Abstract
Aim:
To describe the biomarker profiles in elderly Panamanians diagnosed with Alzheimer's disease (AD), mild cognitive impairment (MCI) or no impairment using serum-based biomarkers.
Methods:
Twenty-four proteins were analyzed using an electrochemiluminescence-based multiplex biomarker assay platform. A biomarker profile was generated using random forest analyses.
Results:
Two proteins differed among groups: IL-18 and T-lymphocyte-secreted protein I-309. The AD profile was highly accurate and independent of age, gender, education and Apolipoprotein E ε4 status. AD and MCI profiles had substantial overlap among the top markers, suggesting common functions in AD and MCI but differences in their relative importance.
Conclusion:
Our results underscore the potential influence of genetic and environmental differences within Hispanic populations on the proteomic profile of AD.
KEYWORDS : aging, blood-based biomarkers, cognition, dementia, Hispanic, Latin America
Summary points.
The search for blood biomarkers that correlate with pathological changes in Alzheimer's disease has yielded evidence that suggests it is a viable approach to early diagnosis.
The present and recent reports indicate a significant impact of race and ethnicity on biomarkers of disease status, thus underscoring the importance of this line of research.
Overlap among the top markers of Alzheimer's disease and mild cognitive impairment suggest common functions across disease stages but differences in their relative importance.
Blood-based biomarkers are promising cost- and time-effective strategies for primary care clinical settings particularly in low-resource settings.
Aim
Alzheimer's disease (AD) is the most common cause of dementia in the elderly. Currently, a large proportion of people with dementia live in low- and middle-income countries (LMIC) where population aging is increasing at unprecedented rates. The number of people at risk for dementia in LMIC will increase rapidly over the next decades for various reasons. For one, age is the greatest risk factor for dementia, and older adults aged 60+ years constitute the fastest growing group in LMIC [1]. Secondly, improvements in healthcare services in LMIC have decreased infant mortality rates and increased the number of years that the elderly survive with dementia [2]. Lastly, a high prevalence of modifiable risk factors for dementia associated with low educational and socioeconomic levels increases the number of people at risk for dementia [3]. Of the LMIC, those in the Latin America and Caribbean (LAC) region are experiencing some of the fastest rates of population aging [4–6]. Recently published population-based studies confirm that rates of AD and mild cognitive impairment (MCI) are similar to those of developed countries [7–9]. These studies indicate that dementia rates in LAC countries are exacerbated by high rates of modifiable risk factors such as diabetes, obesity, cardiovascular risk factors and illiteracy. Consequently, the burden of AD and MCI is expected to be especially high in LAC countries in the coming decades.
The development of cost-effective approaches for detecting dementia early in the course of the disease is an essential step toward reducing the burden of disease. A wealth of evidence indicates that the underlying neuropathological signs of AD, namely extracellular accumulation of amyloid-β (Aβ) in senile plaques, are likely to begin decades before the clinical symptoms appear [10]. Thus, potential disease-modifying treatments will likely be most effective during these preclinical disease stages [11,12]. Presently, a principal barrier to effective diagnosis and treatment of AD is the lack of readily available biomarkers. Evidence shows that brain imaging and cerebrospinal fluid biomarkers are highly accurate in detecting disease presence; however, these methods are not cost- and time-effective strategies for primary care clinical settings particularly in low-resource settings. In this regard, recent research into blood-based biomarkers of AD has produced encouraging results that suggest that blood-based screening is a viable approach to early diagnosis [13–20]. Together, the results of these studies provide strong evidence that a blood-based screening approach can be useful in discriminating AD from healthy controls as well as from other dementias [21].
While a great deal of recent literature has focused on the generation of blood-based tools for the detection of AD, little attention has been paid to the impact of ethnicity on these blood-based biomarker profiles of AD. However, recent work from our group and others clearly demonstrates the need for investigation of this question. A meta-analysis of genome-wide association studies confirmed that expression of the ApoE ε4 allele, the strongest genetic risk factor for sporadic AD, was associated with AD in whites and nonwhite ethnic groups, whereas various other genotypes were associated with AD only in whites [22]. Likewise, in the only study explicitly examining a blood-based biomarker profile of AD conducted specifically among a Latino population to date, our group found that the blood-based biomarker profile approach is highly accurate in detecting AD among US Mexican Americans and the profile was significantly different than that observed among non-Hispanic whites [23]. Additional work is needed in this area.
In the present study, we conducted the first examination of serum-based biomarker profiles of AD and MCI among Panamanians. Our aim was to examine the link between blood-based biomarkers and dementia in Hispanics by examining cases of AD and MCI among the elderly population in Panama, an upper middle-income country in the LAC region that is advancing toward an aged society. We describe the demographic, cognitive and biomarker profiles of Panamanians with AD and MCI. We examined 24 proteins from our previously established algorithm in serum across diagnostic groups and generated a biomarker profile of disease status. The battery of proteins from our previously generated and cross-validated AD algorithm [17,18] consists of various proteins responsible for a wide range of physiological characteristics including chemotactic activity, hematopoiesis, growth factors, tissue markers (receptor and stimulation of B cells and T cells), including proteins that promote the expression and secretion of chemokines, that function as hormones, proteins of acute phase mechanism, proteins involved in inflammatory responses and disease markers and others involved in the development of neurons and axons. Based on previous reports, we hypothesized that our previously generated blood-based profile would yield an algorithm highly accurate in detecting AD. We also sought to conduct preliminary analyses to generate blood-based profiles of MCI in this cohort.
Methods
• Participants
Data from this study came from the PARI study, the first-ever study of Panamanian aging. PARI participants were recruited from the outpatient geriatric services of the largest public hospital of the Social Security (CSS) located in Panama, the capital city of Panama. Inclusion criteria included being 65 years or older, willingness to participate in the baseline interview and three follow-up visits over the course of 12–18 months and provision of informed consent. Exclusion criteria included any medical condition that required hospitalization and participation in an ongoing clinical study at the time of enrollment. The study protocol was approved by the National Bioethics Committee of the Instituto Conmemorativo Gorgas de Estudios de la Salud and the Institutional Bioethics Committee of the CSS. Each participant (or informant/caregiver) signed informed consent forms and patient confidentiality was not breached in accordance with the Declaration of Helsinki (1964).
Data for this study were obtained from 135 participants in the PARI sample diagnosed with Alzheimer's (AD; n = 28), MCI (n = 30) or no impairment (controls; n = 77) that were not institutionalized at the time of enrollment. Each participant underwent an interview, physical exam, clinical interview and nonfasting blood draw. The 30-item Spanish version of the Mini-Mental State Examination (MMSE) was used as a measure of global cognition [24]. The reverse spelling of the word ‘world’ in the attention item was used instead of the backward serial sevens. MMSE scores were adjusted for age and level of education [25]. Executive function was assessed with the clock drawing test using the 10-point Sunderland scale [26]. Depressive symptoms were assessed with the 30-item Geriatric Depression Scale (GDS-30) [27]. Informants and participants provided information regarding difficulties with basic and instrumental activities of daily living. Disease stage was rated according to the Global Deterioration Scale [28]. Diagnosis of probable AD was based on NINCDS-ADRDA criteria [29]. MCI diagnosis was based on core clinical criteria described in Alberts et al. [30]. Inclusion in the normal control group required a corrected MMSE score ≥24, a score of ≤2 in the Global Deterioration Scale and 10 points or less in the GDS-30. Demographic characteristics of the sample are summarized in Table 1.
Table 1. . Demographic characteristics and group comparisons.
| Variable | AD (n = 28) | MCI (n = 30) | Controls (n = 77) | p-value† |
|---|---|---|---|---|
| Gender (% female) |
78.6 |
66.7 |
64.9 |
0.58 |
| Age, years (SD) |
81.9 (9.2)‡ |
81.2 (7.8)‡ |
76.5 (6.7) |
0.001 |
| Education, years (SD) |
6.8 (3.4)‡ |
6.8 (3.0)‡ |
9.4 (4.2) |
0.001 |
| MMSE (SD) |
16.2 (4.0)‡ |
25.1 (2.7)‡ |
28.3 (1.8) |
<0.001 |
| Clock drawing (SD) |
3.0 (2.8)‡ |
5.9 (3.1)‡ |
8.5 (1.7) |
<0.001 |
| Global deterioration scale (SD) |
5.3 (0.8)‡ |
2.7 (1.0)‡ |
1.3 (0.5) |
<0.001 |
| GDS-30 (SD) |
5.3 (4.0) |
9.8 (5.6)‡ |
4.6 (2.9) |
<0.001 |
| Hypertension (% yes) |
75.0 |
80.0 |
83.1 |
0.55 |
| Elevated cholesterol (% yes) |
35.7 |
50.0 |
37.7 |
0.47 |
| Diabetes (% yes) |
25.0 |
43.3 |
27.3 |
0.18 |
| Obesity (% yes) |
18.2 |
8.7 |
29.0 |
0.07 |
| ApoEε4 carrier (% yes) | 57.1‡ | 50.0‡ | 20.8 | 0.001 |
†p-value for trend over the three diagnostic categories was calculated using ANOVA for continuous variables and Pearson chi-square for categorical variables. ANOVA post-hoc comparisons were conducted with Bonferroni tests. p < 0.05 was considered statistically significant.
‡Statistically different from control group.
AD: Alzheimer's disease; GDS-30: Geriatric depression scale (30-item); MCI: Mild cognitive impairment; MMSE: Mini Mental State Examination; SD: Standard deviation.
• Assays
Samples were collected with 10 ml serum-separating vacutainer tubes and allowed to clot at room temperature before being centrifuged at 1300 × g for 10 min, aliquoted (1 ml) into polypropylene cryovial tubes and stored at -80°C. All samples were assayed in duplicate via a multiplex biomarker assay platform using electrochemiluminescence on the SECTOR Imager 2400A from Meso Scale Discovery (MSD) [31] per our previously published methods [18]. The MSD platform has been used extensively to assay biomarkers associated with a range of human diseases including AD and have well-established properties of being more sensitive and requiring less sample volume than conventional ELISAs. The markers assayed were from our previously generated and cross-validated AD algorithm [17,18] and included: FABP, B2M, PPY, CA-125, CRP, sVCAM, THPO, A2M, TNF-α, TN-C, IL-5, IL-6, IL-7, IL-10, IL-18, I309, FVII, TARC, SAA, sICAM, eotaxin 3, adinoponectin, IL-1β and MIP-1α. To be included in the study, analyte concentrations should exceed the limit of detection in >75% of all the samples for each respective analyte. These criteria were fulfilled by all 24 analytes in the present study. In cases where there were values below the limit of detection, we used imputation to assign a value that was 1% below the lowest detectable value for that analyte. Lastly, for ApoE genotyping, DNA samples from leukocytes were extracted from EDTA plasma collection tubes using QIAmp DNA Mini Kit (Qiagen) according to manufacturer recommendations. DNA was extracted from whole blood and ApoE genotyping was conducted according to standardized PCR procedures.
In our laboratory, the assay results were stable for up to three freeze–thaw cycles. Our standard protocol is to utilize only samples with two or fewer freeze–thaw cycles. The long-term stability of MSD assays is excellent though we have not assayed samples stored for longer than 10 years. Short-term stability studies have not yet been conducted. Multiple standards are utilized for each marker and standard curves created for all markers and kits. The markers were assayed via single or multiplex kits from MSD as follows: human vascular injury kit – CRP (LOD = 0.01 ng/ml), SAA (LOD = 0.02 ng/ml, sICAM (LOD = 0.03 ng/ml), sVCAM1 (LOD = 0.01 ng/ml) multiplex, 1:200 dilution; Adiponectin kit – sample dilution 1:1000, LOD = 0.002 ng/ml; CA-125 kit – dilution = neat, LOD = 0.324 pg/ml; Alzheimer's 4-Plex custom multiplex kit – dilution = 1:6000, A2M (LOD = 1116703400 pg/ml), B2M (LOD = 2400 pg/ml), FVII (LOD = 364950 pg/ml), TN-C (LOD = 11470 pg/ml); NeuroD custom multiplex – dilution = 1:2, FABP (LOD = 15000 pg/ml), I309 (LOD = 1.15 pg/ml), IL-18 (LOD = 23.01 pg/ml), PPY (LOD = 240.0 pg/ml), THPO (LOD = 244.0 pg/ml), Custom9 plex – dilution = neat, eotaxin3 (LOD = 0.04 pg/ml), IL-1β (LOD = 0.00001 ng/ml), IL-5 (LOD = 0.05 ng/ml), IL-6 (0.25 ng/ml), IL-7 (LOD = 2.30 ng/ml), IL-10 (LOD = 0.25 ng/ml), MIP-1α (LOD = 0.00001 ng/ml), TARC (22.5 ng/ml), TNF-α (LOD = 0.20 ng/ml). All kits/plates procedures are standardized across runs, laboratory personnel and orders.
• Statistical analysis
Chi square and one-way analysis of variance (ANOVA) tests were used to compare groups for categorical variables and continuous variables, respectively. Multivariate analysis of covariance (MANCOVA) with age, years of education and expression of ApoEε4 as covariates was used to examine differences in individual protein markers across groups. Significant ANOVA and MANCOVA tests were followed by Bonferroni post-hoc tests and p-values less than 0.05 were considered statistically significant. The blood-based profile was generated using random forest (RF) analyses as was done in our prior work. The biomarker data were transformed using the Box-Cox transformation. RF prediction model was performed using R package randomForest (V 4.5) with all software default settings. The receiver operation characteristic curves were analyzed using R package and area under the curve (AUC) was calculated using R package DiagnosisMed (V 0.2.2.2). Age, gender, education and APOEε4 genotype were entered into the RF model per our prior methods.
Results
The demographic characteristics of the cohort and results of statistical analyses can be found in Table 1. Normal control participants were significantly younger than AD (p = 0.004) and MCI groups (p = 0.013), which did not differ from one another. The control group achieved significantly more education than the other diagnostic groups (p < 0.01), which were not different from one another. The AD group scored significantly worse on the MMSE and clock drawing test than the control and MCI groups (p < 0.001), and the MCI group scored significantly worse than the control group (p < 0.001). The MCI group endorsed significantly greater numbers of depressive symptoms on the GDS-30 than AD and control groups (p < 0.001), which did not differ from one another. There was no difference among groups in the frequency of hypertension, elevated cholesterol, diabetes or obesity, although there was a trend toward a greater frequency of obesity among controls (p = 0.07). Lastly, the expression of at least one copy of the ApoEε4 allele differed among groups (X 2 = 17.7; p = 0.001), with at least half of AD (57%) and MCI (50%) groups expressing one or two copies.
The 24 proteins examined and their links to aging- and brain-related conditions are summarized in Table 2 and protein concentrations across diagnostic groups are presented in Table 3. When examining individual protein marker levels, MANCOVA revealed that two proteins were significantly different across groups: IL-18 and I309 (see Figure 1). The AD group had significantly lower levels of IL-18 than controls (p = 0.009), whereas AD (p < 0.001) and MCI (p = 0.014) groups had significantly higher levels of I309 than controls.
Table 2. . Biomarkers and their association with aging- and brain-related conditions.
| Serum biomarkers | Link to aging- and brain-related conditions | Ref. |
|---|---|---|
| FABP |
Neuropathological and genetic link to PD, DLB, CJD and AD in serum and CSF |
[32] |
| B2M |
Chronic kidney disease-related dysfunction and presence of low-grade inflammation in older adults; upregulated in AD and MCI |
[33,34] |
| PPY |
Increased levels in plasma and CSF in subjects with AD and MCI |
[35] |
| CA-125 |
Mainly upregulated in ovarian cancer, in older women it is associated with worse functional status; to date no association has been found with AD |
[36] |
| CRP |
Associated with neurofibrillary tangles and senile plaques in AD brain tissue |
[37] |
| sVCAM1 |
Member of the immunoglobulin superfamily, elevated levels have been found in plasma of AD subjects |
[38] |
| THPO |
Induces the proliferation and maturation of megakaryocytes; plasma levels show difference in subjects with dementia compared with controls |
[39] |
| A2M |
Mediates Aβ toxicity, clearance and degradation; upregulated in AD subjects |
[40,41] |
| Eotaxin3 |
Together with ApoE it discriminates between control and AD; increased levels have been found in plasma and CSF in AD subjects |
[35,42] |
| TNF-α |
Involved in neuronal apoptosis and formation of intracellular neurofibrillary tangles and amyloid plaques; higher blood concentrations were detected in AD subjects |
[43,44] |
| TN-C |
Involved in the development of neurons and axons, neuronal regeneration, microglial activation and inflammatory responses; increased levels have been found in plasma and CSF in AD subjects |
[34–35,45] |
| IL-5 |
Specific hematopoietic growth factor that together with other proteins forms a biomarker profile that can distinguish AD from controls |
[18] |
| IL-6 |
Involved in neuronal apoptosis and formation of intracellular neurofibrillary tangles and amyloid plaques; elevated levels have been found in blood but not in CSF in AD subjects |
[43,44] |
| IL-7 |
Induces the synthesis of inflammatory mediators such as IL-1 and IL-6; in plasma there is no difference between AD subjects compared with controls, but in CSF there is a downregulation in MCI subjects |
[46] |
| IL-10 |
Anti-inflammatory properties and may play a role in schizophrenia pathogenesis; concentrations did not differ between AD subjects and controls |
[44,47] |
| IL-18 |
Probable mediator of cerebral pathogenic processes, microglia activator, involved in neuroendocrine and neuroimmune functions; higher blood concentrations were detected in AD subjects |
[43–44,48] |
| I309 |
Increased levels in tauopathy in mouse model; this small glycoprotein was found elevated in CSF and plasma in AD subjects |
[38,49–50] |
| FVII |
Involved in the coagulation cascade and required for thrombin generation, which has also been linked to AD |
[38] |
| TARC |
Involved in the inflammatory response and the induction of cell migration; together with other proteins forms a biomarker profile that can distinguish AD versus controls |
[18] |
| SAA |
High levels in plasma has been found in cardiovascular disease but the evidence for an association with cognitive decline is not clear |
[51] |
| sICAM1 |
Has been found in amyloid plaques and others brain structures with low levels of amyloid beta deposits; peripheral role in AD |
[52] |
| Adiponectin |
Modulates certain metabolic processes; may be related to changes in prodromal and early stage AD |
[38] |
| IL-1β |
Proinflammatory cytokine overexpressed in AD brains near to amyloid plaques; higher blood concentrations were detected in AD subjects |
[44] |
| MIP-1α | Possesses inflammatory, pyrogenic and chemokinetic properties; probable involvement in microglial activation | [53] |
AD: Alzheimer's disease; CJD: Creutzfeldt-Jakob disease; CSF: Cerebrospinal fluid; DLB: Dementia with Lewy bodies; PD: Parkinson's disease.
Table 3. . Blood biomarker levels across diagnostic groups.
| Serum biomarkers mean (SD) | AD (n = 28) | MCI (n = 30) | Controls (n = 77) | p-value† |
|---|---|---|---|---|
| FABP (ng/ml) |
69.4 (32.4) |
102.2 (87.0) |
73.9 (39.0) |
0.04 |
| B2M (μg/ml) |
3.1 (1.0) |
3.2 (2.6) |
2.5 (1.2) |
0.34 |
| PPY (pg/ml) |
979.7 (300.5) |
896.1 (264.5) |
876.1 (263.2) |
0.42 |
| CA-125 (U) |
25.0 (16.3) |
23.8 (16.7) |
25.0 (19.8) |
0.90 |
| CRP (ng/ml) |
4.1 (10.2) |
4.5 (8.2) |
4.1 (5.8) |
0.75 |
| sVCAM1 (pg/ml) |
484.8 (225.0) |
487.6 (138.4) |
464.1 (159.8) |
0.91 |
| THPO (pg/ml) |
780.5 (626.6) |
862.5 (275.8) |
844.9 (285.7) |
0.71 |
| A2M (mg/ml) |
2.7 (0.7) |
2.2 (0.5) |
2.3 (0.8) |
0.03 |
| Eotaxin3 (pg/ml) |
1.9 (6.1) |
0.8 (2.0) |
1.7 (10.6) |
0.86 |
| TNF-α (pg/ml) |
2.1 (1.8) |
2.4 (1.0) |
2.0 (0.9) |
0.47 |
| TN-C (ng/ml) |
45.7 (14.8) |
41.3 (9.9) |
37.8 (11.7) |
0.15 |
| IL-5 (pg/ml) |
1.2 (1.8) |
1.1 (1.3) |
1.6 (2.5) |
0.39 |
| IL-6 (pg/ml) |
3.3 (4.0) |
9.3 (31.2) |
4.4 (8.5) |
0.32 |
| IL-7 (pg/ml) |
10.0 (5.7) |
9.9 (4.2) |
12.2 (6.0) |
0.49 |
| IL-10 (pg/ml) |
1.6 (1.7) |
24.6 (125.7) |
2.8 (6.0) |
0.15 |
| IL-18 (pg/ml) |
168.5 (91.2)† |
200.1 (74.0) |
234.1 (141.6) |
0.03 |
| I309 (pg/ml) |
9.3 (6.1)† |
6.4 (6.6)† |
3.4 (2.4) |
<0.001 |
| FVII (μg/ml) |
0.9 (0.2) |
0.8 (0.2) |
0.9 (0.3) |
0.12 |
| TARC (pg/ml) |
199.8 (125.5) |
248.2 (172.5) |
254.4 (286.1) |
0.41 |
| SAA (ng/ml) |
18.1 (49.5) |
16.4 (34.6) |
12.8 (21.4) |
0.50 |
| sICAM1 (pg/ml) |
301.2 (81.5) |
311.5 (61.2) |
317.4 (84.6) |
0.33 |
| Adiponectin (μg/ml) |
22.2 (15.0) |
25.2 (17.8) |
19.5 (14.8) |
0.39 |
| IL-1β (pg/ml) |
0.2 (0.3) |
0.1 (0.3) |
0.2 (0.5) |
0.25 |
| MIP-1α (pg/ml) | 160.1 (156.0) | 148.0 (118.0) | 448.2 (1853.0) | 0.79 |
†p-value for trend over the three diagnostic categories was calculated using MANCOVA with age, years of education and expression of ApoEε4 as covariates. Post-hoc comparisons were conducted with Bonferroni tests. p< 0.05 was considered statistically significant.
‡Statistically different from control group.
AD: Alzheimer's disease; MCI: Mild cognitive impairment; SD: Standard deviation.
Figure 1. . Two serum proteins were significantly different among groups: IL-18 and I309.
(A) The AD group had significantly lower levels of IL-18 than controls (p = 0.009). (B) AD (p < 0.001) and MCI (p = 0.014) groups had significantly higher levels of I309 than controls.
*p < 0.05; **p < 0.01; ***p < 0.001.
AD: Alzheimer's disease; MCI: Mild cognitive impairment.
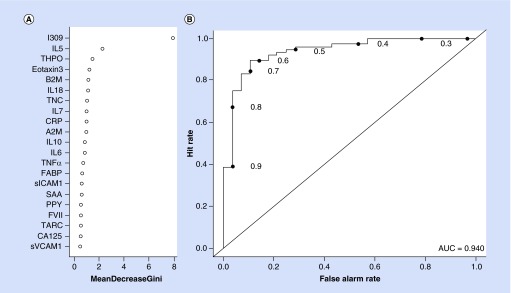
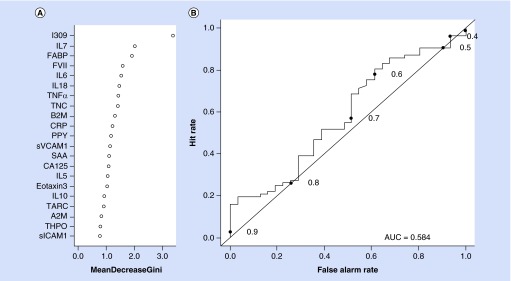
The RF analysis revealed that within the AD group, the biomarker profile was highly accurate in diagnosis with an AUC of 0.94, sensitivity (SN) of 0.86 and specificity (SP) of 0.90. The relative importance of the proteins in the profile can be found in Figure 2A (Gini Plot for AD protein profile) and the ROC curve is found in Figure 2B. The diagnostic accuracy statistics did not change with the addition of age, gender, education and ApoE ε4 status. For the MCI group, the accuracy was poor with an AUC = 0.58 (SN = 0.30, SP = 0.71) (see Figure 3). Addition of the demographic characteristics only minimally changed the diagnostic accuracy statistics (AUC = 0.65; SN = 0.44; SP = 0.73).
Figure 2. . Gini plot and diagnostic accuracy for Alzheimer's disease serum biomarker profile.
(A) Gini plot from random forest biomarker model demonstrating variable importance and differential expression of the top 21 markers of Alzheimer's disease among Panamanians. (B) Receiver operation characteristic plot for the Alzheimer's disease serum biomarker profile using 21 serum analytes. The AUC was 0.94, sensitivity was 0.86 and specificity was 0.90.
AUC: Area under the curve.
Figure 3. . Gini plot and diagnostic accuracy for mild cognitive impairment serum biomarker profile.
(A) Gini plot from random forest biomarker model demonstrating variable importance and differential expression of the top 21 markers of mild cognitive impairment among Panamanians. (B) Receiver operation characteristic plot for the mild cognitive impairment serum biomarker profile using 21 serum analytes. The AUC was 0.58, sensitivity was 0.30 and specificity was 0.71.
AUC: Area under the curve; ROC: Receiver operation characteristic.
Discussion
The present results indicate that AD individuals differ from cognitively healthy controls in the expression of at least two blood-based proteins: IL-18 and I309. In agreement with these results, in a previous study we reported that the cytokine IL-18, one of the key mediators of inflammation and immune response, was decreased in AD subjects relative to controls in a mostly non-Hispanic white cohort [17]. Neuroinflammation has been shown to play a role in AD pathogenesis [54], and we expected proinflammatory molecules to be elevated in AD subjects. The finding that IL-18 is decreased in AD in our cohort is in contrast to a recent meta-analysis of cytokines in AD that revealed that higher concentrations of peripheral IL-18 were associated with significantly increased odds of AD [44]. However, an earlier study that made a distinction between stages of AD progression found that IL-18 levels in AD patients varied depending on whether the disease was in mild, moderate or advanced stages [48]. Lastly, in our cohort I309 levels were significantly elevated in both AD and MCI subjects relative to controls. I309 levels in cerebrospinal fluid have been shown to correlate with the severity of cognitive impairment [49], and also in serum/plasma I309 levels were significantly associated with disease status in AD [38].
When combined into an algorithm RF analyses revealed that 21 proteins reliably distinguish AD subjects from healthy controls. The biomarker profile for AD subjects was highly accurate (AUC = 0.94) and did not change with the addition of age, gender, education and ApoE ε4 status. This work cross-validates our prior work across an independent Latino population. Notably, in the Panamanian cohort six of the top 10 markers associated with AD are also among the top 10 MCI markers (I309, B2M, IL-18, TN-C, IL-7 and CRP). In the current study, the number one marker in the risk score for AD and MCI groups was I309. I309 is associated with the inflammatory response, suggesting AD and MCI associations with immune system dysfunction [55]. Together, these findings are supportive of overlap in the functions of top markers for AD and MCI but also point to differences in the relative importance of these biomarkers.
Several of the markers in the Panamanian AD profile also overlap with those identified using our biomarker assay platform among US Mexican Americans and US non-Hispanic whites. In the current study, 5 of the top 10 AD markers (I309, IL-5, TN-C, IL-7 and CRP) overlapped with the top 10 markers from our recently cross-validated 21 serum-based biomarker panel [18]. An additional 4 biomarkers of the top 10 AD markers (THPO, eotaxin 3, TN-C, and IL-7) overlapped with the top 10 markers among 30 markers associated with AD among non-Hispanic whites [16]. In contrast, Panamanians and US Mexican Americans have one marker (B2M) in common among the top 10 markers of AD. Taken together, when considering ApoEε4 genotype, medical comorbidities and blood-based biomarker profiles, it appears that the profile of AD among Panamanians falls somewhere between that of US Mexican Americans and US non-Hispanic whites.
Recent literature suggests that a blood-based biomarker profile can potentially serve as the first step in the multistage diagnostic process [56,57]. Moreover, our prior work clearly demonstrates the need to take ethnicity and related comorbidities into account in this line of work. The current study is only the second study to ever specifically examine a blood-based biomarker profile for detecting AD among a Latino population and is the first such study in elderly Panamanians. As with our prior work, the biomarker profile was significantly different among this Latino population as compared with our recently published work among non-Hispanic whites [18]. However, the biomarker profile of AD among a Panamanian cohort was also different from that which we observed in our Mexican American cohort. This finding is not entirely surprising as the frequency of diabetes and obesity is lower among the Panamanian cohort as compared with Mexican Americans. Additionally, in our previous work ApoEε4 expression was lower in Mexican Americans with AD (38%) and MCI (26%) [58] than in Panamanians (57 and 50%, respectively), although expression among control subjects is comparable (19–21%). Expression of the ApoEε4 allele has been shown to vary by ethnic group [59], which may contribute to differences in etiology of dementia among different ethnic groups. In Hispanic populations, the variation among studies is likely due to the fact that Hispanics are a mixture of Amerindian, African and European descent with relative compositions varying by country, a notion that is supported by a recent report of variations in neurocognitive performance across different US Hispanic/Latino groups [60]. In light of this, the genetic admixture of Mexican Americans and Panamanians is likely very different and this is a line of investigation the current team is beginning to examine (i.e., the influence of genetic admixture on the blood-based proteomic profile of AD).
This study also sought to take an initial step toward applying our previous methods to the identification of a blood-based profile of MCI. The overall profile was significant; however, the accuracy was lower than anticipated. For the MCI analyses, it is likely that the heterogeneity of the diagnostic classification is partially to blame. MCI, the symptomatic predementia phase of AD, may comprise impairment in more than one cognitive domain, which is challenging from a diagnostic perspective [61]. In fact, in an independent study we have begun to examine blood-based profiles of amnestic versus non-amnestic MCI. Additionally, in our prior work one can incorporate select cognitive screens (single instruments) to the blood-based profiles to refine the algorithms, which we have termed molecular neuropsychology [62]. It is likely that this approach will significantly improve upon the MCI algorithm.
An important limitation of the present study is the small sample size, so results should be considered preliminary, particularly in the case of blood-based biomarkers of MCI where the use of brief cognitive tests limited our ability to examine specific cognitive deficits in this group and their association with biomarker profiles. Also, our results are cross-sectional and longitudinal studies are necessary to study the relationship between biomarker profiles and progression of cognitive decline across groups. Each of these limitations is being addressed in ongoing studies.
Conclusion
The current study is the first report of a proteomic profile of AD among a Panamanian cohort and only the second such study specifically among Latino populations. Given the rapidly growing segment of the Latino population worldwide and the extant literature documenting a significant impact of race and ethnicity on biomarkers of disease status, this line of research requires additional investigation. The search for blood biomarkers that correlate with pathological changes in AD has yielded evidence that suggests it is a viable approach to early diagnosis. AD and MCI biomarker profiles had six markers in common among the top ten markers: I309, B2M, IL-18, TN-C, IL-7 and CRP, suggesting common functions of top markers in AD and MCI but differences in the relative importance of the markers. The current study supports the hypothesis that a blood-based profile of AD exists among Panamanian elders and sets the stage for additional studies examining MCI.
Footnotes
Financial and competing interests disclosure
Research reported in this publication was supported by the National Institute on Aging under Award Numbers AG039389 and AG12300. AE Villarreal and GB Britton are supported by grants from the National Secretariat of Science Technology and Innovation (SENACYT) of Panama and the Melo Brain Research Project. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Disclaimer
The NIH had no role in the design and conduct of the study: collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.New York: United Nations; 2001. World population aging: 1950–2050.www.un.org/esa/population/publications/worldageing19502050/ [Google Scholar]
- 2.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinsella K, Velkoff V. Washington DC, USA: US Government Printing Office; 2001. An Aging World: 2001. Bureau USC.www.census.gov/prod/2001pubs/p95–01–1.pdf [Google Scholar]
- 5.Brea J. Population Reference Bureau; 2003. Population dynamics in Latin America.www.igwg.org/Source/58.1PopulDynamicsLatinAmer.pdf Population Bulletin 58. [Google Scholar]
- 6.Palloni A, Pinto-Aguirre G, Pelaez M. Demographic and health conditions of ageing in Latin America and the Caribbean. Int. J. Epidemiol. 2002;31(4):762–771. doi: 10.1093/ije/31.4.762. [DOI] [PubMed] [Google Scholar]
- 7.Nitrini R, Bottino CM, Albala C, et al. Prevalence of dementia in Latin America: a collaborative study of population-based cohorts. Int. Psychogeriatr. 2009;21(4):622–630. doi: 10.1017/S1041610209009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince M, Acosta D, Ferri CP, et al. Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. Lancet. 2012;380(9836):50–58. doi: 10.1016/S0140-6736(12)60399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosa AL, Albanese E, Stephan BC, et al. Prevalence, distribution, and impact of mild cognitive impairment in Latin America, China, and India: a 10/66 population-based study. PLoS Med. 2012;9(2):e1001170. doi: 10.1371/journal.pmed.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blennow K, De Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey HJ, Mattila KM, Korolainen MA, Pirttila T. Problems associated with biological markers of Alzheimer's disease. Neurochem. Res. 2005;30(12):1501–1510. doi: 10.1007/s11064-005-8827-7. [DOI] [PubMed] [Google Scholar]
- 13.Hye A, Lynham S, Thambisetty M, et al. Proteome-based plasma biomarkers for Alzheimer's disease. Brain. 2006;129(Pt 11):3042–3050. doi: 10.1093/brain/awl279. [DOI] [PubMed] [Google Scholar]; •• First large-scale plasma biomarker study.
- 14.Doecke JD, Laws SM, Faux NG, et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch. Neurol. 2012;69(10):1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laske C, Leyhe T, Stransky E, Hoffmann N, Fallgatter AJ, Dietzsch J. Identification of a blood-based biomarker panel for classification of Alzheimer's disease. Int. J. Neuropsychopharmacol. 2011;14(9):1147–1155. doi: 10.1017/S1461145711000459. [DOI] [PubMed] [Google Scholar]
- 16.O'Bryant SE, Xiao G, Barber R, et al. A blood-based algorithm for the detection of Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2011;32(1):55–62. doi: 10.1159/000330750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Bryant SE, Xiao G, Barber R, et al. A serum protein-based algorithm for the detection of Alzheimer disease. Arch. Neurol. 2010;67(9):1077–1081. doi: 10.1001/archneurol.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Bryant SE, Xiao G, Zhang F, et al. Validation of a serum screen for Alzheimer's disease across assay platforms, species, and tissues. J. Alzheimers Dis. 2014;42(4):1325–1335. doi: 10.3233/JAD-141041. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Study that validated our approach across biomarker assay platforms, species (humans, mouse model) and tissue (blood and brain microvessels).
- 19.O'Bryant SE, Gupta V, Henriksen K, et al. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer's disease research. Alzheimers Dement. 2015;11(5):549–560. doi: 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laske C, Schmohl M, Leyhe T, et al. Immune profiling in blood identifies sTNF-R1 performing comparably well as biomarker panels for classification of Alzheimer's disease patients. J. Alzheimers Dis. 2013;34(2):367–375. doi: 10.3233/JAD-121558. [DOI] [PubMed] [Google Scholar]
- 21.Thambisetty M, Lovestone S. Blood-based biomarkers of Alzheimer's disease: challenging but feasible. Biomark. Med. 2010;4(1):65–79. doi: 10.2217/bmm.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review of approaches, challenges and promising findings related to the discovery of blood-based biomarkers for diagnosis and disease progression.
- 22.Jun G, Naj AC, Beecham GW, et al. Meta-analysis confirms CR1, CLU, and PICALM as alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch. Neurol. 2010;67(12):1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Bryant SE, Xiao G, Edwards M, et al. Biomarkers of Alzheimer's disease among Mexican Americans. J. Alzheimers Dis. 2013;34(4):841–849. doi: 10.3233/JAD-122074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, Mchugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Blesa R, Pujol M, Aguilar M, et al. Clinical validity of the ‘mini-mental state’ for Spanish speaking communities. Neuropsychologia. 2001;39(11):1150–1157. doi: 10.1016/s0028-3932(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 26.Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer's disease. A novel measure of dementia severity. J. Am. Geriatr. Soc. 1989;37(8):725–729. doi: 10.1111/j.1532-5415.1989.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 27.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 28.Reisberg B, Ferris SH, De Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry. 1982;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 29.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 30.Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.www.mesoscale.com Mesoscale.
- 32.Mollenhauer B, Steinacker P, Bahn E, et al. Serum heart-type fatty acid-binding protein and cerebrospinal fluid tau: marker candidates for dementia with Lewy bodies. Neurodegener. Dis. 2007;4(5):366–375. doi: 10.1159/000105157. [DOI] [PubMed] [Google Scholar]
- 33.Shinkai S, Chaves PH, Fujiwara Y, et al. Beta2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Arch. Intern. Med. 2008;168(2):200–206. doi: 10.1001/archinternmed.2007.64. [DOI] [PubMed] [Google Scholar]
- 34.Hall JR, Johnson LA, Barber RC, et al. Biomarkers of basic activities of daily living in Alzheimer's disease. J. Alzheimers Dis. 2012;31(2):429–437. doi: 10.3233/JAD-2012-111481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soares HD, Potter WZ, Pickering E, et al. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch. Neurol. 2012;69(10):1310–1317. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Won E, Hurria A, Feng T, et al. CA125 level association with chemotherapy toxicity and functional status in older women with ovarian cancer. Int. J. Gynecol. Cancer. 2013;23(6):1022–1028. doi: 10.1097/IGC.0b013e318299438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Bryant SE, Johnson L, Edwards M, et al. The link between C-reactive protein and Alzheimer's disease among Mexican Americans. J. Alzheimers Dis. 2013;34(3):701–706. doi: 10.3233/JAD-122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Bryant SE, Xiao G, Barber R, et al. A blood-based screening tool for Alzheimer's disease that spans serum and plasma: findings from TARC and ADNI. PLoS ONE. 2011;6(12):e28092. doi: 10.1371/journal.pone.0028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Royall DR, Palmer RF, Texas Alzheimer's Research and Care Consortium Does ethnicity moderate dementia's biomarkers? Neurobiol. Aging. 2014;35(2):336–344. doi: 10.1016/j.neurobiolaging.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Saunders AJ, Tanzi RE. Welcome to the complex disease world. Alpha2-macroglobulin and Alzheimer's disease. Exp. Neurol. 2003;184(1):50–53. doi: 10.1016/j.expneurol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Zellner M, Veitinger M, Umlauf E. The role of proteomics in dementia and Alzheimer's disease. Acta Neuropathol. 2009;118(1):181–195. doi: 10.1007/s00401-009-0502-7. [DOI] [PubMed] [Google Scholar]
- 42.Johnstone D, Milward EA, Berretta R, Moscato P Alzheimer's Disease Neuroimaging I. Multivariate protein signatures of pre-clinical Alzheimer's disease in the Alzheimer's disease neuroimaging initiative (ADNI) plasma proteome dataset. PLoS ONE. 2012;7(4):e34341. doi: 10.1371/journal.pone.0034341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bossu P, Ciaramella A, Salani F, et al. Interleukin-18, from neuroinflammation to Alzheimer's disease. Curr. Pharm. Des. 2010;16(38):4213–4224. doi: 10.2174/138161210794519147. [DOI] [PubMed] [Google Scholar]
- 44.Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol. Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Xie K, Liu Y, Hao W, et al. Tenascin-C deficiency ameliorates Alzheimer's disease-related pathology in mice. Neurobiol. Aging. 2013;34(10):2389–2398. doi: 10.1016/j.neurobiolaging.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer's disease: a comparative overview. Mol. Neurobiol. 2014;50(2):534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Witte L, Tomasik J, Schwarz E, et al. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr. Res. 2014;154(1):23–29. doi: 10.1016/j.schres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Motta M, Imbesi R, Di Rosa M, Stivala F, Malaguarnera L. Altered plasma cytokine levels in Alzheimer's disease: correlation with the disease progression. Immunol. Lett. 2007;114(1):46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Hu WT, Chen-Plotkin A, Arnold SE, et al. Novel CSF biomarkers for Alzheimer's disease and mild cognitive impairment. Acta. Neuropathol. 2010;119(6):669–678. doi: 10.1007/s00401-010-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garwood CJ, Cooper JD, Hanger DP, Noble W. Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Front Psychiatry. 2010;1:136. doi: 10.3389/fpsyt.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jordanova V, Stewart R, Davies E, Sherwood R, Prince M. Markers of inflammation and cognitive decline in an African–Caribbean population. Int. J. Geriatr. Psychiatry. 2007;22(10):966–973. doi: 10.1002/gps.1772. [DOI] [PubMed] [Google Scholar]
- 52.Reale M, Kamal MA, Velluto L, Gambi D, Di Nicola M, Greig NH. Relationship between inflammatory mediators, Abeta levels and ApoE genotype in Alzheimer disease. Curr. Alzheimer Res. 2012;9(4):447–457. doi: 10.2174/156720512800492549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer's disease. Front Integr. Neurosci. 2013;7:59. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem. Res. 2007;32(10):1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 55.Monson NL, Ireland SJ, Ligocki AJ, et al. Elevated CNS inflammation in patients with preclinical Alzheimer's disease. J. Cereb. Blood Flow Metab. 2014;34(1):30–33. doi: 10.1038/jcbfm.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henriksen K, O'Bryant SE, Hampel H, et al. The future of blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014;10(1):115–131. doi: 10.1016/j.jalz.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snyder HM, Carrillo MC, Grodstein F, et al. Developing novel blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014;10(1):109–114. doi: 10.1016/j.jalz.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Bryant SE, Johnson L, Balldin V, et al. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer's disease. J. Alzheimers Dis. 2013;33(2):373–379. doi: 10.3233/JAD-2012-121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crean S, Ward A, Mercaldi CJ, et al. Apolipoprotein E epsilon4 prevalence in Alzheimer's disease patients varies across global populations: a systematic literature review and meta-analysis. Dement. Geriatr. Cogn. Disord. 2011;31(1):20–30. doi: 10.1159/000321984. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez HM, Tarraf W, Gouskova N, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch. Clin. Neuropsychol. 2015;30(1):68–77. doi: 10.1093/arclin/acu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bazenet C, Lovestone S. Plasma biomarkers for Alzheimer's disease: much needed but tough to find. Biomark. Med. 2012;6(4):441–454. doi: 10.2217/bmm.12.48. [DOI] [PubMed] [Google Scholar]
- 62.O'Bryant SE, Xiao G, Barber R, et al. Molecular neuropsychology: creation of test-specific blood biomarker algorithms. Dement. Geriatr. Cogn. Disord. 2014;37(1):45–57. doi: 10.1159/000345605. [DOI] [PMC free article] [PubMed] [Google Scholar]