Abstract
Aim:
Assess the relationship of serum calcium and serum albumin to tumor stage and other clinical characteristics in patients with cutaneous malignant melanoma (MM).
Patients & methods:
A cross-sectional study to evaluate serum calcium as a marker of disease progression (n = 644) in MM.
Results:
Serum albumin was significantly lower among men (p < 0.01) and among patients with stage 4 disease (p < 0.05). In a multivariable regression model adjusted for age, gender and site, albumin-corrected calcium was positively associated with disease stage (odds ratio: 1.46; 95% CI: 1.02–2.07; p = 0.04). The odds of higher stage increased 60% for each 1.0 mg/dl increase in albumin-corrected calcium.
Conclusion:
Higher albumin-corrected serum calcium may be a marker of disease progression in MM.
KEYWORDS : albumin, albumin-corrected calcium, malignant melanoma, serum calcium
Malignant melanoma (MM) is the 5th and 7th leading cancer diagnosed among US men and women, respectively [1], with approximately 76,380 new cases and 10,130 deaths expected in 2016 [2]. Survival from MM is strongly dependent on stage [3]. Because micrometastases from MM often are not readily detectable, the ability to predict which patients have advanced stage, and thereby to initiate therapies earlier, is limited. Although serum markers such as lactate dehydrogenase (LDH), S100-beta, melanoma-inhibiting activity (MIA) and VEGF are under investigation as prognostic tools, there are no specific serum markers that reflect micrometastatic tumor burden [4,5]. Only LDH has been validated as a biomarker in MM and is incorporated in the American Joint Committee on Cancer MM staging [5].
In addition to markers that are shed from cancer cells themselves, many cancers produce paraneoplastic effects (effects distant from the tumor) that are detectable in blood. For example, elevations in serum calcium resulting in hypercalcemia are a frequent complication of many cancers, including breast, lung and renal cell carcinomas [6–8]. Although hypercalcemia (i.e., serum calcium beyond the normal reference range: 8.5–10.5 mg/dl) is rare in MM, with incidence rates ranging from 1 to 12% [9,10], we reasoned that serum calcium levels that are high but that do not meet the threshold for hypercalcemia (i.e., ‘high normocalcemia’) might be more frequent and reflect tumor growth. For example, high normocalcemia strongly predicted malignancy among women who presented with pelvic masses [11] and predicted cancer-specific death in prostate and ovarian cancers even though hypercalcemia in these cancers is rare [12–14]. The association between high normocalcemia and prostate and ovarian cancers is likely caused by PTHrP, an oncofetal protein that is overexpressed in many cancers and acts like parathyroid hormone to raise calcium levels in serum [15]. PTHrP is expressed in melanoma cells and is implicated in the promotion, invasion and metastasis in MM [16,17]. Here we investigated the relationship of serum calcium and albumin to tumor stage and other clinical characteristics in patients with MM.
Patients & methods
We conducted a cross-sectional study of serum calcium, albumin and patient and tumor characteristics in patients diagnosed with cutaneous MM between January 2000 and December 2010 at Wake Forest University Baptist Medical Center (WFUBMC) in Winston-Salem (NC, USA). A list of patients treated at WFUBMC who were assigned an International Classification of Diseases (9th revision) code 172 (malignant melanoma of the skin) was obtained from the Comprehensive Cancer Center of Wake Forest University cancer registry. In addition to serum calcium and albumin, we obtained data on clinical characteristics including: age, gender, ethnicity, stage, histology, anatomic site, Breslow thickness, presence/absence of metastases, history of parathyroid disease or primary hyperparathyroidism, mortality status and in women of childbearing age (<45 years), pregnancy at the time of diagnosis.
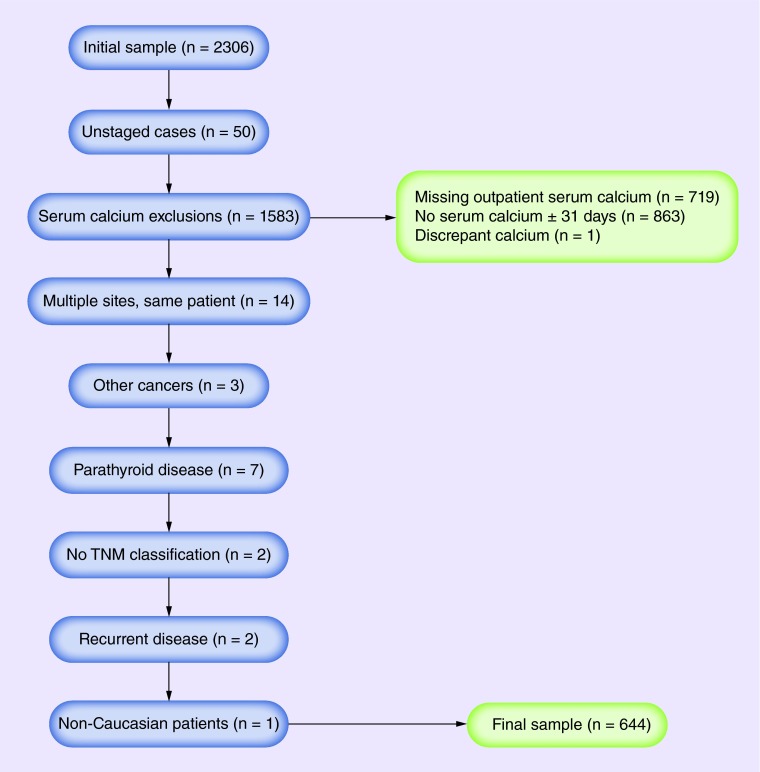
The major eligibility criteria were a diagnosis of cutaneous MM and outpatient serum calcium levels available within 31 days of diagnosis (selected in order to limit the effect of chemotherapy on serum albumin and calcium levels). We excluded patients with unstaged MM; missing outpatient serum calcium or serum calcium values greater than 31 days from the date of initial diagnosis; and a history of primary hyperparathyroidism and/or parathyroid disease. Patients with recurrent disease, other cancers and those with no TNM classification were excluded. Patients with discrepant data (e.g., with multiple serum calcium levels available for the same day) were also excluded. In order to control for fluid shifts that could transiently affect serum calcium levels, we used data for serum calcium from outpatient visits only. For participants with multiple MM sites/stages diagnosed on the same day, the highest stage was used. The protocol for this study was approved by the Wake Forest University Internal Review Board (IRB# 00021288). Participant eligibility, exclusions and final sample population are shown in Figure 1.
Figure 1. . Participant eligibility criteria.
Because different automated clinical chemistry analyzers were used during the decade from which our patient list originated, we used the reference range established by the hospital laboratory which undergoes routine quality control and is certified by Joint Commission, the College of American Pathologists, and the Commission on Office Laboratory Accreditation. Serum calcium was analyzed via the absorbance of o-cresolphthalein complexone and calcium ion complex. A bromocresol green reagent is added to the serum in order to measure the absorbance of the albumin–bromocresol green complex. The absorbance strength was measured on a Beckman Coulter Clinical Chemistry analyzer (Beckman Coulter, Inc., CA, USA) and is proportional to the amount of calcium present in the sample. We used the common formula to estimate the biologically active fraction of serum calcium: serum albumin-corrected calcium (mg/dl) = total calcium (mg/dl) + 0.8 × (4-albumin [g/dl]) [18].
• Statistical analysis
Descriptive statistics are reported as means ± standard deviation. We compared variables of interest (serum albumin, serum total and albumin-corrected calcium, age at diagnosis, Breslow thickness) between MM stages, sites and by quintiles of age at diagnosis with analysis of variance (ANOVA). Comparisons between genders were made by t-test. To evaluate the predictive power of serum albumin, total serum calcium and albumin-corrected serum calcium on MM stage, we used multivariable ordinal logistic regression models adjusting for age, gender and site only. We adjusted for age as a continuous linear term and gender and site as categorical variables. With a sample size of 644, adjustment for three common variables as age and gender, does not represent overadjustment. No other models were investigated. All statistical tests were two-tailed and a p-value of 0.05 was used throughout.
Results
We identified 2306 patients with cutaneous MM. Exclusions were as follows: serum calcium greater than 31 days from the date of initial MM diagnosis (n = 863); no outpatient serum calcium (n = 719); patients with unstaged MM (n = 50); history of primary hyperparathyroidism or parathyroid disease (n = 7); other cancers (n = 3), recurrent disease (n = 2), those with no TNM classification (n = 2), and patients with discrepant data (n = 1). When patients were observed with multiple MM sites/stages diagnosed on the same day (n = 14) the highest stage was used and the rest were excluded. There was only one African–American participant in the near final Caucasian dataset who was also excluded. Participant eligibility and the final sample population are shown in Figure 1.
Six hundred forty four (n = 644, 28%) patients remained after inclusion and exclusion criteria were applied. Sixty percent (60%) of participants in the final dataset were male. The average age at diagnosis was 61 years (range: 11–96 years). The most common anatomic site was the head and neck (30%) followed by the trunk (25%) and the upper limb and shoulder (24%). The most prevalent subtype was MM, not otherwise specified (NOS; 82%). In total, 14% were MM in situ (Table 1).
Table 1. . Tumor and demographic characteristics of patients with melanoma.
| Variables (n = 644 unless otherwise specified) | n (%) or mean ± SD (range) |
|---|---|
| Age (years) |
60.94 ± 15.59 (range: 11–96) |
| Gender: | |
| – Male | 390 (60) |
| – Female |
254 (40) |
| TNM classification/stage: | |
| – T0N0M0 | 92 (14) |
| – T1N0M0 | 166 (26) |
| – T2N0M0 | 273 (42) |
| – T3 (any T, N1+, M0) | 82 (13) |
| – T4 (any T, any N, M1+) |
31 (5) |
| Current status (n = 576): | |
| – Alive | 449 (78) |
| – Deceased |
127 (22) |
| Cancer status (n = 576): | |
| – No evidence of cancer | 473 (73) |
| – Evidence of cancer | 102 (16) |
| – Unknown |
1 (0.2) |
| Histologic subtype: | |
| – Malignant melanoma NOS | 527 (82) |
| – Melanoma in situ | 89 (14) |
| – Desmoplastic | 16 (3) |
| – Superficial spreading | 6 (0.9) |
| – Spindle cell | 3 (0.5) |
| – Malignant melanoma in giant pigmented nevus | 2 (0.3) |
| – Nodular |
1 (0.2) |
| Site: | |
| – Head and neck: | 191 (30) |
| • Face/ears | 31 (5) |
| • Other and unspecified parts of face | 83 (13) |
| • Scalp/neck | 78 (12) |
| – Trunk | 159 (25) |
| – Upper limb and shoulder | 154 (24) |
| – Lower limb and hip | 113 (18) |
| – NOS/overlapping |
27 (4) |
| Breslow thickness (mm) (n = 490) |
2.02 ± 2.05 (range: 0–9.80) |
| Serum albumin (g/dl) (n = 564) |
4.13 ± 0.35 (range: 2.5–5.1) |
| Serum total calcium (mg/dl) (n = 644) |
9.46 ± 0.50 (range: 7.20–11.30) |
| Serum albumin-corrected calcium (mg/dl) (n = 564) | 9.55 ± 0.44 (range: 8.20–11.70) |
NOS: Not otherwise specified; SD: Standard deviation.
• Gender
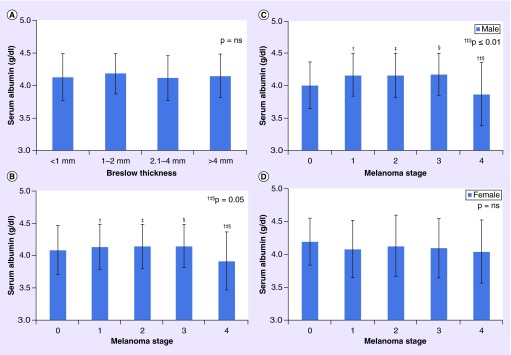
Men were significantly older than women at diagnosis (62.13 vs 59.12 years; p = 0.02; Table 2) and had a higher proportion of MM located on the head and neck (37 vs 18%; p ≤ 0.05). Women had a higher proportion of MM diagnosed at the lower limb and hip (32 vs 8%; p ≤ 0.05). No significant differences were observed in serum albumin, total serum calcium or albumin-corrected calcium (p = 0.07) between men and women (Table 2). However, there was a gender difference in serum albumin by stage. In men, serum albumin in stage 4 was significantly lower than in stages 1–3 (p ≤ 0.01), but not stage 0 (p = 1.00) (Table 2 & Figure 2C & D). Serum albumin in women with stage 4 MM was nonstatistically lower than other stages (p = 1.00).
Table 2. . Key demographic characteristics, serum albumin, total calcium and albumin-corrected calcium by gender.
| Variables | Male (mean ± SD) | Female (mean ± SD) | p-value |
|---|---|---|---|
| n |
390 |
254 |
|
| Age at diagnosis (years) |
62.13 ± 14.94 |
59.12 ± 16.40 |
0.02 |
| TNM classification (n): | |||
| – T0N0M0 | 51 | 41 | |
| – T1N0M0 | 104 | 62 | |
| – T2N0M0 | 160 | 113 | |
| – T3 (any T, N1+, M0) | 54 | 28 | |
| – T4 (any T, any N, M1+) |
21 |
10 |
|
| Breslow thickness (n = 490) |
2.11 ± 2.13 |
1.88 ± 1.94 |
0.23 |
| Current status (n = 578), n (%): | |||
| – Alive (n = 449) | 263 (76) | 186 (80) | |
| – Deceased (n = 127) |
82 (24) |
45 (20) |
|
| Mean serum albumin (g/dl) (n = 564): | 4.13 ± 0.35 | 4.12 ± 0.35 | 0.63 |
| – Stage 0 | 4.01 ± 0.36 | 4.19 ± 0.37 | |
| – Stage 1 | 4.17 ± 0.33† | 4.08 ± 0.37 | |
| – Stage 2 | 4.16 ± 0.34‡ | 4.13 ± 0.34 | |
| – Stage 3 | 4.18 ± 0.33§ | 4.10 ± 0.34 | |
| – Stage 4 |
3.87 ± 0.49†,‡,§ |
4.04 ± 0.37 |
|
| Mean serum calcium (mg/dl) (n = 644): | 9.44 ± 0.48 | 9.50 ± 0.52 | 0.18 |
| – Stage 0 | 9.31 ± 0.49 | 9.40 ± 0.54 | |
| – Stage 1 | 9.47 ± 0.44 | 9.49 ± 0.52 | |
| – Stage 2 | 9.45 ± 0.49 | 9.56 ± 0.49 | |
| – Stage 3 | 9.52 ± 0.48 | 9.49 ± 0.56 | |
| – Stage 4 |
9.37 ± 0.59 |
9.26 ± 0.66 |
|
| Mean albumin-corrected calcium (mg/dl) (n = 564): | 9.53 ± 0.44 | 9.60 ± 0.44 | 0.07 |
| – Stage 0 | 9.40 ± 0.33 | 9.51 ± 0.37 | |
| – Stage 1 | 9.53 ± 0.40 | 9.60 ± 0.44 | |
| – Stage 2 | 9.53 ± 0.48 | 9.61 ± 0.46 | |
| – Stage 3 | 9.57 ± 0.48 | 9.66 ± 0.45 | |
| – Stage 4 | 9.68 ± 0.36 | 9.49 ± 0.48 |
†p = 0.01; ‡p = 0.009; §p = 0.013.
SD: Standard deviation.
Figure 2. . Serum albumin level by (A) Breslow thickness, (B) melanoma stage, (C) melanoma stage in men and (D) melanoma stage in women.
Error bars present the standard error of the mean.
ns: Not significant.
• Total serum calcium
The mean total serum calcium was 9.46 ± 0.50 mg/dl (range: 7.20–11.30 mg/dl; reference range: 8.5–10.5 mg/dl; Table 1). Only 4% (n = 29) of total serum calcium values were beyond the reference range (<8.5 mg/dl: n = 17 and >10.5 mg/dl: n = 12). There was no significant difference in total serum calcium by MM site (Table 3), patient age (Table 4), Breslow thickness or melanoma stage (Table 5). After adjusting for age, gender and site in a multivariable ordinal logistic regression, total serum calcium had a borderline, nonsignificant association with MM stage (p = 0.08; Table 6).
Table 3. . Serum albumin, total calcium and albumin-corrected calcium by melanoma site.
| Site | Albumin (g/dl), n = 564 (mean ± SD) | Calcium (mg/dl), n = 644 (mean ± SD) | Albumin-corrected calcium (mg/dl), n = 564 (mean ± SD) |
|---|---|---|---|
| Head and neck† |
4.11 ± 0.33‡ |
9.41 ± 0.50 |
9.50 ± 0.43 |
| Trunk |
4.14 ± 0.34§ |
9.49 ± 0.49 |
9.57 ± 0.44 |
| Upper limb and shoulders |
4.14 ± 0.32§ |
9.46 ± 0.50 |
9.52 ± 0.49 |
| Lower limb and hip |
4.19 ± 0.39§ |
9.57 ± 0.46 |
9.65 ± 0.42 |
| NOS/overlapping | 3.81 ± 0.48§ | 9.29 ± 0.56 | 9.57 ± 0.33 |
†Head and neck includes face/ears, scalp/neck and other/unspecified parts of face.
‡p = 0.002; §p ≥ 0.0003.
NOS: not otherwise specified ; SD: Standard deviation.
Table 4. . Serum albumin, total and albumin-corrected calcium and Breslow thickness according to age quintiles.
| Variables | ≤45 years (mean ± SD) | 46–57 years (mean ± SD) | 58–66 years (mean ± SD) | 67–75 years (mean ± SD) | >75 years (mean ± SD) |
|---|---|---|---|---|---|
| Albumin (g/dl) |
4.18 ± 0.38† (n = 108) |
4.18 ± 0.32‡ (n = 121) |
4.16 ± 0.37§ (n = 116) |
4.13 ± 0.34¶ (n = 114) |
3.98 ± 0.33†,‡,§,¶ (n = 105) |
| Total calcium (mg/dl) |
9.44 ± 0.46 (n = 116) |
9.48 ± 0.46 (n = 137) |
9.47 ± 0.51 (n = 131) |
9.47 ± 0.54 (n = 134) |
9.45 ± 0.50 (n =126) |
| Albumin-corrected calcium (mg/dl) |
9.49 ± 0.41 (n = 108) |
9.55 ± 0.39 (n = 121) |
9.55 ± 0.45 (n = 116) |
9.55 ± 0.45 (n = 114) |
9.64 ± 0.49 (n = 105) |
| Breslow thickness (mm) | 2.13 ± 2.36 (n = 116) | 1.91 ± 1.78 (n = 137) | 1.81 ± 2.03 (n = 131) | 1.88 ± 1.83 (n = 134) | 2.41 ± 2.27 (n = 126) |
†p = 0.0003; ‡p = 0.0001; §p = 0.001; ¶p = 0.02.
SD: Standard deviation.
Table 5. . Diagnosis age, serum albumin, total and albumin-corrected calcium and Breslow thickness by melanoma stage.
| Stage | Age (years), n = 644 (mean ± SD) | Albumin (g/dl), n = 564 (mean ± SD) | Calcium (mg/dl), n = 644 (mean ± SD) | Albumin-corrected calcium (mg/dl), n = 564 (mean ± SD) | Breslow thickness (mm), n = 490 (mean ± SD) |
|---|---|---|---|---|---|
| 0 |
64.59 ± 14.36 |
4.09 ± 0.38 |
9.35 ± 0.51 |
9.45 ± 0.35 |
0.87 ± 1.04¶,# |
| 1 |
60.53 ± 15.32 |
4.14 ± 0.35† |
9.48 ± 0.45 |
9.56 ± 0.42 |
0.76 ± 0.72††,‡‡ |
| 2 |
60.88 ± 15.54 |
4.15 ± 0.34‡ |
9.49 ± 0.50 |
9.56 ± 0.47 |
2.42 ± 1.91¶,††,§§ |
| 3 |
58.07 ± 17.26 |
4.15 ± 0.33§ |
9.51 ± 0.51 |
9.60 ± 0.47 |
3.52 ± 2.71#,‡‡,§§,¶¶ |
| 4 | 60.45 ± 15.38 | 3.92 ± 0.45†,‡,§ | 9.34 ± 0.60 | 9.61 ± 0.40 | 1.27 ± 3.06¶¶ |
†p = 0.05; ‡p = 0.019; §p = 0.041; ¶p = 0.002; #p < 0.0001; ††p < 0.0001; ‡‡p < 0.0001; §§p < 0.0001; ¶¶p = 0.003.
SD: Standard deviation.
Table 6. . Odds ratios for albumin-corrected and total calcium and serum albumin from ordinal logistic regression adjusted for age, gender and site.
| Serum marker | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Albumin |
1.02 |
0.65–1.61 |
0.92 |
| Total calcium |
1.30 |
0.97–1.74 |
0.08 |
| Albumin-corrected calcium | 1.46 | 1.02–2.07 | 0.04 |
• Serum albumin
The mean serum albumin (n = 564) was 4.13 ± 0.35 g/dl (observed range: 2.5–5.1 g/dl; reference range: 3.2–5.0 g/dl; Table 1). 27% of serum albumins were < 4.0 g/dl but only 4.3% of patients had serum albumin <3.5 g/dl (a common cut point for hypoalbuminemia) [19,20]. Serum albumin was significantly lower in stage 4 versus stages 1–3 (p ≤ 0.05; Table 5). Serum albumin among participants with overlapping skin lesions or where the site was NOS, was significantly lower (p ≤ 0.002) than among patients with MM diagnosed at the head and neck (Table 3). Serum albumin was significantly lower among older participants (albumin ≤45 years: 4.18 ± 0.38 g/dl vs albumin >75 years: 3.98 ± 0.33 g/dl; p = 0.0003; Table 4). Serum albumin did not vary significantly by Breslow thickness (Figure 2A) and was not significantly associated with MM stage after adjusting for age, gender and site in a multivariable logistic regression model (p = 0.92; Table 6).
• Albumin-corrected serum calcium
Mean albumin-corrected serum calcium was 9.55 ± 0.44 mg/dl (n = 564; range: 8.2–11.7 mg/dl; Table 1). Only 3% (n = 15) of albumin-corrected serum calcium values were beyond the normal reference range (<8.5 mg/dl: n = 4 and >10.5 mg/dl: n = 11). However, none of the participants in stage 4 had albumin-corrected serum calcium values greater than 10.5 mg/dl. Higher albumin-corrected calcium was observed with increasing MM stage, but this was not significant in a univariate analysis. However, when adjusted for age, gender and site by logistic regression, albumin-corrected serum calcium was significantly associated with stage (OR: 1.46; 95% CI: 1.02–2.07; p = 0.04; Table 6). For each 1.0 mg/dl increase in albumin-corrected serum calcium the odds of higher stage increased by 60%.
Discussion
We examined the cross-sectional relationships between tumor characteristics and serum albumin, total and albumin-corrected calcium in MM patients. Adjusted for age, gender and site of cancer, we found that albumin-adjusted serum calcium was significantly higher with higher tumor stage. To our knowledge, this is the largest series of MM patients (n = 644) examined with measurements of serum calcium and albumin at the time of diagnosis. Several case reports [10,21–24] and case series of relatively small numbers of patients have reported hypercalcemia in MM patients [9]. For example, Kageshita et al. reported that 7/59 patients with melanoma had hypercalcemia. All seven of these patients had stage 4 disease and hypercalcemia in that study were associated with increased serum levels of PTHrP [9]. Although hypercalcemia, an adjusted serum calcium >10.5 mg/dl was rare (˜2%), ‘high normocalcemia’, which we defined as an adjusted serum calcium that is high but within the normal range (between 10 and 10.5 mg/dl) was relatively common (74/564, 13%). In our previous study of 514 women with adnexal masses, in whom some of the masses were malignant but most of which were not, high normocalcemia was highly predictive of malignancy, with ORs >14.0 (95% CI: 5.7–32.1) [11].
We observed that serum albumin levels overall were significantly lower in stage 4 versus stages 1–3 (p ≤ 0.05; Table 2). Albumin is a negative acute phase protein and its serum levels in cancer may decrease due to decreased synthesis, increased catabolism and other mechanisms [25–29]. In our study, a multivariate regression analysis adjusting for age, gender and MM site, every 1.0 mg/dl increase in albumin-corrected serum calcium increased the odds of higher MM stage by 60%. In addition to the lower serum albumin, which would increase the ‘free’ fraction of serum calcium, it is likely that serum calcium levels were increased due to the actions of PTHrP, the major cause of hypercalcemia of malignancy [30,31], which is known to be upregulated in melanoma [32]. PTHrP shares the eight of its first amino acids with parathyroid hormone (PTH), and this shared homology leads to shared receptor binding [33]. The binding of PTHrP to PTH receptors in bone leads to an increase in serum calcium caused by bone resorption and decreased urinary excretion of calcium [18].
Our study has several limitations. This study is vulnerable to selection bias as we excluded patients with missing or outdated data on outpatient serum albumin, total and albumin-corrected calcium. A very small subset of the patients had data for LDH which precluded its inclusion in this study. None of the patients had PTHrP levels available to correlate increasing serum calcium levels with increasing stage. However, our recent work in women with ovarian cancer has clearly shown that in the absence of PTHrP, serum calcium and albumin alone may provide significant clinical information. In women with pelvic masses who presented for surgical resection, high normocalcemia was associated with an approximately 14-fold increase in malignancy (OR: 13.57; 95% CI: 5.7–32.1) and albumin-corrected serum calcium explained 20% of the variance in outcome [11]. It is conceivable that serum albumin levels in our study may have been affected by differences in BMI, for which patient data were not available. However, analysis of data from NHANES I indicates that serum albumin levels varied only very modestly with BMI and showed opposite effects in men and women [34]. Our sample was comprised primarily of non-Hispanic Caucasians, which limits its generalizability to patients of other ethnicities. We also did not screen for liver and renal dysfunction, the presence of inflammation or other medical conditions that conceivably could affect serum albumin levels. Pregnancy is associated with alterations in the calcium axis. Although we were unable to verify pregnancy status in the women of childbearing age, the relatively small number of women in this age group in our study (n = 51) suggests that it is unlikely to have greatly influenced our results.
Conclusion
In this cross-sectional study of 644 patients with MM, after adjusting for age, gender and site, serum levels of albumin-corrected calcium were positively associated with disease stage (OR: 1.46; 95% CI: 1.02–2.07; p = 0.04). The odds of higher stage increased 60% for each 1.0 mg/dl increase in albumin-corrected calcium. These data suggest that higher albumin-corrected serum calcium, a very inexpensive marker, may indicate disease progression before overt signs of progression are evident clinically. If confirmed in longitudinal studies, this finding may be useful clinically to help guide the initiation of therapeutic interventions, especially since there presently are no biomarkers that predict progression and relapse in MM [35]. Total serum calcium that is adjusted for serum albumin gives an approximation of ionized calcium, the biologically active fraction of total serum calcium. However, this approximation is relatively insensitive to true calcemia, which is more accurately measured using ionized calcium [36,37]. Serum levels of ionized calcium and serum albumin merit investigation in prospective studies of patients with MM.
Future perspective
Serum calcium is an inexpensive laboratory test that is routinely ordered. We found that the odds of higher stage increased 60% for each 1.0 mg/dl increase in albumin-corrected calcium. Although total serum calcium that is adjusted for serum albumin (albumin-corrected calcium) gives an approximation of ionized calcium, it is relatively insensitive to true calcemia, which is more accurately measured using ionized calcium. Prospective studies are needed to confirm the clinical utility of albumin-corrected serum calcium, ionized calcium and serum albumin in predicting disease progression in MM.
EXECUTIVE SUMMARY.
Malignant melanoma is the 5th and 7th leading cancer diagnosed among US men and women, respectively.
Although serum markers are under investigation as prognostic tools, there are no specific serum markers that reflect micrometastatic tumor burden in melanoma patients.
Patients & methods
We conducted a cross-sectional study to evaluate serum calcium as a marker of disease progression in patients diagnosed with cutaneous malignant melanoma between January 2000 and December 2010 at an academic medical center.
Results
Serum albumin was significantly lower among men (p < 0.01) and patients with stage 4 disease (p < 0.05).
The odds of higher melanoma stage increased 60% for each 1.0 mg/dl increase in albumin-corrected calcium.
Discussion
Adjusted for age, gender and site of cancer, albumin-corrected serum calcium was positively associated with higher tumor stage.
Increasing albumin-corrected serum calcium, a very inexpensive marker, may indicate melanoma progression before overt signs of progression are evident clinically.
Acknowledgements
The authors wish to thank Ms Inez Inman, Ms Julia Robertson and Mr Bob Morrell for help with data extraction from the electronic medical records.
Footnotes
Financial & competing interests disclosure
M Datta was supported by the Comprehensive Cancer Center of Wake Forest University Cancer Control Traineeship – NCI/NIH Grant# R25CA122061. Biostatistical services were supported by the Comprehensive Cancer Center of Wake Forest University NCI CCSG P30CA012197 grant. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures. 2016. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- 3.Balch CM, Buzaid AC, Soong S-J, et al. New TNM melanoma staging system: linking biology and natural history to clinical outcomes. Semin. Surg. Oncol. 2003;21(1):43–52. doi: 10.1002/ssu.10020. [DOI] [PubMed] [Google Scholar]
- 4.Brochez L, Naeyaert JM. Serological markers for melanoma. Br. J. Dermatol. 2000;143(2):256–268. doi: 10.1046/j.1365-2133.2000.03649.x. [DOI] [PubMed] [Google Scholar]
- 5.Palmer SR, Erickson LA, Ichetovkin I, Knauer DJ, Markovic SN. Circulating serologic and molecular biomarkers in malignant melanoma. Mayo Clin. Proc. 2011;86(10):981–990. doi: 10.4065/mcp.2011.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumachi F, Brunello A, Roma A, Basso U. Cancer-induced hypercalcemia. Anticancer Res. 2009;29(5):1551–1555. [PubMed] [Google Scholar]
- 7.Yao M, Murakami T, Shioi K, et al. Tumor signatures of PTHLH overexpression, high serum calcium, and poor prognosis were observed exclusively in clear cell but not non clear cell renal carcinomas. Cancer Med. 2014;3(4):845–854. doi: 10.1002/cam4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaw SSH, Sahmoun A, Schwartz GG. Serum calcium, tumor size, and hormone receptor status in women with untreated breast cancer. Cancer Biol. Ther. 2012;13(7):457–461. doi: 10.4161/cbt.19606. [DOI] [PubMed] [Google Scholar]
- 9.Kageshita T, Matsui T, Hirai S, Fukuda Y, Ono T. Hypercalcaemia in melanoma patients associated with increased levels of parathyroid hormone-related protein. Melanoma Res. 1999;9(1):69–73. doi: 10.1097/00008390-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Burt ME, Brennan MF. Hypercalcemia and malignant melanoma. Am. J. Surg. 1979;137(6):790–794. doi: 10.1016/0002-9610(79)90095-3. [DOI] [PubMed] [Google Scholar]
- 11.Kelly MG, Winkle SS, Lentz SS, et al. Serum calcium and serum albumin are biomarkers that can discriminate malignant from benign pelvic masses. Cancer Epidemiol. Biomarkers Prev. 2015;24(10):1593–1598. doi: 10.1158/1055-9965.EPI-15-0443. [DOI] [PubMed] [Google Scholar]
- 12.Skinner HG, Schwartz GG. Serum calcium and incident and fatal prostate cancer in the National Health and Nutrition Examination Survey. Cancer Epidemiol. Biomarkers Prev. 2008;17(9):2302–2305. doi: 10.1158/1055-9965.EPI-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GG, Skinner HG. Prospective studies of total and ionized serum calcium in relation to incident and fatal ovarian cancer. Gynecol. Oncol. 2013;129(1):169–172. doi: 10.1016/j.ygyno.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 14.Van Dijk JM. Hypercalcemia in prostatic carcinoma: case report and review of the literature. Am. J. Clin. Oncol. 1993;16(4):329–331. [PubMed] [Google Scholar]
- 15.Luparello C. Parathyroid hormone-related protein (PTHrP): a key regulator of life/death decisions by tumor cells with potential clinical applications. Cancers. 2011;3(1):396–407. doi: 10.3390/cancers3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang DC, Yang XF, Ochietti B, Fadhil I, Camirand A, Kremer R. Parathyroid hormone-related protein: potential therapeutic target for melanoma invasion and metastasis. Endocrinology. 2014;155(10):3739–3749. doi: 10.1210/en.2013-1803. [DOI] [PubMed] [Google Scholar]
- 17.Danks JA, Ebeling PR, Hayman J, et al. Parathyroid hormone-related protein: immunohistochemical localization in cancers and in normal skin. J. Bone Miner. Res. 1989;4(2):273–278. doi: 10.1002/jbmr.5650040221. [DOI] [PubMed] [Google Scholar]
- 18.Baird GS. Ionized calcium. Clin. Chim. Acta. 2011;412(9–10):696–701. doi: 10.1016/j.cca.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Caraceni P, Domenicali M, Tovoli A, et al. Clinical indications for the albumin use: still a controversial issue. Eur. J. Intern. Med. 2013;24(8):721–728. doi: 10.1016/j.ejim.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern. Emerg. Med. 2012;7(3):193–199. doi: 10.1007/s11739-012-0802-0. [DOI] [PubMed] [Google Scholar]
- 21.Matsui T, Kageshita T, Ishihara T, Tomiguchi S, Takahashi M, Ono T. Hypercalcemia in a patient with malignant melanoma arising in congenital giant pigmented nevus. Dermatology. 1998;197(1):65–68. doi: 10.1159/000017960. [DOI] [PubMed] [Google Scholar]
- 22.Yeung S-CJ, Eton O, Burton DW, Deftos LJ, Vassilopoulou-Sellin R, Gagel RF. Hypercalcemia due to parathyroid hormone-related protein secretion by melanoma. Horm. Res. 1998;49(6):288–291. doi: 10.1159/000023188. [DOI] [PubMed] [Google Scholar]
- 23.Attia P, Phan GQ, Duray PH, Rosenberg SA. Parathyroid hormone-related protein and hypercalcemia in patients with metastatic melanoma: case report and review. Am. J. Clin. Oncol. 2003;26(1):42–45. doi: 10.1097/00000421-200302000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batsis JA, Barry MJ. Metastatic malignant melanoma presenting with hypercalcaemia and bone marrow involvement. J. Eur. Acad. Dermatol. Venereol. 2006;20(4):432–434. doi: 10.1111/j.1468-3083.2006.01435.x. [DOI] [PubMed] [Google Scholar]
- 25.Margarson MP, Soni N. Serum albumin: touchstone or totem? Anaesthesia. 1998;53(8):789–803. doi: 10.1046/j.1365-2044.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 26.Mendez CM, Mcclain CJ, Marsano LS. Albumin therapy in clinical practice. Nutr. Clin. Pract. 2005;20(3):314–320. doi: 10.1177/0115426505020003314. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Yoo C, Lee D, Kim S-W, Lee J-S, Suh C. Serum albumin level is a significant prognostic factor reflecting disease severity in symptomatic multiple myeloma. Ann. Hematol. 2010;89(4):391–397. doi: 10.1007/s00277-009-0841-4. [DOI] [PubMed] [Google Scholar]
- 28.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin. Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 29.Stehle G, Sinn H, Wunder A, et al. Plasma protein (albumin) catabolism by the tumor itself – implications for tumor metabolism and the genesis of cachexia. Crit. Rev. Oncol. Hematol. 1997;26(2):77–100. doi: 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 30.Mundy GR, Edwards JR. PTH-related peptide (PTHrP) in hypercalcemia. J. Am. Soc. Nephrol. 2008;19(4):672–675. doi: 10.1681/ASN.2007090981. [DOI] [PubMed] [Google Scholar]
- 31.Grill V, Rankin W, Martin TJ. Parathyroid hormone-related protein (PTHrP) and hypercalcaemia. Eur. J. Cancer. 1998;34(2):222–229. doi: 10.1016/s0959-8049(97)10130-7. [DOI] [PubMed] [Google Scholar]
- 32.El Abdaimi K, Papavasiliou V, Goltzman D, Kremer R. Expression and regulation of parathyroid hormone-related peptide in normal and malignant melanocytes. Am. J. Physiol. Cell Physiol. 2000;279(4):C1230–C1238. doi: 10.1152/ajpcell.2000.279.4.C1230. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson S, Waxman J. Clues from hypercalcaemia. Br. J. Cancer. 2002;86(7):1021–1022. doi: 10.1038/sj.bjc.6600220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Micozzi MS, Albanes D, Stevens RG. Relation of body size and composition to clinical biochemical and hematologic indices in US men and women. Am. J. Clin. Nutr. 1989;50(6):1276–1281. doi: 10.1093/ajcn/50.6.1276. [DOI] [PubMed] [Google Scholar]
- 35.Levine D, Fisher D. Current status of diagnostic and prognostic markers in melanoma. In: Thurin M, Marincola FM, editors. Molecular Diagnostics for Melanoma. Humana Press; NY, USA: 2014. pp. 177–197. [DOI] [PubMed] [Google Scholar]
- 36.Sorva A, Elfving S, Pohja P, Tilvis RS. Assessment of calcaemic status in geriatric hospital patients: serum ionized calcium versus albumin-adjusted total calcium. Scand. J. Clin. Lab. Invest. 1988;48(6):489–494. doi: 10.3109/00365518809085762. [DOI] [PubMed] [Google Scholar]
- 37.Björkman MP, Sorva AJ, Tilvis RS. Calculated serum calcium is an insufficient surrogate for measured ionized calcium. Arch. Gerontol. Geriatr. 2009;49(3):348–350. doi: 10.1016/j.archger.2008.11.014. [DOI] [PubMed] [Google Scholar]