Abstract
There is growing concern about the late effects of traumatic brain injury (TBI). This scoping review summarizes clinical research from the past 10 years that evaluates the relationship between TBI and Alzheimer's disease. This review identified five studies that found increased risk for dementia after TBI, two studies that found no increased risk and four studies that found a relationship only under certain conditions or in specified subsamples. Methodological differences across studies preclude direct comparison of results, and discrepant findings elucidate the complex course of post-TBI neurodegeneration. We discuss the factors that influence the strength and direction of the relationship between TBI and Alzheimer's disease, and the implications of this body of research for patient care and future research.
KEYWORDS : Alzheimer's disease, dementia, traumatic brain injury
Practice points.
Background
Case studies dating back to the early 20th century provide evidence of progressive dementia many years after a traumatic brain injury (TBI) with loss of consciousness as well as histopathological evidence of Alzheimer's disease (AD) postmortem.
Many epidemiological studies provide compelling evidence that sustaining a TBI is associated with increased risk for degenerative conditions that may result in dementia including AD; however, other high-quality epidemiological studies have demonstrated no increased risk for dementia following TBI.
AD is by far the most commonly diagnosed subtype of dementia, accounting for up to 80% of all dementia cases, and thus is the focus of the current review.
Studies on the risk for dementia following TBI are very difficult to compare due to differences in study design, duration of follow-up, operational definition of both TBI and dementia, and differences in the extent to which other dementia risk factors are controlled.
In order to understand the nuanced differences between findings, reviews must examine both the clinical and demographic factors that influence the strength and direction of the relationship between TBI and AD.
Results of the current scoping review
Several recent studies reported that TBI is a risk factor for later development of AD and remains so after adjustment for other demographic and risk factors.
Other high-quality studies reported no relationship between TBI and AD; however, studies that enrolled older adults may have excluded people with TBI who died or became demented before study entry.
Studies reporting a conditional relationship between TBI and dementia suggest that factors such as APOE ε4 genotype, more severe injury and age at injury may impact dementia risk.
Conclusion
Given the evidence that dementia risk may increase with injury severity and frequency, a detailed account of age at injury, injury severity and number of lifetime TBI exposure is essential to document for future studies.
The magnitude of risk for dementia associated with TBI varied considerably across studies, and differences were largely attenuated by adjusting for known demographic and clinical risk factors for dementia.
Several studies found that history of TBI was associated with significantly more co-morbid medical conditions compared with uninjured controls, including conditions that are well-known independent risk factors for dementia. It is important to consider the implications of adjusting for conditions that may represent early or prodromal signs of dementia or that may represent downstream or secondary consequences of TBI such as metabolic or endocrine disorders.
Most recent evidence suggests that post-TBI neurodegeneration may represent a disease process that is clinically and pathologically distinct from AD; it is possible that delayed post-TBI cognitive and functional decline may be misclassified as AD in part due to the absence of a more appropriate diagnostic label.
Multiple distinct pathological processes may contribute to the late-life neurodegeneration experienced by some TBI survivors; more research is needed to define and validate diagnostic criteria for the clinical and neuropathological signatures of post-TBI neurodegeneration.
It is essential for future research to investigate postmortem neuropathological data in well-characterized clinical TBI samples in order to understand the population-based prevalence of post-TBI neurodegeneration, its relationships to known dementia subtypes and factors that may modify risk or protection.
Future perspective: implications for patient care
The complex relationship between TBI and AD highlights potential opportunities for intervention, prevention and improved clinical management of older adults living with a history of TBI.
Clinicians and others caring for individuals with TBI can improve long-term health management by providing reminders for appointments and follow-up care, writing down recommendations and instructions and involving trusted others in healthcare decisions when appropriate.
Factors that increase risk for dementia among TBI survivors, such as medical comorbidity, substance use and psychiatric illness, can and should be treated or managed using existing empirically supported clinical management protocols.
The late-life effects of traumatic brain injury (TBI) have received unprecedented attention in recent years. In 2005, a syndrome of progressive cognitive, motor and neuropsychiatric symptoms initially described almost 80 years prior [1,2] found renewed interest when these symptoms were described in two professional American football players whose brains showed an apparently unique distribution of tau pathology, or neurofibrillary tangles (NFTs) [3,4]. This tauopathy called chronic traumatic encephalopathy (CTE) has since been identified postmortem in a small but growing number of individuals [4–7], resulting in widespread media attention and public health concern about the possible neurodegenerative effects of TBI. Based on preliminary neuropathological diagnostic published earlier this year [8], CTE has thus far only been found postmortem in individuals who were exposed to repetitive concussive or subconcussive head trauma during life [6].
However, the link between single TBI and dementia has been a topic of interest among researchers and clinicians for decades. Published case studies dating back to the early 20th century describe individuals who developed a progressive dementia many years after surviving a TBI with loss of consciousness (LOC) and showed histopathological evidence of Alzheimer's disease (AD) postmortem [9,10]. Throughout the 1980–1990s, several epidemiological studies provided compelling evidence that single TBI is associated with increased risk for degenerative neurocognitive conditions resulting in dementia [11–15]. AD is by far the most commonly diagnosed subtype of dementia, accounting for up to 80% of all dementia cases [16]. The terms ‘dementia’ and ‘Alzheimer's disease’ are used synonymously in some epidemiological studies, the former referring to all-cause dementia (ACD) diagnosis as determined by consensus conference [17], or perhaps even medical record or self-reported ‘dementia’ of unknown type, the latter term referring to probable and possible AD diagnoses determined using consensus criteria [18] or otherwise defined AD-type dementia. Based on these epidemiological studies, TBI became widely recognized as the strongest environmental risk factor for dementia in general and for AD in particular.
This apparent link between TBI and AD stimulated investigation into potential mechanisms underlying the association, and the most carefully studied pathophysiological mechanism is the production and clearance of amyloid-β (Aβ) after TBI [19]. However, studies of Aβ peptide levels in humans with TBI have produced contradictory results [20,21]. Additional research has demonstrated increased levels of hyperphosphorylated tau protein, the same protein involved in the NFTs in AD, are found in individuals who have experienced a severe TBI [22]; however, it remains unclear whether or how TBI-associated plaques identified acutely after injury develop into the dense neuritic plaques that are the hallmark pathological features of AD.
Importantly, several large-scale high-quality epidemiological studies have found no increased risk for dementia associated with TBI [23–30]. Differences across studies in design, duration of follow-up, operational definition of both TBI and dementia, and differences in the extent to which other dementia risk factors are controlled to make the results of these studies very difficult to compare. An exhaustive review of the topic was undertaken by the Institute of Medicine (IOM) who convened a panel of experts to review the relevant literature published prior to 2005. The IOM issued a report in which the panel rated the evidence available and summarized the long-term health consequences of TBI [31]. Ten studies on TBI and AD were reviewed, and the IOM concluded that there is ‘sufficient evidence of an association’ between moderate or severe TBI and AD. Results were mixed with regard to a relationship between mild TBI (mTBI) and AD, reporting ‘suggestive evidence of an association’ between mTBI with LOC and AD. There was ‘inadequate evidence’ to determine whether an association exists between mTBI without LOC and AD [31]. The IOM review also investigated the relationship between TBI and other neurodegenerative pathologies and found sufficient evidence of an association between TBI and dementia pugilistica as well as TBI and parkinsonism [31]. The relationships between TBI and these other neurodegenerative pathologies are not investigated in this review, however, because this review is focused solely on the relationship between TBI and AD.
Another decade has passed since the IOM review was completed, and during that time the size of the older adult population in the USA – defined as persons aged 65 years or older – has grown steadily, becoming the fastest-growing segment of the population [32]. Updated statistics on TBI from the Centers for Disease Control and Prevention indicate that at least 3.5 million people sustain a TBI in the USA [33], and at least 25% of those injuries are classified as moderate or severe [34]. It is therefore urgently important to understand and characterize the late effects of TBI and to identify the factors that may impact risk for late-life neurodegeneration among TBI survivors. The editorial board of Neurodegenerative Disease Management invited the current authors to characterize the current understanding of TBI as a contributor to AD and implications of recent research for clinical practice. Accordingly, this manuscript presents a scoping review of studies on the relationship between TBI and AD published since the IOM report was completed. We then discuss the relevance and implications of these findings for TBI survivors and their caregivers.
Methods
A scoping review involves a search of a predefined area of the literature in order to synthesize current knowledge relevant to a given research question, identify gaps in that knowledge and inform clinical implications of a particular area of study [35,36]. Like systematic reviews, scoping reviews rely on clear and reproducible methods for reviewing the literature and clear criteria for study inclusion and exclusion. Unlike systematic reviews, scoping reviews do not provide an appraisal of study methods and do not rate the level of evidence provided by each study. The goal of the current scoping review is to summarize the literature on the relationship between TBI and AD that has been published in the last 10 years since the IOM report [31]. In addition to summarizing the results of original studies identified through the scoping review, we summarize the review papers that have been published during this time period.
• Search strategy
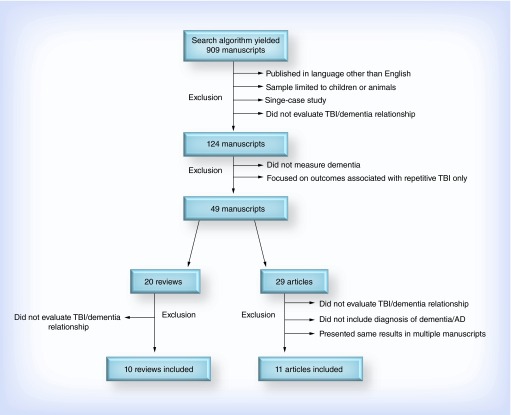
Articles were identified through a search of manuscripts in the PubMed scientific database between December 2005 and February 2015. The search combined two strings with the following terms: brain injury or traumatic brain injury or concussion or head injury or head trauma or brain trauma and Alzheimer's disease or dementia or mild cognitive impairment or mild cognitive impairment (MCI) or cognitive impairment or neurocognitive impairment. Moreover, these terms were required to appear in the title or abstract of eligible manuscripts. Filters were added to limit the range of publication dates to the specified 10-year span, to limit the species to human and to limit the language to English. This search identified 909 potential manuscripts.
In the first round of review, studies were excluded if: first, they were published in any language other than English; second, the sample was limited to children or animals; third, they described a single-case study and fourth, they did not evaluate the relationship between TBI and dementia. At this stage, 785 studies were excluded; most of these were single-case studies, intervention studies that did not evaluate the relationship between TBI and dementia, or focused only the relationship between TBI and non-AD neurodegenerative diseases (e.g., CTE, Parkinson's disease, vascular dementia). Studies were also excluded if they focused exclusively on outcomes associated with repetitive TBI or subconcussive head injury exposure but did not consider single TBI. Studies that examined the relationship between TBI and ACD were retained.
The next stages of review entailed either an abstract or full-text review to verify inclusion eligibility of the remaining 124 manuscripts. A total of 93 papers were excluded through these stages; the studies either did not measure dementia (e.g., studies of cognitive decline in general that did not include AD or ACD as an outcome), did not quantify the relationship between TBI and diagnosis of dementia (e.g., studies that simply described rates of TBI in an AD sample without quantifying associated risks) or focused exclusively on outcomes associated with repetitive TBI (without also considering single TBI). Of the 31 remaining articles, 20 were literature reviews that did not include original data analysis; ten of these were excluded because they did not characterize or quantify the relationship between TBI and dementia. Review papers that otherwise met inclusion criteria were retained and summarized separately from studies that directly evaluated the relationship between TBI and AD using original or archived data.
The remaining 11 scientific studies (Figure 1) were thoroughly reviewed to extract information necessary to describe the following elements: study design, sample size, methods of TBI and dementia ascertainment, overall findings and relevant statistics (e.g., odds ratio, hazard ratio [HR]). Review papers that provided additional information relevant to this relationship are also summarized below.
Figure 1. . Stages of manuscript exclusion.
AD: Alzheimer's disease; TBI: Traumatic brain injury.
Results
• Studies reporting a clear relationship between TBI & dementia
Five of the 11 retained papers concluded a positive relationship between TBI and dementia (Table 1). Two of these studies [37,38] were retrospective cohort studies of adults who were aged 55 years or older and dementia-free at baseline, and diagnosis of TBI and subsequent dementia were obtained through medical record review. The sample studied by Barnes et al. [37] consisted of veterans and available records did not include the date of TBI, which means it was not known whether the TBI was recent or remote. Over a 7-year period, veterans with any history of TBI were more likely (16%) to have a dementia diagnoses recorded in the medical record than those with no history of TBI (10%) after adjusting for the competing risk of death, demographics, and medical and psychiatric co-morbidities (adjusted HR [95% CI]: 1.57 [1.35–1.83]) [37]. In the Gardner et al. study [38], however, state-wide administrative health data were used to identify a sample of individuals whose records indicated emergency department or inpatient hospital treatment for TBI over a 2-year period, and these cases were compared with a control group of individuals who were treated for non-TBI trauma over this same period. Both groups were followed for 5–7 years, and those with a recent TBI were more likely than their non-TBI trauma counterparts to have a diagnosis of dementia recorded in the medical record during an emergency department or inpatient hospital visit, after adjusting for age, sex and medical and psychiatric co-morbidities (adjusted cox ratio [95% CI]: 1.26 [1.21–1.32]) [38]. After stratifying by age and TBI severity, the authors reported that moderate–severe TBI was associated with increased dementia risk across all age groups, though mTBI was only associated with increased dementia risk above aged 65 years.
Table 1. . Studies finding a relationship between traumatic brain injury and Alzheimer's disease.
| Study (year) | Design | Sample size | TBI identification | Dementia identification | Type of dementia | Ref. |
|---|---|---|---|---|---|---|
| Barnes et al. (2014) |
Cohort retrospective |
188,764 veterans aged 55 years and over and dementia-free at baseline |
Medical record, using ICD-9-CM codes |
Medical record, using ICD-9-CM codes |
ACD, AD |
[37] |
| Gardner et al. (2014) |
Cohort retrospective |
51,799 adults aged 55 years and over |
Medical record, using ICD-9 codes |
Medical record, using ICD-9 codes |
ACD |
[38] |
| Lee et al. (2013) |
Cohort retrospective |
28,551 with mTBI 692,382 without mTBI |
National health insurance claims data; ICD-9 codes |
National health insurance claims data; ICD-9 codes |
ACD, AD |
[39] |
| Wang et al. (2012) |
Cohort retrospective, case–control |
44,925 with TBI 224,625 without TBI |
National health insurance longitudinal database; ICD-9-CM |
National health insurance longitudinal database; ICD-9-CM |
AD, VaD, dementia (unspecified) |
[40] |
| Suhanov et al. (2006) | Case–control | 260 patients with AD 260 matched controls |
Self-report; medical record | DSM-IV criteria for dementia; NINCDS-ADRDA criteria for AD | AD | [41] |
ACD: All-cause dementia; AD: Alzheimer's disease; ICD: International Statistical Classification of Diseases and Related Health Problems; LOC: Loss of consciousness; mTBI: mild TBI; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer's Disease and Related Disorders Association; TBI: Traumatic brain injury; VaD: Vascular dementia.
Two additional retrospective cohort studies [39,40] that found a positive relationship between TBI and dementia used the same national health insurance database but approached the analyses somewhat differently. The first of these studies [39] identified individuals who sought care for mTBI over a 3-year period, and examined risk for dementia over a 5-year period. Patients were excluded if they were diagnosed with dementia prior to follow-up or if they were ever hospitalized for TBI (implying a more severe injury), and dementia ascertainment required that patients had either a catastrophic illness certificate for dementia (which implies advanced disease) or were receiving pharmacological interventions for dementia. After controlling for demographics, medical co-morbidities and other risk factors, adjusted HR (95% CI) for dementia associated with mTBI was 3.26 (2.69–3.94) [39]. However, the average time between mTBI and dementia diagnosis was 1 year, suggesting reverse causation may at least partially explain results. The other study using this national health claims database [40] identified individuals who received care for a TBI of any severity over a 5-year period, identified matched comparison cases by age, sex and year of index (non-TBI) ambulatory care visit and tracked both groups for 5 years to identify those who were subsequently diagnosed with dementia. After controlling for demographics and select co-morbidities, the adjusted HR (95% CI) for dementia associated with TBI was 1.68 (1.57–1.80). Stratified analyses found the highest adjusted risk for dementia related to TBI (HR [95% CI]: 3.87 [1.98–7.57]) in the youngest (15–29 years) age group which may reflect enduring cognitive sequelae of severe TBI given the short duration of follow-up, followed by the oldest (75+ years) age group (95% CI): 2.60 (2.33–2.91) in which reverse causation may partially explain findings. The authors noted that AD was not the most common subtype of dementia diagnosed in cases with a history of TBI [40]. Moreover, in each of these four studies that compared individuals with and without TBI, individuals with TBI had significantly higher rates of co-morbid medical conditions at baseline [37–40].
The final study in this category included two groups of individuals living in nursing homes or other long-term care facilities: those with prevalent AD (based on validated consensus criteria) and a matched dementia-free comparison group [41]. People were excluded from the control group if they had a history of psychiatric disorder or alcohol/drug abuse, which, given the high co-occurrence of psychiatric and substance use disorders in individuals with TBI [42], may have actually excluded some TBI cases from this group. A history of TBI with LOC was queried via structured interview but had to be documented in the medical records, which may suggest that more recent TBIs were more likely to be ascertained. In a multivariate model that included all significant univariate predictors, TBI with LOC was significantly, albeit marginally, associated with an increased risk for AD (HR [95% CI]: 1.7 [1.0–2.8]) [41]. The authors noted that the results of this study of institutionalized older adults may not generalize to the community-dwelling population.
• Studies reporting no relationship between TBI & dementia
Two of the 11 retained papers concluded that there is no evidence of a relationship between TBI and AD (Table 2). The first study describes a population-based cohort study of randomly selected individuals over the age of 65 years who were dementia-free at baseline and were followed at 2-year intervals for an average of 7.4 years [43]. Lifetime history of TBI with LOC was gathered using a structured interview at baseline, and participants who showed cognitive decline from a prior study visit underwent a consensus-based diagnostic evaluation for dementia. Results showed that lifetime history of TBI with LOC was not associated with risk for incident dementia or incident AD in particular and there was no interaction between lifetime history of TBI with LOC and APOE ε4 in increasing dementia or AD risk after adjusting for age, sex and education [43]. Not even individuals who reported a recent TBI (i.e., at their last study visit) were at increased risk for dementia [43] in this study.
Table 2. . Studies finding no relationship between traumatic brain injury and Alzheimer's disease.
| Study (year) | Design | Sample size | TBI identification | Dementia identification | Type of dementia | Ref. |
|---|---|---|---|---|---|---|
| Dams-O'Connor et al. (2013) |
Cohort prospective |
4225 dementia-free individuals, 606 (14.3%) with history of TBI with LOC |
Self-report |
DSM-IV criteria for dementia; NINCDS-ADRDA criteria for AD |
Incident ACD, probable or possible AD |
[43] |
| Helmes et al. (2011) | Cohort prospective | 648 (23.5%) with TBI, 2115 (76.5%) without TBI | Medical record or self-report or informant report | Clinical consensus conference, DSM-III-R criteria | ACD | [44] |
ACD: All-cause dementia; AD: Alzheimer's disease; LOC: Loss of consciousness; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer's Disease and Related Disorders Association; TBI: Traumatic brain injury.
The second study describes another prospective cohort study of individuals who were over the age of 65 years and dementia-free at baseline [44]. TBI history was queried at baseline and was considered positive if a participant or informant reported a history of head injury with or without LOC, and on average the TBI occurred nearly 28-year prior, and 40% of TBI cases had not experienced LOC but some participants reported up to ten prior TBIs with LOC lasting up to 87 days. Dementia diagnosis was based on consensus conference criteria at 5-year follow-up. Results indicated that a history of TBI was not associated with an increased risk for dementia beyond the risk explained by age alone. These analyses were stratified by injury severity, and neither mild nor moderate–severe TBI improved prediction of dementia outcome after adjusting for age [44]. While these two studies boast large sample sizes and lengthy follow-up into late life, it is worth noting that people who died or became demented early in life (i.e., before the age of 65 years) would have been excluded.
• Studies reporting a conditional relationship between TBI & dementia
Four of the 11 retained papers found evidence of a conditional association between TBI and AD (Table 3). Papers were categorized into this group if a relationship to AD was supported only in subgroups of the study sample, or was supported in crude or partially adjusted models but not when additional covariates were included.
Table 3. . Studies finding a conditional relationship between traumatic brain injury and Alzheimer's disease.
| Study (year) | Design | Sample size | TBI identification | Dementia identification | Type of dementia | Ref. |
|---|---|---|---|---|---|---|
| Abner et al. (2014) |
Cohort prospective |
649 adults aged 60 years and over, 166 with history of TBI Among the 166 with TBI, 34 met criteria for MCI and 27 met criteria for dementia at follow-up |
Self-report |
Reduced performance on cognitive tests; medical records; AD-positive pathology |
AD |
[45] |
| Nordstrom et al. (2014) |
Cohort prospective |
811,622 men |
Medical record, using ICD-8, 9, 10 codes |
Medical record, using ICD-8, 9, 10 codes |
YOD/ACD |
[46] |
| Sundstrom et al. (2007) |
Cohort prospective |
543 adults aged 40–80 years, 181 developed dementia |
Self-report; medical record |
DSM-IV criteria for dementia |
ACD |
[47] |
| Sayed et al. (2013) | Case–control | 8381 adults with dementia, 7862 controls; 878 had history of TBI | Self-report | Consensus-based clinical diagnosis | AD, LBD, VaD, FTD | [48] |
ACD: All-cause dementia; AD: Alzheimer's disease; FTD: Frontotemporal dementia; ICD: International Statistical Classification of Diseases and Related Health Problems; LBD: Lewy body dementia; LOC: Loss of consciousness; TBI: Traumatic brain injury; VaD: Vascular dementia; YOD: Young onset dementia.
One paper described a prospective cohort study of 649 adults who were over the age of 60 years and dementia-free at baseline and followed annually [45]. Lifetime history of TBI with or without LOC was gathered via self-report during baseline and follow-up using a structured interview. History of TBI was not reported to be an independent predictor of dementia, but the interaction between TBI history and older age was significant for transition from normal cognition to MCI and also for transition from MCI to dementia. This study was somewhat unique in that 82 participants with a history of TBI died and underwent autopsy, and neuropathological data indicated that fewer than half of the cases who were clinically demented at death had neuropathologically confirmed AD, while remaining cases had mixed pathology [45].
The second paper described a case–control study using data from a population-based prospective longitudinal study of 543 individuals over the age of 40 years who were dementia-free at baseline and were re-evaluated within 5 years [47]. ACD was diagnosed by clinical evaluation conducted with all participants who showed evidence of cognitive impairment or decline on the Mini-mental State Examination at follow-up, and a control group included 2:1 age- and gender-matched non-demented subjects who scored 24 or higher on the Mini-mental State Examination. Lifetime history of TBI was determined at baseline based on subjects’ self-report of “head injury which required medical care”, and cases were excluded if there was an indication that the TBI occurred less than 5 years prior to a dementia diagnosis. The participants were subdivided by APOE ε4 carrier status; results indicated no increased risk for dementia among noncarriers but found a significant association between TBI and dementia risk for APOE ε4 carriers [47].
Another case–control study used data from the National Alzheimer's Coordinating Center Uniform Data Set to evaluate risk for dementia associated with TBI in 8381 adults with clinical consensus-based diagnosis of dementia and 7862 nondemented healthy controls. History of TBI was ascertained in structured interview at the baseline visit, and TBIs were categorized as resulting in either a brief LOC, extended LOC or ‘chronic deficit or dysfunction’. There was no increased risk for dementia associated with a TBI that resulted in a brief or extended LOC, but risk for ACD significantly increased when TBI resulted in chronic deficit or dysfunction (HR [95% CI]: 3.06 [1.83–5.1]). In additional analyses, the available neurobehavioral data for 62 individuals with ACD and a history of TBI resulting in chronic deficit or dysfunction were compared with 122 gender- and education-matched individuals with probable AD and no history of TBI. Those with chronic TBI were more likely to have symptoms of depression, agitation, irritability and motor dysfunction, which was interpreted as evidence that dementia following TBI differs in clinical presentation from typical AD [48].
The final paper in this category presents a large retrospective cohort study that examined the relationship between TBI and dementia risk among men who conscripted for military service at (on average) 18 years of age between 1969 and 1986 [46]. Given the young age of the sample at enrollment (national healthcare data were available through 2011), this study focused specifically on young onset dementia (YOD) associated with TBI. Characterization of TBI and dementia status after military conscription was based on the presence of diagnosis in the medical register. After adjusting for demographics, family history and select co-morbid conditions, results found that TBI (regardless of severity) was not associated with YOD of the Alzheimer's type. The authors did find increased risk for non-AD YOD associated with one or two mTBIs, and also with severe TBI, but these associations were markedly attenuated after adjustment for covariates (HRs [95% CI] ranged from 1.7 [1.2–2.3] for one mTBI to 2.6 [1.6–4.1] for severe TBI). Less than 1% of the sample was diagnosed with YOD during follow-up, and from a total sample of 811,622, there were only 14 cases of YOD among individuals with TBI. Thus, despite statistically significant HRs, the absolute risk for YOD attributable to TBI in this cohort is exceedingly low.
• Review papers commenting on the relationship between TBI & AD
Ten review articles met inclusion criteria for the current scoping review but did not directly evaluate the relationship between TBI and AD using new or archived data; these papers are summarized here. Six of these papers concluded that a relationship exists between TBI and AD. In these reviews, TBI is described as a prominent risk factor for AD [49–51], and authors conclude that TBI is associated with both higher frequency of AD and younger onset [52,53]. Age at injury and injury severity were also recognized contributors to increasing AD risk, with one paper concluding that even mTBI increases risk for dementia in adults over aged 65 years [54]. Three of the review articles suggested a conditional relationship between TBI and AD. One such paper concluded that moderate–severe TBI is associated with increased AD risk, but only mTBI with LOC (not mTBI without LOC) increases AD risk [55]. Another paper recognizes a possible link between TBI and dementia, but asserts that additional work is necessary to determine whether the dementia associated with TBI is consistent with clinically diagnosed AD or other types of dementia [56]. Other authors question whether a direct link exists between TBI and dementia, and instead suggest that a history of TBI in late life, combined with the brain changes associated with normal aging, might lead to more rapid cognitive decline in older adults [57]. The final review paper, the met inclusion criteria, was a systematic review which found insufficient evidence to draw any conclusion about a potential risk for dementia after mTBI [58].
Conclusion
Numerous studies have explored the relationship between TBI and AD over the past decade, and given the tremendous variability across studies examining the relationship between TBI and dementia, it is not at all surprising that their results are inconsistent. The results of the current scoping review are overall similar to the conclusions of the IOM report [31]. In this scoping review of the literature from 2005 to 2015, there is further evidence that moderate–severe TBI is associated with increased risk for dementia, and particularly AD, and some indication that mTBI may be associated with dementia risk. Overall, the magnitude of risk for dementia associated with TBI varies across studies, and adjusting for known demographic and clinical dementia risk factors tends to attenuate the relationship considerably.
The current review highlights several key methodological factors that may influence the significance and/or strength of the relationship between TBI and dementia. First, TBI diagnosis ascertainment methods vary considerably across studies. Research studies that rely on medical records or national health registries for TBI case identification are undoubtedly undercounting the true prevalence and/or incidence of TBI, as evidence suggests nearly 30% of people who sustained a TBI never sought medical care [59]. Studies that rely on a single-item self-report question about TBI history are similarly likely to identify only a fraction of lifetime TBIs that might be elicited by a more comprehensive TBI screening method [60]. Given the evidence that dementia risk may increase with injury severity and frequency, a detailed account of the number, timing and severity of lifetime TBI exposure is essential. Dementia ascertainment methods are similarly variable across studies, and using medical records or self-report for case identification (as opposed to clinical consensus-based dementia diagnosis) likely results in undercounts and/or misclassification of dementia subtype.
Age at injury appears to be influential in dementia risk [54]; there is evidence that even milder injuries sustained in late life may increase dementia risk, and reverse causality may at least partially explain this finding. Additionally, several studies have found that individuals with a history of TBI experienced significantly more co-morbid medical conditions at baseline compared with uninjured controls, which is consistent with a growing body of research describing the chronic health consequences of TBI [61,62]. Of course, many of these chronic medical conditions are well-known independent risk factors for dementia, and disease burden also increases the risk for premature death. It is therefore not surprising that when dementia risk models are adjusted for medical co-morbidities, the risk associated with TBI tends to be weakened. However, it is worth considering the implications of adjusting for conditions that may represent early or prodromal signs of dementia (such as cerebrovascular disease or depression), or of adjusting for conditions that may represent downstream or secondary consequences of TBI such as metabolic or endocrine disorders. Further research is required to better understand the ways in which disease burden impacts and interacts with dementia risk among survivors of TBI.
Another important factor to consider in evaluating the relationship between TBI and dementia is study inclusion and exclusion criteria. It may seem reasonable to exclude people from a ‘healthy control’ group who achieve low scores on cognitive screening tests in an effort to rule out preclinical dementia cases, but this could exclude nondemented patients who have TBI-related cognitive impairments. Similarly, given the preponderance of substance abuse and psychiatric illness among TBI survivors [42], excluding people with such histories from a nondemented control group may also preferentially exclude nondemented TBI patients from the study.
Finally, the studies reported here vary in the subtype(s) of dementia considered, and a steadily growing body of evidence suggests that post-TBI neurodegeneration may represent a distinct disease process that is clinically [48,63] and pathologically [45–46,56] distinct from AD. The enduring cognitive sequelae of moderate–severe TBI may mask further late-life decline and may contribute to diagnostic uncertainty when individuals with TBI undergo consensus-based dementia evaluations [63]. Data from the National Alzheimer's disease Coordinating Center database suggest that despite meeting consensus-based diagnostic criteria, the clinical profiles of older adults with dementia differ among demented adults with and without a history of TBI, suggesting that when TBI does result in dementia it may be distinguishable from known dementia subtypes, including AD [48,63]. Clinical criteria for the diagnosis of dementia due to TBI are poorly defined and clinical diagnostic criteria for CTE or ‘TBI-associated dementia’ do not yet exist, so it is possible that delayed post-TBI cognitive and functional decline may be misclassified as AD in part due to the absence of a more appropriate diagnostic label. Pathological data gathered from individuals who survive more than a year after moderate–severe TBI increasingly suggest that post-TBI neurodegeneration is best described as a polypathology [64]; in other words, multiple distinct pathological processes may underlie and contribute to the late-life neurodegeneration experienced by some TBI survivors. As discussed above, research has also demonstrated increased levels of hyperphosphorylated tau protein after severe TBI [22,65], hypoxia-related changes in gene expression [66], injury-related vascular damage [67], chronically upregulated systemic inflammatory markers [68,69] and other potentially relevant processes [70], each of which can contribute to distinct neurodegenerative pathological processes that may evolve over time after TBI. Indeed, the largest study to date on late-life TBI outcomes was published in July 2016; this study pooled data from 7130 adults over the age of 65 years who were alive and dementia-free at the time of entry into prospective longitudinal cohort studies to evaluate associations between TBI and late-life clinical dementia outcomes and neuropathological findings for those with autopsy data [71]. There was no relationship between TBI with LOC and clinically diagnosed AD, but there was a strong association between TBI with LOC and Parkinson's disease. Neuropathological findings in 1652 autopsy cases showed no association between TBI with LOC and Aβ plaques or NFTs, the hallmark indicators of AD, though there was an increased risk for Lewy bodies and cerebral microinfarcts among cases with a history of TBI [71]. The results of this recent large-scale study suggest that TBI does appear to be associated with late-life clinical and neuropathological outcomes, as concluded by several of the studies included in this review, but that the late-life neurodegeneration seen in some TBI survivors is something other than AD-type dementia. It is clear that more research is needed to define and validate diagnostic criteria for the clinical and neuropathological signatures of post-TBI neurodegeneration.
Future perspective
Uncertainty surrounding the ability to recognize and diagnose post-TBI neurodegeneration highlights a key gap in current knowledge. TBI is a disease that manifests and evolves with tremendous heterogeneity across individuals over time, and at the present time we are unable to classify subtypes of this disease in a meaningful way. Long-term neuropathologic effects of TBI are largely unknown, particularly as they relate to the development of neurodegenerative processes. As such, further research is required to better understand the underlying mechanisms and exacerbating factors (particularly those risk factors that may be modifiable) of late or delayed neurodegeneration following TBI. Postmortem neuropathological data in well-characterized clinical TBI samples are essential to understanding the population-based prevalence of post-TBI neurodegeneration, its relationships to known dementia subtypes and factors that may modify risk or protection. Future research in this area can inform the development of disease-modifying interventions that align with clinical and neuropathological subtypes of chronic TBI.
Despite the inconsistent conclusions across studies with regard to the relationship between TBI and AD, results of the current review highlight potential opportunities for intervention, prevention and improved clinical management of older adults living with a history of TBI. Several factors that increase risk for dementia among TBI survivors, such as medical co-morbidity, substance use and psychiatric illness, can and should be treated or managed using existing empirically supported clinical management protocols. It is important to consider that TBI-related cognitive, behavioral and emotional symptoms may negatively impact a person's ability to manage one's health independently, which can lead to health decline over time. To the extent possible, clinicians and others caring for individuals with TBI can help overcome these challenges by providing reminders for appointments and follow-up care, writing down recommendations and instructions and involving trusted others in healthcare decisions when appropriate. In addition, evidence that late-life TBI is associated with increased risk for dementia, together with the finding that lifetime history of TBI may account for nearly 20% of the population-attributable risk for late-life TBI [72], suggests that older adults with a history of TBI may benefit from targeted fall-prevention programs. Finally, the expanded interest and accelerated pace of research on the association between TBI and dementia over the past 10 years may provide some reassurance to TBI survivors and their caregivers, as growth in this field reflects an unprecedented investment in understanding and improving the long-term outcomes of TBI.
Limitation
The current paper shares results of a scoping review of the literature published in the past 10 years on the relationship between TBI and AD with a goal of synthesizing current knowledge, identifying gaps in that knowledge and highlighting clinical implications of the available literature. There are some limitations to this manuscript that warrant consideration. A scoping review does not evaluate the quality of the primary studies, which may limit the usefulness of the review, if poor-quality studies are not excluded. In the current paper, we have addressed this concern by highlighting the methodological nuances that may account for inconsistent findings across studies. It is also possible that relevant research on the relationship between TBI and AD was not included in the current review as our search methodology was not exhaustive and did not include all available scientific databases, dissertations, conference proceedings or book chapters.
Footnotes
Financial & competing interests disclosure
K Dams-O'Connor conducts research on the comprehensive clinical course of brain injury funded by Grant # K01HD074651-01A1 from the NIH, National Institute of Child Health and Development. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Millspaugh JA. Dementia pugilistica. US Nav. Med. Bull. 1937;35:297–303. [Google Scholar]
- 2.Martland HS. Punch drunk. JAMA. 1928;91(15):1103–1107. [Google Scholar]
- 3.Omalu BI, Dekosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 2006;59(5):1086–1092. doi: 10.1227/01.NEU.0000245601.69451.27. [DOI] [PubMed] [Google Scholar]
- 4.Omalu BI, Dekosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- 5.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130(6):877–889. doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omalu BI, Fitzsimmons RP, Hammers J, Bailes J. Chronic traumatic encephalopathy in a professional American wrestler. J. Forensic Nurs. 2010;6(3):130–136. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 8.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claude H, Cuel J. Demence pre-senile post-traumatique apres fracture de crane: considerations medicos-legales. Annales Medico-Legales. 1939;19:173–184. [Google Scholar]
- 10.Rudelli R, Strom JO, Welch PT, Ambler MW. Posttraumatic premature Alzheimer's disease. Neuropathologic findings and pathogenetic considerations. Arch. Neurol. 1982;39(9):570–575. doi: 10.1001/archneur.1982.00510210040009. [DOI] [PubMed] [Google Scholar]
- 11.Molgaard CA, Stanford EP, Morton DJ, Ryden LA, Schubert KR, Golbeck AL. Epidemiology of head trauma and neurocognitive impairment in a multi-ethnic population. Neuroepidemiology. 1990;9(5):233–242. doi: 10.1159/000110778. [DOI] [PubMed] [Google Scholar]
- 12.Mortimer JA, French LR, Hutton JT, Schuman LM. Head injury as a risk factor for Alzheimer's disease. Neurology. 1985;35(2):264–267. doi: 10.1212/wnl.35.2.264. [DOI] [PubMed] [Google Scholar]
- 13.Mortimer JA, Van Duijn CM, Chandra V, et al. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case–control studies. EURODEM Risk Factors Research Group. Int. J. Epidemiol. 1991;20(Suppl. 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 14.Graves AB, White E, Koepsell TD, et al. The association between head trauma and Alzheimer's disease. Am. J. Epidemiol. 1990;131(3):491–501. doi: 10.1093/oxfordjournals.aje.a115523. [DOI] [PubMed] [Google Scholar]
- 15.O'Meara ES, Kukull WA, Sheppard L, et al. Head injury and risk of Alzheimer's disease by apolipoprotein E genotype. Am. J. Epidemiol. 1997;146(5):373–384. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 16.Alzheimer's Association. What We Know Today About Alzheimer's Disease and Dementia. 2016. www.alz.org/research/science/alzheimers_research.asp
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. American Psychiatric Press, Inc.; Washington, DC, USA: 2000. [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease? Nat. Rev. Neurosci. 2010;11(5):361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raby CA, Morganti-Kossmann MC, Kossmann T, et al. Traumatic brain injury increases beta-amyloid peptide 1–42 in cerebrospinal fluid. J. Neurochem. 1998;71(6):2505–2509. doi: 10.1046/j.1471-4159.1998.71062505.x. [DOI] [PubMed] [Google Scholar]
- 21.Kay AD, Petzold A, Kerr M, Keir G, Thompson E, Nicoll JA. Alterations in cerebrospinal fluid apolipoprotein E and amyloid beta-protein after traumatic brain injury. J. Neurotrauma. 2003;20(10):943–952. doi: 10.1089/089771503770195795. [DOI] [PubMed] [Google Scholar]
- 22.Tsitsopoulos PP, Marklund N. Amyloid-beta peptides and tau protein as biomarkers in cerebrospinal and interstitial fluid following traumatic brain injury: a review of experimental and clinical studies. Front. Neurol. 2013;4:79. doi: 10.3389/fneur.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta KM, Ott A, Kalmijn S, et al. Head trauma and risk of dementia and Alzheimer's disease: the Rotterdam study. Neurology. 1999;53(9):1959–1962. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 24.Katzman R, Aronson M, Fuld P, et al. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann. Neurol. 1989;25(4):317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- 25.Williams DB, Annegers JF, Kokmen E, O'Brien PC, Kurland LT. Brain injury and neurologic sequelae: a cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology. 1991;41(10):1554–1557. doi: 10.1212/wnl.41.10.1554. [DOI] [PubMed] [Google Scholar]
- 26.Chandra V, Philipose V, Bell PA, Lazaroff A, Schoenberg BS. Case–control study of late onset “probable Alzheimer's disease”. Neurology. 1987;37(8):1295–1300. doi: 10.1212/wnl.37.8.1295. [DOI] [PubMed] [Google Scholar]
- 27.Amaducci LA, Fratiglioni L, Rocca WA, et al. Risk factors for clinically diagnosed Alzheimer's disease: a case–control study of an Italian population. Neurology. 1986;36(7):922–931. doi: 10.1212/wnl.36.7.922. [DOI] [PubMed] [Google Scholar]
- 28.Broe GA, Henderson AS, Creasey H, et al. A case–control study of Alzheimer's disease in Australia. Neurology. 1990;40(11):1698–1707. doi: 10.1212/wnl.40.11.1698. [DOI] [PubMed] [Google Scholar]
- 29.Ferini-Strambi L, Smirne S, Garancini P, Pinto P, Franceschi M. Clinical and epidemiological aspects of Alzheimer's disease with presenile onset: a case–control study. Neuroepidemiology. 1990;9(1):39–49. doi: 10.1159/000110750. [DOI] [PubMed] [Google Scholar]
- 30.Launer LJ, Andersen K, Dewey ME, et al. Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999;52(1):78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 31.Health IOM COGWA. Gulf War and Health. National Academies Press; Washington, DC, USA: 2008. Long-term consequences of traumatic brain injury, Volume 7. [PubMed] [Google Scholar]
- 32.Administration on Aging. Aging statistics. 2016. http://www.aoa.acl.gov/Aging_Statistics/index.aspx
- 33.Coronado VG, McGuire LC, Sarmiento K, et al. Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J. Safety Res. 2012;43(4):299–307. doi: 10.1016/j.jsr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Faul M, Xu L, Wald MM, Coronado VG. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention Control; 2010. Traumatic brain injury in the united states: emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- 35.Arksey H, O'malley L. Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. 2005;8(1):19–32. [Google Scholar]
- 36.Dijkers M. What is a scoping review? KT Update. 2015 ktdrr.org/products/update/v4n1/dijkers_ktupdate_v4n1_12-15.pdf [Google Scholar]
- 37.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71(12):1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS ONE. 2013;8(5):e62422. doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HK, Lin SH, Sung PS, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J. Neurol. Neurosurg. Psychiatr. 2012;83(11):1080–1085. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- 41.Suhanov AV, Pilipenko PI, Korczyn AD, et al. Risk factors for Alzheimer's disease in Russia: a case–control study. Eur. J. Neurol. 2006;13(9):990–995. doi: 10.1111/j.1468-1331.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 42.Rogers JM, Read CA. Psychiatric comorbidity following traumatic brain injury. Brain Inj. 2007;21(13–14):1321–1333. doi: 10.1080/02699050701765700. [DOI] [PubMed] [Google Scholar]
- 43.Dams-O'Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J. Neurol. Neurosurg. Psychiatry. 2013;84(2):177–182. doi: 10.1136/jnnp-2012-303938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helmes E, Ostbye T, Steenhuis RE. Incremental contribution of reported previous head injury to the prediction of diagnosis and cognitive functioning in older adults. Brain Inj. 2011;25(4):338–347. doi: 10.3109/02699052.2011.556104. [DOI] [PubMed] [Google Scholar]
- 45.Abner EL, Nelson PT, Schmitt FA, et al. Self-reported head injury and risk of late-life impairment and AD pathology in an AD center cohort. Dement. Geriatr. Cogn. Disord. 2014;37(5–6):294–306. doi: 10.1159/000355478. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this study, participants with traumatic brain injury (TBI) who died underwent autopsy; neuropathological data indicated that less than half of cases who were clinically demented at death had neuropathologically confirmed Alzheimer's disease (AD), while remaining cases had mixed pathology.
- 46.Nordström P, Michaëlsson K, Gustafson Y, Nordström A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann. Neurol. 2014;75(3):374–381. doi: 10.1002/ana.24101. [DOI] [PubMed] [Google Scholar]; • In this study, TBI was associated with increased risk of young onset non-AD dementia regardless of severity; however, this association was markedly attenuated by covariates (e.g., demographics, family history and selected co-morbid conditions) and the overall risk for young onset dementia following TBI was low.
- 47.Sundstrom A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int. Psychogeriatr. 2007;19(1):159–165. doi: 10.1017/S1041610206003498. [DOI] [PubMed] [Google Scholar]; • In this study, TBI was associated with all-cause dementia when the ApoE ε4 genotype was present, but those with TBI who did not carry to ApoE ε4 genotype were not at increased risk for dementia.
- 48.Sayed N, Culver C, Dams-O'Connor K, Hammond F, Diaz-Arrastia R. Clinical phenotype of dementia after traumatic brain injury. J. Neurotrauma. 2013;30(13):1117–1122. doi: 10.1089/neu.2012.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this study, TBI was associated with increased risk for all-cause dementia only when TBI resulted in loss of consciousness and chronic deficits or dysfunction. Moreover, those with chronic TBI were more likely to have a clinical presentation that differs from typical AD.
- 49.Anstey KJ, Cherbuin N, Herath PM. Development of a new method for assessing global risk of Alzheimer's disease for use in population health approaches to prevention. Prev. Sci. 2013;14(4):411–421. doi: 10.1007/s11121-012-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chauhan NB. Chronic neurodegenerative consequences of traumatic brain injury. Restor. Neurol. Neurosci. 2014;32(2):337–365. doi: 10.3233/RNN-130354. [DOI] [PubMed] [Google Scholar]
- 51.Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog. Brain Res. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- 52.Filer W, Harris M. Falls and traumatic brain injury among older adults. N. C. Med. J. 2015;76(2):111–114. doi: 10.18043/ncm.76.2.111. [DOI] [PubMed] [Google Scholar]
- 53.Gardner RC, Yaffe K. Traumatic brain injury may increase risk of young onset dementia. Ann. Neurol. 2014;75(3):339–341. doi: 10.1002/ana.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson VE, Stewart W. Traumatic brain injury: age at injury influences dementia risk after TBI. Nat. Rev. Neurol. 2015;11(3):128–130. doi: 10.1038/nrneurol.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J. Head Trauma Rehabil. 2009;24(6):439–451. doi: 10.1097/HTR.0b013e3181c15600. [DOI] [PubMed] [Google Scholar]
- 56.Daneshvar DH, Riley DO, Nowinski CJ, McKee AC, Stern RA, Cantu RC. Long-term consequences: effects on normal development profile after concussion. Phys. Med. Rehabil. Clin. N. Am. 2011;22(4):683–700. ix. doi: 10.1016/j.pmr.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11(12):1103–1112. doi: 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
- 58.Godbolt AK, Cancelliere C, Hincapie CA, et al. Systematic review of the risk of dementia and chronic cognitive impairment after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 2014;95(3 Suppl.):S245–S256. doi: 10.1016/j.apmr.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 59.Whiteneck GG, Cuthbert JP, Corrigan JD, Bogner JA. Risk of negative outcomes after traumatic brain injury: a statewide population-based survey. J. Head Trauma Rehabil. 2016;31(1):E43–E54. doi: 10.1097/HTR.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 60.Dams-O'Connor K, Cantor JB, Brown M, Dijkers MP, Spielman LA, Gordon WA. Screening for traumatic brain injury: findings and public health implications. J. Head Trauma Rehabil. 2014;29(6):479–489. doi: 10.1097/HTR.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masel BE, Dewitt DS. Traumatic brain injury: a disease process, not an event. J. Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 62.Masel BE. The chronic consequences of neurotrauma. J. Neurotrauma. 2015;32(23):1833. doi: 10.1089/neu.2015.29004.bm. [DOI] [PubMed] [Google Scholar]
- 63.Dams-O'Connor K, Spielman L, Hammond FM, Sayed N, Culver C, Diaz-Arrastia R. An exploration of clinical dementia phenotypes among individuals with and without traumatic brain injury. NeuroRehabilitation. 2013;32(2):199–209. doi: 10.3233/NRE-130838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 2013;9(4):211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22(2):142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci. Biobehav. Rev. 2012;36(5):1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Franzblau M, Gonzales-Portillo C, Gonzales-Portillo GS, et al. Vascular damage: a persisting pathology common to Alzheimer's disease and traumatic brain injury. Med. Hypotheses. 2013;81(5):842–845. doi: 10.1016/j.mehy.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giunta B, Obregon D, Velisetty R, Sanberg PR, Borlongan CV, Tan J. The immunology of traumatic brain injury: a prime target for Alzheimer's disease prevention. J. Neuroinflammation. 2012;9:185. doi: 10.1186/1742-2094-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(Pt 1):28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson VE, Stewart W, Trojanowski JQ, Smith DH. Acute and chronically increased immunoreactivity to phosphorylation-independent but not pathological TDP-43 after a single traumatic brain injury in humans. Acta Neuropathol. 2011;122(6):715–726. doi: 10.1007/s00401-011-0909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crane PK, Gibbons LE, Dams-O'Connor K, et al. Association between traumatic brain injury and late life neurodegenerative conditions and neuropathological findings. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.1948. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dams-O'Connor K, Gibbons LE, Landau A, Larson EB, Crane PK. Health problems precede traumatic brain injury in older adults. J. Am. Geriatr. Soc. 2016;64(4):844–848. doi: 10.1111/jgs.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]