Abstract
This contribution reports the synthesis of some novel bioconjugates with anticancer activity and able to release nitric oxide (NO) under visible light excitation. The 4-nitro-2-(trifluoromethyl)aniline derivative, a suitable NO photodonor, was conjugated with 2′-deoxyadenosine and urso- and cheno-deoxycholic acid derivatives, through a thioalkylic chain or the 4-alkyl-1,2,3-triazole moiety. Photochemical experiments demonstrated the effective release of NO from 2′-deoxyadenosine and ursodeoxycholic acid conjugates under the exclusive control of visible light inputs. Studies for the in vitro antiproliferative activity against leukemic K562 and colon carcinoma HCT116 cell lines are reported for all the compounds as well as a case study of photocytotoxicity against HCT116.
Keywords: NO photorelease, 2′-deoxyadenosine conjugates, bile acid conjugates, photochemotherapeutics
The biological relevance of nitric oxide (NO) in many bioregulatory systems such as neurotransmission, vasodilatation, hormone secretion, and immune stimulation is nowadays well-established.1,2 Recent studies demonstrated that NO is a very efficient antimicrobial and antioxidant agent that can be employed to tackle bacterial infections and cardiovascular diseases.3,4 Moreover NO can be used as an anticancer agent or as a chemosensitizer enhancing the effect of traditional cancer therapies.3−7 In this view, some conventional drugs may be in principle modified with a NO releasing moiety in order to produce synergistic effects due to the combined active species. Although several therapies are based on the regulation of NO synthase activity responsible for the endogenous NO concentration,7−9 there is an increasing interest in innovative therapies based on exogenous NO releasing agents.10 The effect of a NO releasing drug is strongly related to the concentration and dose of NO delivered. For instance, NO concentrations in the micromolar range inhibit the growth of tumor cells, whereas picomolar NO concentrations encourage cell proliferation.11−13 Strategies to control the NO release can be based on the use of an external trigger that allow the NO release under a specific stimulus (luminous, chemical, or electrochemical).14,15 Among these, light is the most appealing due to its peculiar advantages of not perturbing the physiological values of parameters such as temperature, pH, and ionic strength, fundamental prerequisites for biomedical applications.16 These unique features make photoactivated NO donors (photocages) a powerful arsenal in the burgeoning field of nanomedicine with intriguing potential to tackle cancer diseases in a noninvasive way.17−20 In the last years we have developed a number of molecular and supramolecular systems with anticancer activity based on the 1-nitro-2-(trifluoromethyl)aniline as suitable photocage releasing NO upon irradiation with visible light.16,17,21−23 The mechanism of NO release involves a nitro-to-nitrite photorearrangement followed by the rupture of the O–NO bond with formation of NO and a phenoxy radical intermediate.21 Our interest in the study of bioconjugate functional compounds for biosensoring and pharmacological applications prompted us to scout new molecules integrating a photocaged NO radical with 2′-deoxyadenosine and bile acid derivatives in the same molecular skeleton. This was motivated by the already assessed cytotoxic activity of 2′-deoxyadenosine and bile acid based compounds as well as on their chemical properties. It is well established that modified 2′-deoxyadenosines are important cytotoxic agents against lymphoid and myeloid tumors.24,25 Bile acid derivatives, with particular regard to chenodeoxycholic (CDC) and ursodeoxycholic (UDC) bile acids, are well-known to inhibit the growth cells and to induce apoptosis in many human cancer cell lines.26−29 From the chemical point of view, 2′-deoxyadenosine can be modified at C-8 position keeping unchanged its intrinsic characteristic of recognition of natural nucleic acids through specific hydrogen bond patterns (Watson–Crick and Hoogsteen). However, the intrinsic properties of bile acids such as rigidity, chirality, amphiphilicity, etc., can be fine-tuned. Moreover, 2′-deossiadenosine/bile acid conjugates embodying the 1,2,3-triazole ring as well as a S-alkyl unit were found to exhibit a good antiproliferative activity selectively toward both leukemic T Jurkat and K562 cells.30 Along these lines, we adopted similar molecular skeletons but we changed one by one the 2′-deoxyadenosine and bile acid derivatives with a photocage unit.
Therefore, we designed the synthesis of some novel bioconjugates of 4-nitro-3-(trifluoromethyl)-aniline with 2′-deoxyadenosine, UDC and CDC bile acid moieties, namely, photocage-SdAdo, photocage-UDC, and photocage-CDC that can be considered as progenitors of large variety of new bioconjugates and, combining the intrinsic characteristics of the biological components with that of NO could be powerful tools in cancer therapy. Photochemical studies on the effective release of NO are also reported. A biological screening for the cytotoxic effects toward a selection of two cancer cell lines, leukemic K562 and colon carcinoma HCT116, as well as the normal fibroblast skin cells was reported together with a photobiological study on photocage-SdAdo toward the HCT116 cancer cell line.
Recently we have set up a new methodology for the preparation of 2′-deoxyadenosine modified at C-8 position enabling the conjugation with a variety of functional molecules such as chromophores fluorophores, intercalators, alkylating agents, and functional groups for the click chemistry.31,32 The 2′-deoxyadenosine derivatives synthesized have been demonstrated to be quite powerful derivatives for further applications.30,31,33 Following the procedure previously described31,32 the conjugate photocage-SdAdo was prepared by reaction between the commercial 8-bromo-2′-deoxyadenosine 1 and 5-(4-nitro-3-trifluoromethyl)phenylamino-pentane-1-thiol 2 through a reaction in water mediated by triethylamine (TEA) (Scheme 1, part A). The reaction was performed in a sealed tube at 100 °C for 2 h. A simple and very efficient work up based on the extraction of the warm reaction mixture with ethyl acetate led to the pure target bioconjugate photocage-SdAdo in 20% yield. The thiol 2 was prepared through a multistep synthesis as depicted in Scheme 1, part C, starting from commercial 4-chloro-1-nitro-2- trifluoromethyl-benzene 5 in 34% overall yield. Starting from the same nitrobenzene derivative 5 the terminal alkyne 4 was obtained in good yield (Scheme 1, part C; for details see SI).
Scheme 1. Synthesis.
Reagents and conditions: (a) H2O, TEA, 100 °C, 2 h (20% yield); (b) CuSO4·5H2O, sodium ascorbate, THF/tBuOH/H2O (1.5:1:1), microwaves: 80 °C, 30 min (70–80% yield); (c) DMSO, K2CO3, 85 °C, 24 h (90% yield); (d) DCM, 20 °C, 16 h (50% yield); (e) DMSO, 50 °C, 16 h (80% yield); (f) MeOH, −78 °C, 4 h (95% yield); (g) DMSO, K2CO3, 85 °C, 24 h (65% yield).
The synthesis of photocage-CDC and photocage-UDC was performed via a Cu(I) mediated 1,3-dipolar cycloaddition reaction starting from the appropriate 3-azidobile acid 3a,b with the alkyne 4 (Scheme 1, part B).30 In both cases the reaction performed by reacting under microwave irradiation at 80 °C for 30 min, a 1:1.5:0.4:2 molar ratio of the alkyne 4, the appropriate azide 3a,b, CuSO4·5H2O, and sodium ascorbate, respectively, in THF/tBuOH/H2O mixture (1.5:1:1, v/v) provided the target compound in 70–80% yield after chromatographic purification.
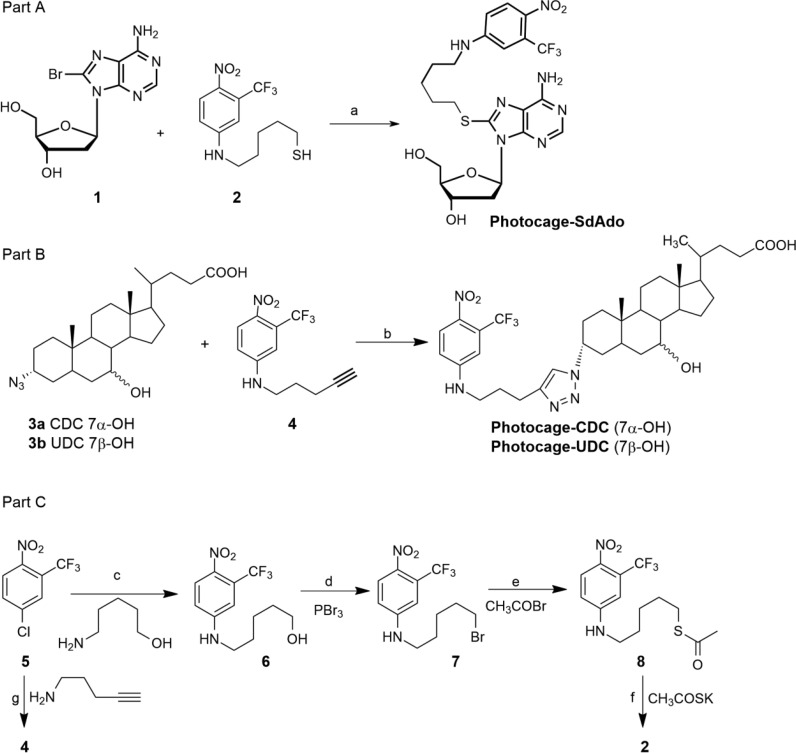
The photochemical experiments were focused on photocage-SdAdo and photocage-UDC. Photocage-CDC was not studied in consideration of its high cytotoxicity against normal fibroblast cells at low micromolar concentrations (see Table 1). Figure 1 shows the absorption spectra of photocage-SdAdo and photocage-UDC. As expected, the nitroaniline chromogenic unit strongly dominates the absorption in the visible range, whereas the UV region is characterized by the typical absorption band of the deoxyadenosine fragment in the case of photocage-SdAdo.
Table 1. IC50 Values after 72 h Incubation Time in the Dark and upon Irradiationa.
| IC50 (μM) |
||||
|---|---|---|---|---|
| compound | K562 | HTC116 | fibroblast | |
| dark | photocage-CDC | 24.0 ± 1.2 | 21.0 ± 0.8 | 32.0 ± 1.0 |
| photocage-UDC | 31.6 ± 1.6 | 14.0 ± 1.0 | ≫100 | |
| photocage-SdAdo | 68.1 ± 1.3 | 66.2 ± 3.3 | ≫100 | |
| light | photocage-SdAdo | 31.0 ± 2.5 | ||
| cisplatin | 5.40 ± 1.0 | 8.5 ± 1.2 | 25.4 ± 3.5 | |
IC50 values were determined from the dose–response curves using MTT assay after 72 h incubation time in the dark and also upon irradiation in the case of photocage-SdAdo. Results are expressed as the mean of three independent experiments ± SD. Cisplatin was used as a reference compound.
Figure 1.
Absorption spectra of photocage-SdAdo (solid) and photocage-UDC (dotted) in phosphate buffer (10 mM, pH = 7.4) with DMSO 0.5%.
Note that the molar absorbivity of the visible band for both conjugates is ca. 10.000 M–1 cm–1. This value is basically the same to that already reported for the NO photocage alone,21,34 ruling out any significant interaction, in the ground state, between the nitroaniline chromophores and the other functional molecular components in both photocage-SdAdo and photocage-UDC. To exploit the cytotoxicity effects of NO along with those of 2′-deoxyadenosines and bile acids, the NO photoreleasing capability of the NO photocage needs to be preserved after its covalent conjugation. Note that, in contrast to nonphotoresponsive compounds, the preservation of the photobehavior of a photoactive center after its covalent linking with other molecular components is not a “trivial result”. In some cases, the response to light of the photoactive unit can be considerably influenced, in both nature and efficiency, by the occurrence of competitive photoprocesses (i.e., photoinduced energy and/or electron transfer, nonradiative deactivation, etc.), occurring upon light absorption. The most convenient methodology to demonstrate the NO generation from the molecular conjugates under visible light stimuli is the direct and in real-time monitoring of this radical species. To this end, we have used an ultrasensitive NO electrode, which directly detects NO, with nanomolar concentration sensitivity, by an amperometric technique.35
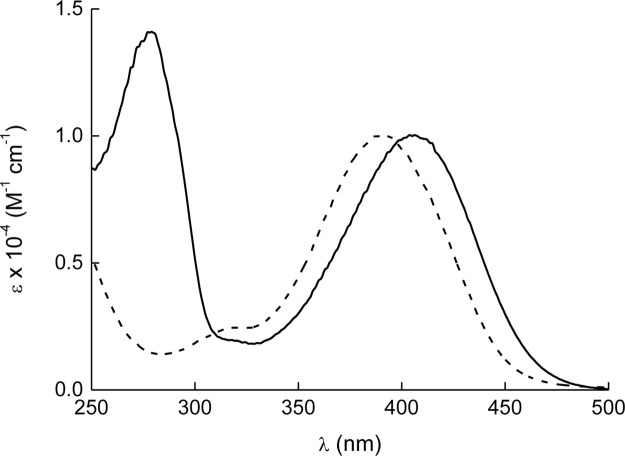
The results illustrated in Figure 2 provide unambiguous evidence that both photocage-SdAdo and photocage-UDC are stable in the dark but supply NO upon illumination with visible light.
Figure 2.
NO release profiles observed upon visible light irradiation (λ = 420 nm, 1.5 mW cm–2) of solutions of photocage-SdAdo (a) and photocage-UDC (b) (50 μM) in phosphate buffer (10 mM, pH = 7.4) with DMSO 0.5%.
The release process is strictly regulated by the external light inputs, as confirmed by the linear NO generation, which promptly stops as the light turns off and restarts as the illumination turns on again. From the slopes of the photoamperograms obtained, one can note that the NO photorelease by photocage-UDC is almost 30% larger than that of photocage-SdAdo.
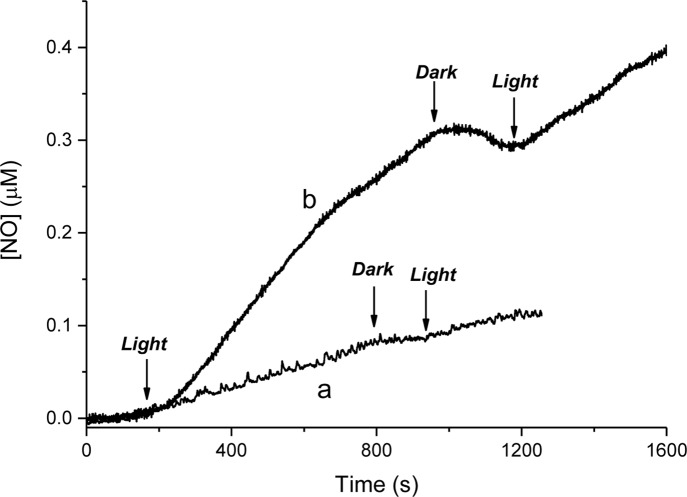
In principle, one can tentatively attribute this different photoreactivity to side reactions occurring in the case of photocage-SdAdo, competitive with NO photorelease. However, this is not the case. In fact, the absorption spectral changes of photocage-SdAdo as a function of the irradiation time show a photobleaching of the visible band without any significant shift in the absorption maximum (Figure 3A).
Figure 3.
(A) Absorption spectral changes observed upon visible light irradiation (λ = 420 nm, 7 mW cm–2) of an aqueous solution of photocage-SdAdo (50 μM) in phosphate buffer (10 mM, pH = 7.4) with DMSO 0.5% from 0 to 70 min. (B) Kinetic profiles, monitored at λmax, and the related linear fittings, observed for 50 μM solutions of photocage-SdAdo (■) and photocage-UDC (●).
This spectral behavior is in excellent agreement with the photochemical pathway leading to the NO release previously proposed in the case of the single NO photodonor unit.21 Comparative photolysis experiments carried out with an optically matched solution of photocage-UDC (Figure 3B) show that the kinetics of the photobleaching in the case of photocage-UDC is faster than that observed for photocage-SdAdo, in good agreement with the NO photoreleasing behavior. On the basis of these results, the higher photochemical reactivity of photocage-UDC could be probably due to an active role of the bile acid substituent in the pathways following the NO release and leading to the steady state coproduct via the intermediate phenoxy radical21 (i.e., intramolecular H-abstraction).
All the biological trials were performed first of all in the dark. All bioconjugates were tested in vitro against a selection of two human cancer cell lines such as K562 leukemia cells and the colon cancer cell line HCT116 and normal human skin fibroblast cells as a control. Cisplatin was chosen as a reference compound. The cytotoxicity was evaluated using MTT assay (see SI).
Cell growth inhibition was determined at concentrations of 10, 25, and 50 μM for the cancer cells and up to 100 μM for fibroblast cells after 72 h. No cytotoxicity was observed with both photocage-SdAdo and photocage-UDC bioconjugates on the fibroblast cells since the percent cell growth inhibition was found under the detection limit up to 100 μM (Table 1). In contrast, a significant concentration-dependent antiproliferative effect was found with both conjugates against the K562 and HCT116 cancer cells lines (Table 1) with photocage-UDC exhibiting a higher cytotoxic effect than photocage-SdAdo and some cytoselectivity. In the case of photocage-CDC, IC50 values similar to photocage-UDC against K562 and HTC116 cancer cells were found. However, the IC50 value of 32.0 μM against fibroblast cells prompted us to exclude this compound from further studies. Based on these results we evaluated the photocage-SdAdo conjugate, with lower, but still good, cytotoxic activity with respect to photocage-UDC, the best candidate to highlight the cytotoxic effect of the light, resulting in the production of NO, toward the HTC116 cancer cells.
Figure 4 shows the cytotoxicity observed for different concentrations of the conjugate photogage-SdAdo at 72 h in the case of samples irradiated for 40 min with visible light and, for comparison, kept in the dark.
Figure 4.
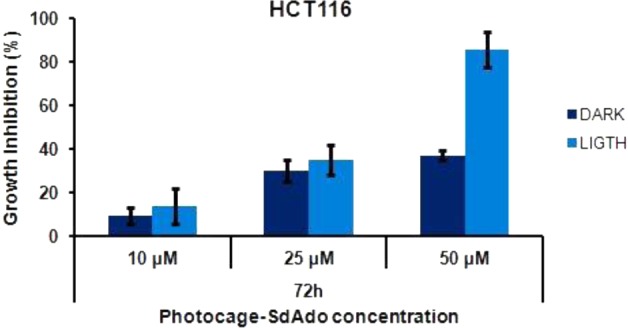
Antiproliferative effect of photocage-SdAdo on HCT116 cell line observed at 72 h after 40 min light irradiation (λ = 420 nm, 7 mW cm–2) and, for comparison, in the dark.
The data obtained show an increase of the growth inhibition at all concentrations. In particular, the percent of growth inhibition observed for 50 μM solution of photocage-SdAdo upon irradiation was around double that observed in the dark.
In conclusion, we have demonstrated that the covalent linking of a NO photoreleaser with modified 2′-deoxyadenosine as well as CDC and UDC bile acid derivatives leads to molecular conjugates displaying an interesting cytotoxicity toward both leukemia K562 and colon carcinoma HCT116 cancer cells. In particular, photocage-SdAdo and photocage-UDC, with no significant cytotoxicity to the human fibroblast skin cells and good antiproliferative effect against both K562 and HCT116 cells, were found to preserve the NO photoreleasing capability of the NO photodonor after its covalent conjugation. In fact, although with different efficiency, both the synthesized compounds exhibited NO delivery exclusively regulated by visible light inputs. This feature offers the possibility to enhance the cytotoxic effects of the conjugates upon light irradiation, combining the chemotherapeutic action with the cytotoxic activity of the photogenerated NO. This was clearly demonstrated in the case of photocage-SdAdo for which, upon illumination, the IC50 value after 72 h of incubation was found more than halved, decreasing from 66.2 μM to 31.0 μM. This result, being the first evidence in vitro of combined chemo- and phototherapeutic activities in the same molecule, represents an intriguing starting point toward the development of a larger number of molecular hybrids.
Acknowledgments
We thank Dr P. Formaglio, Department of Chemical and Pharmaceutical Sciences, University of Ferrara, for running NMR experiments.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00257.
General experimental information; full synthetic procedures and characterization data for all new compounds; experimental procedures for the biological assay (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
This study was financially supported by MIUR (PRIN 2010–2011). ICE SpA supplied bile acids.
The authors declare no competing financial interest.
Supplementary Material
References
- Wang P. G.; Cai T. B.; Taniguchi N. Nitric Oxide Donors For Pharmaceutical and Biological Applications Preface. Nitric Oxide Donors: For Pharmaceutical and Biological Applications 2005, Xv–Xvii. [Google Scholar]
- Kerwin J. F.; Lancaster J. R.; Feldman P. L. Nitric-Oxide - a New Paradigm for 2nd-Messengers. J. Med. Chem. 1995, 38 (22), 4343–4362. 10.1021/jm00022a001. [DOI] [PubMed] [Google Scholar]
- Carpenter A. W.; Schoenfisch M. H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41 (10), 3742–3752. 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpenny G. M.; Mascharak P. K. Emerging Antimicrobial Applications of Nitric Oxide (NO) and NO-Releasing Materials. Anti-Infect. Agents Med. Chem. 2010, 9 (4), 187–197. 10.2174/187152110794785086. [DOI] [Google Scholar]
- Fang F. C.Nitric Oxide and Infections; Kluwer Academic/Plenum Publishers: New York, 1999. [Google Scholar]
- Yasuda H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: Novel strategy for cancer therapy: Nitric oxide donor as a therapeutic enhancer. Nitric Oxide 2008, 19 (2), 205–216. 10.1016/j.niox.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Ostrowski A. D.; Ford P. C. Metal complexes as photochemical nitric oxide precursors: Potential applications in the treatment of tumors. Dalton T 2009, 48, 10660–10669. 10.1039/b912898k. [DOI] [PubMed] [Google Scholar]
- McCarthy H. O.; Coulter J. A.; Robson T.; Hirst D. G. Gene therapy via inducible nitric oxide synthase: a tool for the treatment of a diverse range of pathological conditions. J. Pharm. Pharmacol. 2008, 60 (8), 999–1017. 10.1211/jpp.60.8.0007. [DOI] [PubMed] [Google Scholar]
- Maddaford S.; Annedi S. C.; Ramnauth J.; Rakhit S. Advancements in the Development of Nitric Oxide Synthase Inhibitors. Annu. Rep. Med. Chem. 2009, 44, 27–50. 10.1016/S0065-7743(09)04402-9. [DOI] [Google Scholar]
- Riccio D. A.; Schoenfisch M. H. Nitric oxide release: Part I. Macromolecular scaffolds. Chem. Soc. Rev. 2012, 41 (10), 3731–3741. 10.1039/c2cs15272j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D.; Kashiwagi S.; Jain R. K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6 (7), 521–534. 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- Ridnour L. A.; Thomas D. D.; Donzelli S.; Espey M. G.; Roberts D. D.; Wink D. A.; Isenberg J. S. The biphasic nature of nitric oxide responses in tumor biology. Antioxid. Redox Signaling 2006, 8 (7–8), 1329–1337. 10.1089/ars.2006.8.1329. [DOI] [PubMed] [Google Scholar]
- Chang C. F.; Diers A. R.; Hogg N. Cancer cell metabolism and the modulating effects of nitric oxide. Free Radical Biol. Med. 2015, 79, 324–336. 10.1016/j.freeradbiomed.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalawansa S. J. Nitric oxide: new evidence for novel therapeutic indications. Expert Opin. Pharmacother. 2008, 9 (11), 1935–1954. 10.1517/14656566.9.11.1935. [DOI] [PubMed] [Google Scholar]
- Wang P. G.; Xian M.; Tang X. P.; Wu X. J.; Wen Z.; Cai T. W.; Janczuk A. J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102 (4), 1091–1134. 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- Sortino S. Photoactivated nanomaterials for biomedical release applications. J. Mater. Chem. 2012, 22 (2), 301–318. 10.1039/C1JM13288A. [DOI] [Google Scholar]
- Sortino S. Light-controlled nitric oxide delivering molecular assemblies. Chem. Soc. Rev. 2010, 39 (8), 2903–2913. 10.1039/b908663n. [DOI] [PubMed] [Google Scholar]
- Ford P. C. Polychromophoric metal complexes for generating the bioregulatory agent nitric oxide by single- and two-photon excitation. Acc. Chem. Res. 2008, 41 (2), 190–200. 10.1021/ar700128y. [DOI] [PubMed] [Google Scholar]
- Fry N. L.; Mascharak P. K. Photoactive Ruthenium Nitrosyls as NO Donors: How To Sensitize Them toward Visible Light. Acc. Chem. Res. 2011, 44 (4), 289–298. 10.1021/ar100155t. [DOI] [PubMed] [Google Scholar]
- Ford P. C. Photochemical delivery of nitric oxide. Nitric Oxide 2013, 34, 56–64. 10.1016/j.niox.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Caruso E. B.; Petralia S.; Conoci S.; Giuffrida S.; Sortino S. Photodelivery of nitric oxide from water-soluble platinum nanoparticles. J. Am. Chem. Soc. 2007, 129 (3), 480–481. 10.1021/ja067568d. [DOI] [PubMed] [Google Scholar]
- Fraix A.; Marino N.; Sortino S. Phototherapeutic Release of Nitric Oxide with Engineered Nanoconstructs. Top. Curr. Chem. 2016, 370, 225–257. 10.1007/978-3-319-22942-3_8. [DOI] [PubMed] [Google Scholar]
- Fraix A.; Sortino S. Photoactivable Platforms for Nitric Oxide Delivery with Fluorescence Imaging. Chem. - Asian J. 2015, 10 (5), 1116–1125. 10.1002/asia.201403398. [DOI] [PubMed] [Google Scholar]
- Genini D.; Adachi S.; Chao Q.; Rose D. W.; Carrera C. J.; Cottam H. B.; Carson D. A.; Leoni L. M. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood 2000, 96 (10), 3537–3543. [PubMed] [Google Scholar]
- Van Den Neste E.; Cardoen S.; Offner F.; Bontemps F. Old and new insights into the mechanisms of action of two nucleoside analogs active in lymphoid malignancies: fludarabine and cladribine (Review). Int. J. Oncol. 2005, 27 (4), 1113–1124. 10.3892/ijo.27.4.1113. [DOI] [PubMed] [Google Scholar]
- Choi Y. H.; Im E. O.; Suh H.; Jin Y.; Lee W. H.; Yoo Y. H.; Kim K. W.; Kim N. D. Apoptotic activity of novel bile acid derivatives in human leukemic T cells through the activation of caspases. Int. J. Oncol. 2001, 18 (5), 979–984. 10.3892/ijo.18.5.979. [DOI] [PubMed] [Google Scholar]
- Choi Y. H.; Im E. O.; Suh H.; Jin Y. G.; Yoo Y. H.; Kim N. D. Apoptosis and modulation of cell cycle control by synthetic derivatives of ursodeoxycholic acid and chenodeoxycholic acid in human prostate cancer cells. Cancer Lett. 2003, 199 (2), 157–167. 10.1016/S0304-3835(03)00351-3. [DOI] [PubMed] [Google Scholar]
- Jeong J. H.; Park J. S.; Moon B.; Kim M. C.; Kim J. K.; Lee S.; Suh H.; Kim N. D.; Kim J. M.; Park Y. C.; Yoo Y. H. Orphan nuclear receptor Nur77 translocates to mitochondria in the early phase of apoptosis induced by synthetic chenodeoxycholic acid derivatives in human stomach cancer cell line SNU-1. Ann. N. Y. Acad. Sci. 2003, 1010, 171–177. 10.1196/annals.1299.029. [DOI] [PubMed] [Google Scholar]
- Park S. E.; Choi H. J.; Yee S. B.; Chung H. Y.; Suh H.; Choi Y. H.; Yoo Y. H.; Kim N. D. Synthetic bile acid derivatives inhibit cell proliferation and induce apoptosis in HT-29 human colon cancer cells. Int. J. Oncol. 2004, 25 (1), 231–236. 10.3892/ijo.25.1.231. [DOI] [PubMed] [Google Scholar]
- Perrone D.; Bortolini O.; Fogagnolo M.; Marchesi E.; Mari L.; Massarenti C.; Navacchia M. L.; Sforza F.; Varani K.; Capobianco M. L. Synthesis and in vitro cytotoxicity of deoxyadenosine-bile acid conjugates linked with 1,2,3-triazole. New J. Chem. 2013, 37 (11), 3559–3567. 10.1039/c3nj00513e. [DOI] [Google Scholar]
- Capobianco M. L.; Marchesi E.; Perrone D.; Navacchia M. L. Labeling Deoxyadenosine for the Preparation of Functional Conjugated Oligonucleotides. Bioconjugate Chem. 2013, 24 (8), 1398–1407. 10.1021/bc400243q. [DOI] [PubMed] [Google Scholar]
- Chatgilialoglu C.; Navacchia M. L.; Postigo A. A facile one-pot synthesis of 8-oxo-7,8-dihydro-(2 ′-deoxy)adenosine in water. Tetrahedron Lett. 2006, 47 (5), 711–714. 10.1016/j.tetlet.2005.11.099. [DOI] [Google Scholar]
- Bosi V.; Sarti E.; Navacchia M. L.; Perrone D.; Pasti L.; Cavazzini A.; Capobianco M. L. Gold-nanoparticle extraction and reversed-electrode-polarity stacking mode combined to enhance capillary electrophoresis sensitivity for conjugated nucleosides and oligonucleotides containing thioether linkers. Anal. Bioanal. Chem. 2015, 407 (18), 5405–5415. 10.1007/s00216-015-8702-6. [DOI] [PubMed] [Google Scholar]
- Callari F. L.; Sortino S. Amplified nitric oxide photorelease in DNA proximity. Chem. Commun. 2008, (17), 1971–1973. 10.1039/b800132d. [DOI] [PubMed] [Google Scholar]
- Coneski P. N.; Schoenfisch M. H. Nitric oxide release: Part III. Measurement and reporting. Chem. Soc. Rev. 2012, 41 (10), 3753–3758. 10.1039/c2cs15271a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.