Abstract
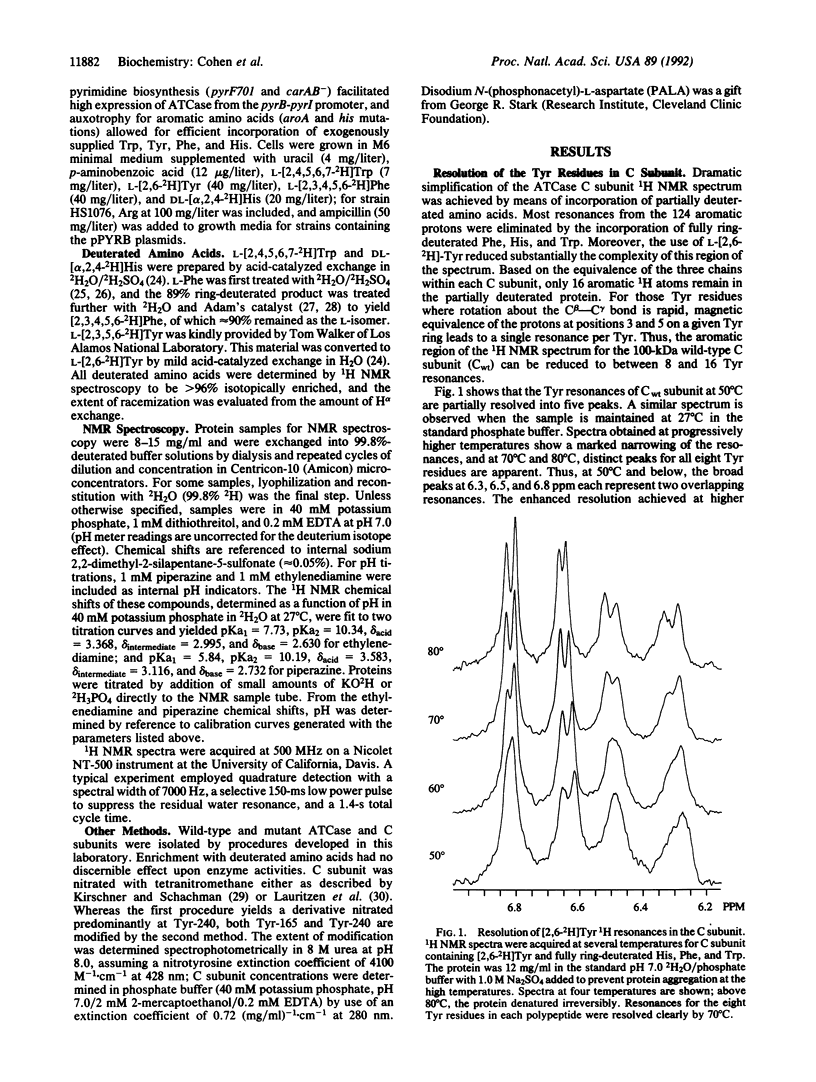
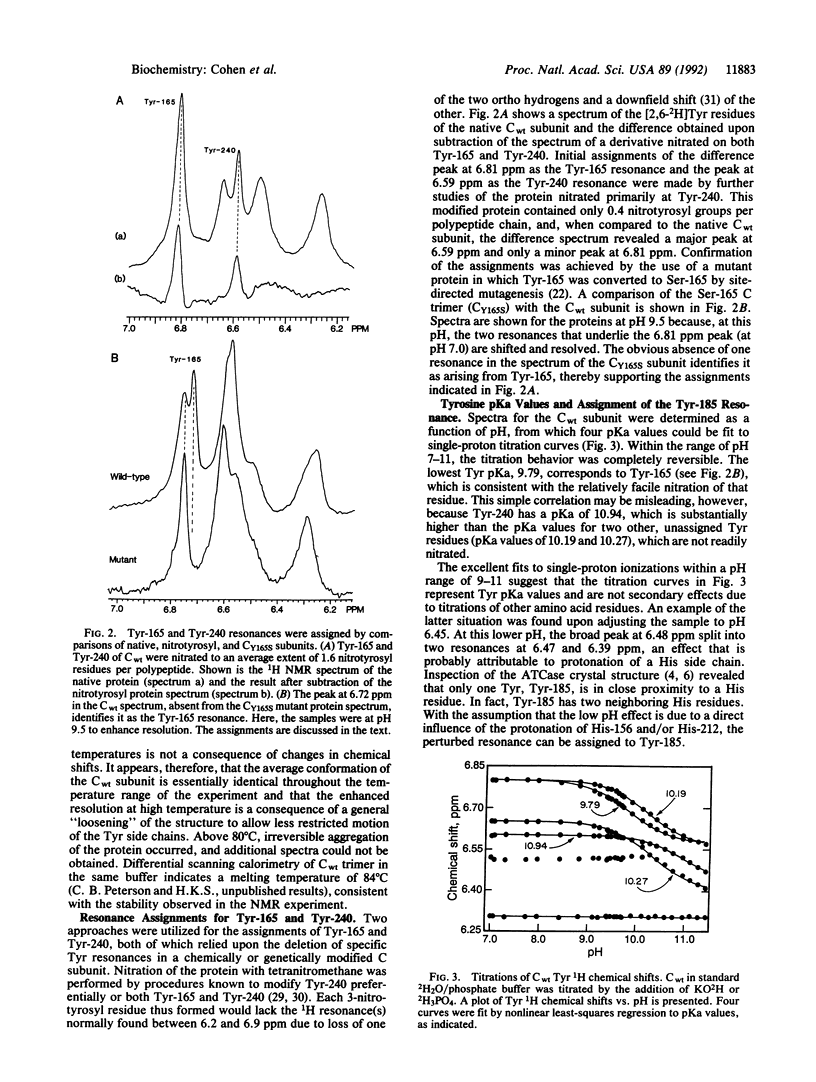
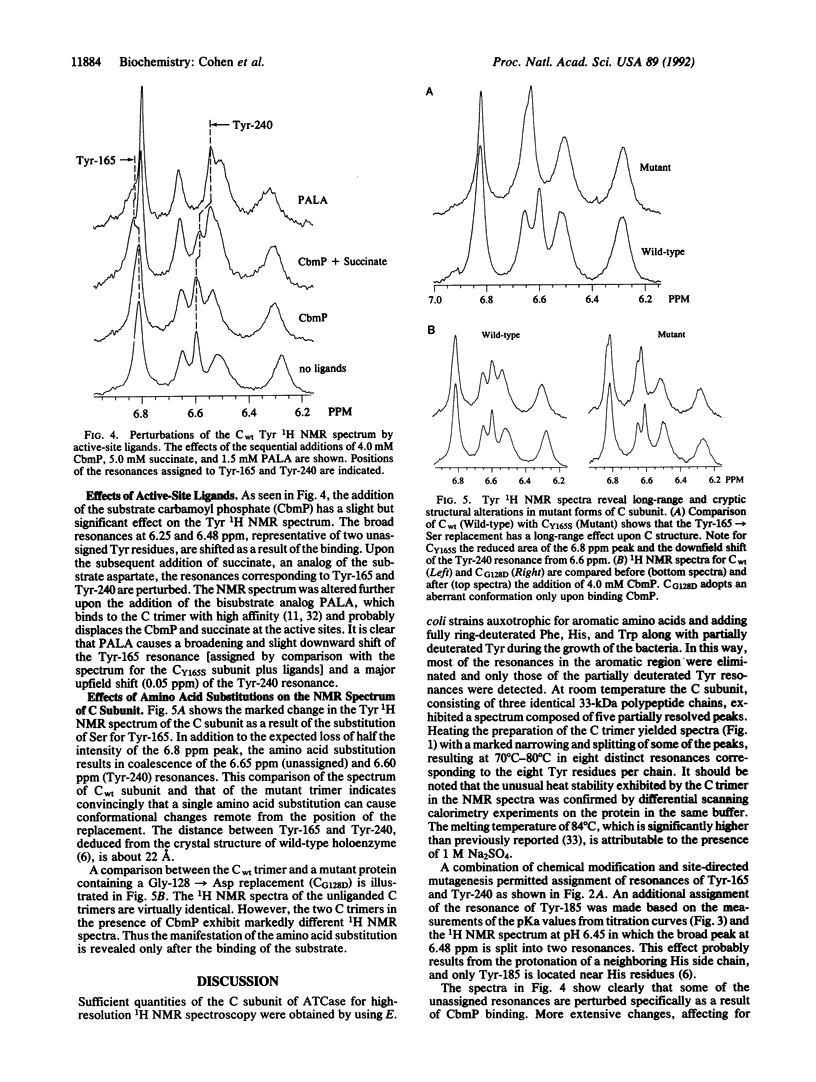
The 1H NMR spectrum of the catalytic subunit of Escherichia coli aspartate transcarbamoylase (EC 2.1.3.2) was simplified by using strains auxotrophic for the aromatic amino acids and a growth medium containing fully deuterated Trp, Phe, and His and partially deuterated Tyr. 1H resonances for Tyr in the catalytic trimer (M(r) = 10(5)) were partially resolved into five peaks at 27 degrees C, which above 50 degrees C were further resolved to give a distinct resonance for each of the eight Tyr residues in the polypeptide chain. Experiments on chemically modified catalytic subunits and on a mutant form in which Tyr-165 was converted to Ser-165 led to the assignment of resonances for Tyr-165, Tyr-240, and Tyr-185. Binding of the substrate, carbamoyl phosphate, caused shifts of two of the unassigned resonances, and the subsequent binding of the aspartate analog succinate perturbed the resonances corresponding to Tyr-165 and Tyr-240. The bisubstrate analog N-(phosphonacetyl)-L-aspartate produced a spectrum differing considerably from that caused by the combination of carbamoyl phosphate and succinate. The NMR spectrum for the Tyr-165-->Ser mutant trimer showed clearly that the single amino acid substitution caused conformational changes affecting the environment of residues remote from the position of the replacement. In contrast, the inactive mutant subunit in which Gly-128 was replaced by Asp exhibited a spectrum virtually identical to that of the wild-type protein. However, addition of the substrate carbamoyl phosphate caused a marked change in the spectrum of the mutant enzyme, whereas that of the wild-type trimer was altered only slightly, showing that the effect of the amino acid substitution was manifested in the NMR spectrum only with the liganded enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allewell N. M. Escherichia coli aspartate transcarbamoylase: structure, energetics, and catalytic and regulatory mechanisms. Annu Rev Biophys Biophys Chem. 1989;18:71–92. doi: 10.1146/annurev.bb.18.060189.000443. [DOI] [PubMed] [Google Scholar]
- Beard C. B., Schmidt P. G. Binding of succinate to aspartate trancarbamylase catalytic subunit. pH and temperature dependence of nuclear magnetic resonance relaxation times. Biochemistry. 1973 Jun 5;12(12):2255–2264. doi: 10.1021/bi00736a012. [DOI] [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-L-aspartate. J Biol Chem. 1971 Nov;246(21):6599–6605. [PubMed] [Google Scholar]
- Edge V., Allewell N. M., Sturtevant J. M. High-resolution differential scanning calorimetric analysis of the subunits of Escherichia coli aspartate transcarbamoylase. Biochemistry. 1985 Oct 8;24(21):5899–5906. doi: 10.1021/bi00342a032. [DOI] [PubMed] [Google Scholar]
- Foote A. M., Winkler F. K., Moody M. F. Crystallization and preliminary x-ray study of the catalytic subunit of aspartate transcarbamylase. J Mol Biol. 1981 Mar 5;146(3):389–391. doi: 10.1016/0022-2836(81)90395-8. [DOI] [PubMed] [Google Scholar]
- Griffiths D. V., Feeney J., Roberts G. C., Burgen A. S. Preparation of selectively deuterated aromatic amino acids for use in 1H NMR studies of proteins. Biochim Biophys Acta. 1976 Oct 28;446(2):479–485. doi: 10.1016/0005-2795(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Kantrowitz E. R., Lipscomb W. N. Escherichia coli aspartate transcarbamoylase: the molecular basis for a concerted allosteric transition. Trends Biochem Sci. 1990 Feb;15(2):53–59. doi: 10.1016/0968-0004(90)90176-c. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Honzatko R. B., Lipscomb W. N. Structure of unligated aspartate carbamoyltransferase of Escherichia coli at 2.6-A resolution. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4037–4040. doi: 10.1073/pnas.81.13.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H. M., Lipscomb W. N., Cho Y. J., Honzatko R. B. Complex of N-phosphonacetyl-L-aspartate with aspartate carbamoyltransferase. X-ray refinement, analysis of conformational changes and catalytic and allosteric mechanisms. J Mol Biol. 1988 Dec 5;204(3):725–747. doi: 10.1016/0022-2836(88)90365-8. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Pan Z. X., Honzatko R. B., Ke H. M., Lipscomb W. N. Structural asymmetry in the CTP-liganded form of aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1987 Aug 20;196(4):853–875. doi: 10.1016/0022-2836(87)90410-4. [DOI] [PubMed] [Google Scholar]
- Kinsey R. A., Kintanar A., Oldfield E. Dynamics of amino acid side chains in membrane proteins by high field solid state deuterium nuclear magnetic resonance spectroscopy. Phenylalanine, tyrosine, and tryptophan. J Biol Chem. 1981 Sep 10;256(17):9028–9036. [PubMed] [Google Scholar]
- Kirschner M. W., Schachman H. K. Conformational studies on the nitrated catalytic subunit of aspartate transcarbamylase. Biochemistry. 1973 Jul 31;12(16):2987–2997. doi: 10.1021/bi00740a007. [DOI] [PubMed] [Google Scholar]
- Kleanthous C., Wemmer D. E., Schachman H. K. The role of an active site histidine in the catalytic mechanism of aspartate transcarbamoylase. J Biol Chem. 1988 Sep 15;263(26):13062–13067. [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. 2.5 A structure of aspartate carbamoyltransferase complexed with the bisubstrate analog N-(phosphonacetyl)-L-aspartate. J Mol Biol. 1987 Feb 5;193(3):527–553. doi: 10.1016/0022-2836(87)90265-8. [DOI] [PubMed] [Google Scholar]
- Lauritzen A. M., Landfear S. M., Lipscomb W. N. Inactivation of the catalytic subunit of aspartate transcarbamylase by nitration with tetranitromethane. J Biol Chem. 1980 Jan 25;255(2):602–607. [PubMed] [Google Scholar]
- London R. E., Schmidt P. G. A nuclear magnetic resonance study of the interaction of inhibitory nucleosides with Escherichia coli aspartate transcarbamylase and its regulatory subunit. Biochemistry. 1974 Mar 12;13(6):1170–1179. doi: 10.1021/bi00703a018. [DOI] [PubMed] [Google Scholar]
- Milstien S., Kaufman S. Studies on the phenylalanine hydroxylase system in vivo. An in vivo assay based on the liberation of deuterium or tritium into the body water from ring-labeled L-phenylalanine. J Biol Chem. 1975 Jun 25;250(12):4782–4785. [PubMed] [Google Scholar]
- Moore A. C., Browne D. T. Binding of regulatory nucleotides to aspartate transcarbamylase: nuclear magnetic resonance studies of selectively enriched carbon-13 regulatory subunit. Biochemistry. 1980 Dec 9;19(25):5768–5773. doi: 10.1021/bi00566a016. [DOI] [PubMed] [Google Scholar]
- Navre M., Schachman H. K. Synthesis of aspartate transcarbamoylase in Escherichia coli: transcriptional regulation of the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1207–1211. doi: 10.1073/pnas.80.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell J. O., Markby D. W., Schachman H. K. Cooperative binding of the bisubstrate analog N-(phosphonacetyl)-L-aspartate to aspartate transcarbamoylase and the heterotropic effects of ATP and CTP. J Biol Chem. 1989 Feb 15;264(5):2476–2481. [PubMed] [Google Scholar]
- Roberts M. F., Opella S. J., Schaffer M. H., Phillips H. M., Stark G. R. Evidence from 13C NMR for protonation of carbamyl-P and N-(phosphonacetyl)-L-aspartate in the active site of aspartate transcarbamylase. J Biol Chem. 1976 Oct 10;251(19):5976–5985. [PubMed] [Google Scholar]
- Robey E. A., Schachman H. K. Site-specific mutagenesis of aspartate transcarbamoylase. Replacement of tyrosine 165 in the catalytic chain by serine reduces enzymatic activity. J Biol Chem. 1984 Sep 25;259(18):11180–11183. [PubMed] [Google Scholar]
- Schachman H. K. Can a simple model account for the allosteric transition of aspartate transcarbamoylase? J Biol Chem. 1988 Dec 15;263(35):18583–18586. [PubMed] [Google Scholar]
- Schmidt P. G., Stark G. R., Baldeschwieler J. D. Aspartate transcarbamylase. A nuclear magnetic resonance study of the binding of inhibitors and substrates to the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1860–1868. [PubMed] [Google Scholar]
- Snyder G. H., Rowan R., 3rd, Karplus S., Sykes B. D. Complete tyrosine assignments in the high field 1H nuclear magnetic resonance spectrum of the bovine pancreatic trypsin inhibitor. Biochemistry. 1975 Aug 26;14(17):3765–3777. doi: 10.1021/bi00688a008. [DOI] [PubMed] [Google Scholar]
- Stevens R. C., Gouaux J. E., Lipscomb W. N. Structural consequences of effector binding to the T state of aspartate carbamoyltransferase: crystal structures of the unligated and ATP- and CTP-complexed enzymes at 2.6-A resolution. Biochemistry. 1990 Aug 21;29(33):7691–7701. doi: 10.1021/bi00485a019. [DOI] [PubMed] [Google Scholar]
- Sykes B. D., Schmidt P. G., Stark G. R. Aspartate transcarbamylase. A study by transient nuclear magnetic resonance of the binding of succinate to the native enzyme and its catalytic subunit. J Biol Chem. 1970 Mar 10;245(5):1180–1189. [PubMed] [Google Scholar]
- Wacks D. B., Schachman H. K. 19F nuclear magnetic resonance studies of fluorotyrosine-labeled aspartate transcarbamoylase. Properties of the enzyme and its catalytic and regulatory subunits. J Biol Chem. 1985 Sep 25;260(21):11651–11658. [PubMed] [Google Scholar]
- Wall K. A., Schachman H. K. Primary structure and properties of an inactive mutant aspartate transcarbamoylase. J Biol Chem. 1979 Dec 10;254(23):11917–11926. [PubMed] [Google Scholar]



