| Summary: |
The invention in this patent application relates to 6,6-difluoro-1-oxo-octahydroisobenzofuran derivatives represented generally by formula (I). These compounds possess activities as PAR-1 antagonists and may provide useful treatments for diseases such as acute coronary syndrome (ACS) and peripheral artery disease (PAD) as well as inhibition of platelet aggregation. |
| The protease-activated receptors (PAR-1, 2, 3, and 4) are members of the seven transmembrane G-protein-coupled receptor superfamily. They are expressed throughout the body and can be activated by the action of serine proteases such as thrombin and trypsin. |
| Thrombin performs a variety of activities in different cell types. For example, its signaling in platelets contributes to hemostasis and thrombosis. PAR-1 receptors are known to exist in cell types such as platelets, vascular smooth muscle cells, endothelial cells, and fibroblasts. They have been implicated in the pro-inflammatory response observed in atherosclerosis and restenosis as well as in muscle growth and bone cell differentiation and proliferation. Therefore, PAR-1 receptor antagonists can potentially be useful for the treatment of thrombotic, inflammatory, atherosclerotic, and fibroproliferative disorders, as well as other disorders in which thrombin and PRA-1 play pathological roles. |
| Cannabinoid receptors are members of the superfamily of G-protein coupled receptors. These receptors function through modulating adenylate cyclase and Ca2+ and K+ currents. They include two kinds, the cannabinoid 1 receptors (CB-1) and cannabinoid 2 receptors (CB-2). Activities of CB-1 receptors are associated mainly with the central nervous system, but CB-2 receptors show peripheral effects related to bronchial constriction, immunomodulation, and inflammation. Studies suggest that selective CB-2 receptor binding agents may potentially have therapeutic utilities in controlling diseases associated with rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, diabetes, osteoporosis, renal ischemia, cerebral stroke, cerebral ischemia, nephritis, inflammatory disorders of the lungs and gastrointestinal tract, and respiratory tract disorders such as reversible airway obstruction, chronic asthma, and bronchitis. |
The piperidine alkaloid himbacine has been identified as a muscarinic receptor antagonist, specifically for the muscarinic acetylcholine receptor M2.
|
| |
Researchers have prepared several himbacine-related bi- and tricyclic structures, mostly through modifications in rings A and D of himbacine. Many of these compounds were identified as thrombin receptor antagonists that can potentially treat thrombin receptor mediated disorders including thrombosis, atherosclerosis, restenosis, hypertension, angina pectoris, angiogenesis related disorders, arrhythmia, heart failure, ACS, myocardial infarction, glomerulonephritis, thrombotic stroke, thromboembolytic stroke, PAD, deep vein thrombosis, venous thromboembolism, disseminated intravascular coagulation syndrome, and cerebral infarction. In addition, there is an indication that these himbacine-related compounds may also function as inhibitors of the CB-2 receptors, and accordingly, they may potentially provide treatments for CB-2 receptor mediated disorders. |
| The inventors mentioned several of the known himbacine-related PAR-1 receptor antagonists including vorapaxar, which is a potent and selective thrombin receptor antagonist. However, in spite of the relatively large number of known PAR-1 receptor antagonists, there is still a need for new antagonists with improved therapeutic profiles such as desirable half-life and reduced drug–drug interactions that can provide better treatments for diseases associated with both PAR-1 and CB2 receptors. The compounds of formula (I) described in this patent application are inhibitors of the PAR-1 receptor. In addition, based upon their structures, they may also possess activities as inhibitors of the CB-2 receptor. Therefore, these compounds are potentially useful as treatments for diseases associated with the inhibition of these two receptors. |
| Important Compound Classes: |
 |
| Key Structures: |
The inventors described the structures and synthesis of 13 compounds of formula (I) including the following representative examples:
|
| Biological Assay: |
|
| Biological Data: |
The biological data from testing the above representative examples are listed in the following table: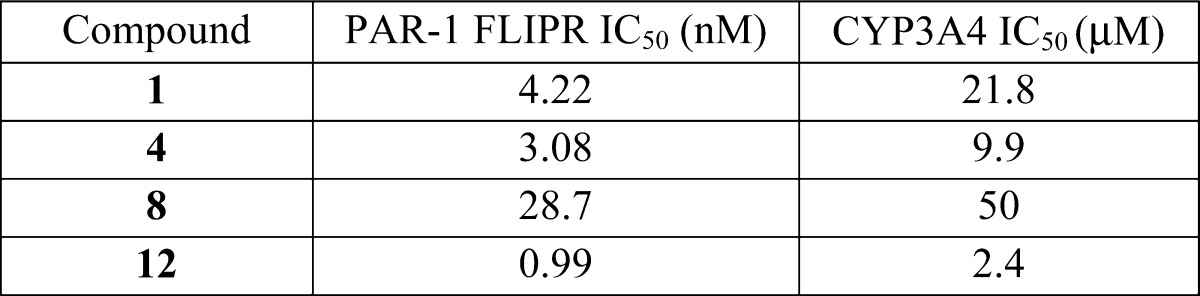
|
| Recent Review Articles: |
1. Gao F.; Shen H.; Wang Z. J.; Yang S. W.; Liu X. L.; Zhou Y. J.. Thromb. Res. 2015, 136 ( (2), ), 243–249. |
| 2. Cui H.; Tan W.; Shi J.; Xia Y.. Open J. Med. Chem. 2012, 2 ( (4), ), 112–118. |
| 3. Capodanno D.; Bhatt D. L.; Goto S.; O’Donoghue M. L.; Moliterno D. J.; Tamburino C.; Angiolillo D. J.. J. Thromb. Hemost. 2012, 10 ( (10), ), 2006–2015. |






