D, Burgess RC, Najm IM, Alexopoulos AV: Voxel-based morphometric MRI post-processing in MRI-negative focal cortical dysplasia followed by simultaneously recorded MEG and stereo-EEG. Epilepsy Res 100: 188– 193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Kim H, Bernasconi N, Bernhardt B, Colliot O, Bernasconi A: Basal temporal sulcal morphology in healthy controls and patients with temporal lobe epilepsy. Neurology 70: 2159– 2165, 2008. [DOI] [PubMed] [Google Scholar]
- 30). Saavalainen T, Jutila L, Mervaala E, Kälviäinen R, Vanninen R, Immonen A: Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology 85: 1467–1474, 2015. [DOI] [PubMed] [Google Scholar]
- 31). Byrne RW, Smith AP, Roh D, Kanner A: Occult middle fossa encephaloceles in patients with temporal lobe epilepsy. World Neurosurg 73: 541– 546, 2010. [DOI] [PubMed] [Google Scholar]
- 32). Abou-Hamden A, Lau M, Fabinyi G, Berkovic SF, Jackson GD, Mitchell LA, Kalnins R, Fitt G, Archer JS: Small temporal pole encephaloceles: a treatable cause of “lesion negative” temporal lobe epilepsy. Epilepsia 51: 2199– 2202, 2010. [DOI] [PubMed] [Google Scholar]
- 33). Salamon N, Kung J, Shaw SJ, Koo J, Koh S, Wu JY, Lerner JT, Sankar R, Shields WD, Engel J, Jr, Fried I, Miyata H, Yong WH, Vinters HV, Mathern GW: FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology 71: 1594– 1601, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Capraz IY, Kurt G, Akdemir Ö, Hirfanoglu T, Oner Y, Sengezer T, Kapucu LO, Serdaroglu A, Bilir E: Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia 53: 342– 348, 2012. 22192050 [Google Scholar]
- 35). Kuba R, Tyrlíková I, Chrastina J, Slaná B, Pažourková M, Hemza J, Brázdil M, Novák Z, Hermanová M, Rektor I: “MRI-negative PET-positive” temporal lobe epilepsy: invasive EEG findings, histopathology, and postoperative outcomes. Epilepsy Behav 22: 537– 541, 2011. [DOI] [PubMed] [Google Scholar]
- 36). Iwasaki M, Nakasato N: MEG in epilepsy and pre-surgical functional mapping. In: Supek S, Aine CJ, eds. Magnetoencephalography from signals to dynamic cortical networks. Berlin, Heidelberg: Springer Berlin Heidelberg; 821–842, 2014. [Google Scholar]
- 37). Iwasaki M, Nakasato N, Shamoto H, Nagamatsu K, Kanno A, Hatanaka K, Yoshimoto T: Surgical implications of neuromagnetic spike localization in temporal lobe epilepsy. Epilepsia 43: 415– 424, 2002. [DOI] [PubMed] [Google Scholar]
- 38). Iida K, Otsubo H, Matsumoto Y, Ochi A, Oishi M, Holowka S, Pang E, Elliott I, Weiss SK, Chuang SH, Snead OC, 3rd, Rutka JT: Characterizing magnetic spike sources by using magnetoencephalography-guided neuronavigation in epilepsy surgery in pediatric patients. J Neurosurg 102: 187– 196, 2005. [DOI] [PubMed] [Google Scholar]
- 39). Stefan H, Rampp S, Knowlton RC: Magnetoencephalography adds to the surgical evaluation process. Epilepsy Behav 20: 172– 177, 2011. [DOI] [PubMed] [Google Scholar]
- 40). Matsuda H, Matsuda K, Nakamura F, Kameyama S, Masuda H, Otsuki T, Nakama H, Shamoto H, Nakazato N, Mizobuchi M, Nakagawara J, Morioka T, Kuwabara Y, Aiba H, Yano M, Kim YJ, Nakase H, Kuji I, Hirata Y, Mizumura S, Imabayashi E, Sato N: Contribution of subtraction ictal SPECT coregistered to MRI to epilepsy surgery: a multicenter study. Ann Nucl Med 23: 283– 291, 2009. [DOI] [PubMed] [Google Scholar]
- 41). Lee JY, Joo EY, Park HS, Song P, Young Byun S, Seo DW, Hong SB: Repeated ictal SPECT in partial epilepsy patients: SISCOM analysis. Epilepsia 52: 2249– 2256, 2011. [DOI] [PubMed] [Google Scholar]
- 42). Shattuck DW, Leahy RM: BrainSuite: an automated cortical surface identification tool. Med Image Anal 6: 129– 142, 2002. [DOI] [PubMed] [Google Scholar]
- 43). Reuter M, Schmansky NJ, Rosas HD, Fischl B: Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61: 1402– 1418, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Helmstaedter C: Neuropsychological aspects of epilepsy surgery. Epilepsy Behav 5: S45– S55, 2004. [DOI] [PubMed] [Google Scholar]
- 45). Khalil AF, Iwasaki M, Nishio Y, Jin K, Nakasato N, Tominaga T: Verbal dominant memory impairment and low risk for post-operative memory worsening in both left and right temporal lobe epilepsy associated with hippocampal sclerosis. Neurol Med Chir (Tokyo) 2016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]