Abstract

High-throughput screening (HTS) of ligand library to find new active molecules for G protein-coupled receptors is still a major interest, as well as an actual challenge. Fluorescence polarization (FP) assay portrays an essential role in HTS; however, in many cases, it was restricted by the absence of FP probes, the narrow measurement window, and low signal-to-noise (S/N) ratio. Herein, based on the modification of our previous probe 1 (QFL), we discovered an FP probe 3 (QGGFL) for α1-adrenergic receptors (α1-ARs), which has satisfactory fluorescence intensity, specific binding ability to receptors, and suitable fluorescence properties that were compatible with the filters in the FP system. Meanwhile, an “ELISA-like” strategy was designed for FP-based HTS assay in which proteins were adhered into a solid phase to improve the measurement window and S/N ratio. With fluorescent antagonist QGGFL and the ELISA strategy, we succeeded in establishing the first competitive binding FP assay for α1-AR antagonists as the alternative of the radioligand binding assay.
Keywords: α1-Adrenergic receptors, fluorescence polarization, ligand screening, fluorescent probe, ELISA, GPCR
G protein-coupled receptors (GPCRs) represent the largest family of transmembrane proteins in the human genome that account for about 40% of therapeutic agents in the prescription pharmaceutical industry.1,2 Unbiased identification of compounds interacting with GPCRs is strongly dependent on high-throughput screening (HTS) of chemical libraries. Therefore, developing analytical methods to find new lead compounds targeting GPCRs is a great interest, as well as an actual challenge.3 Currently, HTS approaches for GPCR ligands mainly involve the ligand–receptor binding assay using radioactive or fluorescent tracers.4 Radioligand binding is a well-established tool for screening at GPCRs, but with drawbacks of safety concerns and costly consumables.5 In parallel to the rapid development of fluorescent techniques, fluorescence assay is becoming a multifaceted tool in life science.6 Among these techniques, the nonradioactive, low-cost, and self-referencing FP assay using fluorescent ligands propose another credible alternative.7,8 Polarization value is defined as the ratio of fluorescence intensities parallel (I∥) and perpendicular (I⊥) with respect to the plane-polarized excitation light.9,10 In general, fast rotation of freely moving fluorophores will scramble polarization, while constrained fluorophores that bind to bulk receptors will emit highly polarized fluorescence.
Fluorescent ligands of GPCRs sprang up in recent decades, but their applications were restricted primarily to receptor visualization and localization.11,12 Although fluorescent ligand-based assay has opened a brand new standpoint in the fields of GPCRs, it still requires further development.13 Due to the absence of FP probes, the narrow measurement window, and low signal-to-noise (S/N) ratio, FP assays have not been extensively applied.13 The key issue in such a matter is that the polarization is not totally or completely inexistent when fluorescent ligands bind to GPCRs because there is not a difference in rotation rate between the free fluorescent ligand and ligand confined by the receptor.
α1-Adrenergic receptors (α1-ARs) are members of GPCR superfamily that mediate many physiological processes such as smooth muscle contraction, myocardial inotropy and chronotropy, and hepatic glucose metabolism.14 As therapeutic targets, the antagonists of α1-AR play critical roles in the treatment of hypertension, benign prostatic hyperplasia (BPH), and lower urinary tract symptoms (LUTS). Moreover, some α1-adrenoceptor antagonists could induce prostate cancer cell apoptosis.15,16 However, the screening of α1-AR antagonists is heavily dependent on radioligand binding assay at present.17,18 Although several fluorescent probes have been synthesized to study α1-ARs in cells, a few of them are qualified for the high-throughput screening of antagonists, and no FP-based assay has been discovered or reported.18−22 In this note, through the structure modification of our previous fluorescent antagonist QFL (1) of α1-AR,21 we designed and synthesized an excellent FP antagonist probe QGGFL (3) with maintained binding affinity and increased fluorescence quantum yield (∼10-fold), which could serve as an efficient toolkit to achieve an FP-based HTS assay (Figure 1). To settle the matters of narrow measurement window and low S/N ratio in FP assay, an “ELISA-like” strategy was designed to amplify FP effect.23,24 This strategy is to adhere α1-AR proteins to the solid phase and then to measure the FP effect of fluorescent ligands binding to these proteins. When bound to the confined solid-phase proteins, the rotation of fluorescent ligands was constrained greatly, which would produce a considerable polarization effect.
Figure 1.
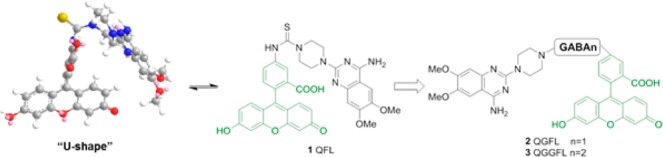
Chemical structure, U-shaped preferential conformation, and structure modification strategy of our previous fluorescent ligand 1 (QFL) of the α1-AR.
To achieve the FP-based ligand binding assay, the first thing we need to do is to afford an excellent FP probe with bright fluorescence and high binding affinity to receptors. In particular, the fluorescence intensity must be strong enough in order to increase S/N ratio and measure up to the microplate reader. Among masses of fluorophores, fluorescein performs brightly as the signal emitter in FP assay by virtue of its high quantum yield.25,26 However, in our previous research on α1-AR, the reported fluorescent quinazoline-fluorescein conjugate QFL (1, Figure 1), possessing nanomolar binding affinity to α1-ARs, showed very low quantum yield (Table 1).21 As a result, this compound failed to be employed in quantitative screening of α1-AR antagonists and could not become an FP probe.21 With the help of Chemdraw 3D software, we found QFL’s essential conformation with a crowded U-shape (Figure 1). In such a conformation, the quinazoline group comes close to the fluorescein core, which may exert an influence on the conjugate plane of fluorophores, and thereby the quantum yield decreases. While the quinazoline moiety represents an interesting pharmacophoric scaffold of antagonist to develop FP agents that bind to the α1-AR receptor tightly, to circumvent the issue about fluorescence intensity, two novel fluorescent ligands, QGFL and QGGFL (2 and 3, Figure 1), were designed by introducing the linker, γ-aminobutyric acid (GABA), to conjugate pharmacophore and fluorophore. Although GABA is the inhibitory neurotransmitter targeted at GABA receptors, the new compounds including GABA linker here will show no agonism activity to GABA receptors because the primary amino group at 4-position in GABA is necessary for its agonism activity.27 As the linker, GABA could increase the flexibility of molecules and reduce the steric hindrance between quinazoline and fluorescein groups. The target compounds were conveniently synthesized as depicted in Scheme 1, in which piperazine-substituted chlorine of 6 afforded compound 7, and then the amide condensation and N-Boc deprotection reactions were involved to introduce one or two GABA(s) to the key intermediates 9 and 10. Finally, the key intermediate reacted with FITC to provide 2 (QGFL) and 3 (QGGFL), respectively.
Table 1. Characterization of Fluorescent Ligands in Spectroscopy and Binding Affinity to α1-ARs.
|
Kia (nM) |
||||||
|---|---|---|---|---|---|---|
| compd | λex (nm) | λem (nm) | Φ (%) | α1A | α1B | α1D |
| phentolamine | 1.3 | 7.7 | 13.8 | |||
| QFL21 | 485 | 517 | 2.6 | 4.7 | 7.3 | 20.7 |
| 2 | 500 | 530 | 17.1 | 33.5 | 8.1 | 31.3 |
| 3 | 500 | 530 | 27.2 | 4.0 | 2.7 | 23.8 |
| atropine | NA | NA | NA | |||
Ki was calculated from IC50 using the Cheng–Prusoff equation (eq S3).
Scheme 1. Synthesis of Fluorescent Ligands 2 and 3.
The affinities of two target compounds 2 (QGFL) and 3 (QGGFL) were assessed routinely by competitive binding assays on three α1-AR subtypes stably expressed in CHO cells, using radiolabeled [3H]prazosin, an established α1-AR competitive antagonist. As expected, each compound revealed up to nanomolar affinities for all α1-AR subtypes (Table 1). This result also indicated that the new compounds could serve as antagonists of α1-AR. It should be noted that our previous research indicated clearly that the large moiety was permitted to conjugate to the N4 position of 2-piperazine group in the quinazoline pharmacophore without losing much binding affinity, but bulk fluorescein was not under consideration for selection.21 These results revealed that introducing a linker between fluorescein and quinazoline groups was beneficial for affinity improvement. Moreover, compound 3 (QGGFL) with the longer linker showed higher affinity than compound 2 (QGFL).
A summary of spectral properties for all compounds is shown in Table 1 as well. Compared to the reported compound 1 (QFL), the newly synthesized fluorescent ligands 2 (QGFL) and 3 (QGGFL) displayed about 15 nm red-shift in wavelength. Significantly, the major improvement in optical properties among them was fluorescence quantum yield, and the quantum yield was positively associated with the length of the linker. In brief, compound 3 (QGGFL) with two GABAs as the linker displayed about 10-fold quantum yield (27.2%) than compound 1 (QFL) without linker (2.6%), and compound 2 (QGFL) with one GABA linker showed 6-fold yield (17.1%).
An essential requirement of a fluorescent ligand of GPCR is to visualize the target protein. As an improved fluorescent ligand, compound 3 (QGGFL) shows the reasonable receptor affinity and appropriate quantum yield, especially the latter. Next, we established the transfected HEK293A cells transiently with α1B-AR and determined the ability of QGGFL to bind to such intact cells expressing α1-AR in culture. In confocal imaging study, the α1B-AR transfected HEK293A cells could be “dyed” by strong green fluorescence when incubated with 300 nM compound 3 (QGGFL) for 20 min, and the strong fluorescence would weaken while a coincubation of QGGFL (300 nM) and an excess of prazosin (3 μM) was carried out (Figure S3). Meanwhile, the untransfected HEK293A cells were also employed as a negative control in which very weak fluorescence was observed. This negative result indicated that nonspecific binding is absent between QGGFL and cell components. The untransfected HEK293A cells also express some β-ARs natively, so these imaging results also indicated that QGGFL showed no interactions with the aminergic β-ARs. As is known, the α1B-AR subtype mainly distributes on cytomembrane.28 In our confocal imaging, most cells with highlighted membrane by QGGFL came into view, indicative of a cell surface localization for α1B-AR, which was highly consistent with the conclusion from Piascik and declared that the probe 3 (QGGFL) could recognize α1-adrenoceptors specifically.28 In addition, to check the specificity or selectivity of QGGFL, we measured its activities to three kinases (BMX, KIT, and ALK) because quinazoline may cause the inhibitory activity against kinases.29 As shown in Figure S5, we found all the GI50 values were greater than 10 μM. Moreover, QGGFL also showed low affinities to other adrenergic receptors (α2- and β1-ARs, Figure S6). As a result, we can conclude that this fluorescent antagonist QGGFL with quinazoline core has few off-target activities.
At present, we have gotten an excellent fluorescent ligand 3 (QGGFL) with improved fluorescence intensity, the specific binding ability to α1-AR, and suitable fluorescent properties that were compatible with the filters in our FP system. In order to identify whether the probe QGGFL may serve as an FP probe or if it could be qualified for the FP-based ligand screening of α1-AR, polarization at 520 nm of QGGFL with various concentrations of α1-AR proteins was measured first using the 96-well or 384-well no-binding flat bottom black plate (Corning, USA) in classical FP assay. However, as shown in Figure S4Aa, the polarization values seemed to have no correlation with the amount of proteins. This set of irregular data incubated that the measurement window was too narrow for FP assay. To amplify the polarization signal, the ELISA strategy was surrogated (Figure S4B), in which the enzyme or antigen was attached to the solid-phase surface.23,24 By adhering to stationary phase, the constrained proteins could slow the spin of fluorescent ligands down, and thus, the fluorescent ligand would produce the obvious polarization effect. To achieve this strategy, 384-well flat bottom black plate with high protein-binding force (Fluotrac 600, Greiner) was employed in 1 h incubation at room temperature. By contrast with the irregular data in Figure S4Aa, three nice saturation curves in Figure S4Ba showed that addition of 16 nM QGGFL into increasing concentrations of three α1-AR proteins gave a saturable increase in FP. We also measured the Z′ factor in both the traditional assay and the ELISA-like assay (Figure S4b), which is a statistical benchmark to judge whether the response in a particular assay is large enough to warrant further attention.30,31
The Z′ factors calculated for our ELISA-based FP assay are 0.84, 0.77, and 0.70 for α1A-, α1B-, and α1D-AR proteins, respectively (Figure S4Bb), and in the traditional FP assay, the Z′ factors are −0.49, −0.64, and −0.56, respectively (Figure S4Ab). A Z′ factor greater than 0.5 means the assay is considered acceptable for a good HTS, while that less than 0 confirms there is too much overlap between the positive and negative controls for the assay to be used. In addition, when treated with excess membrane proteins (0.4 mg/mL), the FP value displays a dose-dependent enhancement, which yields Kd value of 0.56 ± 0.14, 1.21 ± 0.49, and 0.64 ± 0.30 nM for α1A-, α1B-, and α1D-AR, respectively (Figure 2). The results above indicate that compound 3 (QGGFL) is qualified to become a FP probe in the evolutionary ELISA-based FP assay. The specific interaction between QGGFL and α1-AR proteins reached equilibrium in 60 min and was saturable. Therefore, the FITC-derivative compound 3 (QGGFL) is suitable for the measurement of binding affinity of ligands.
Figure 2.
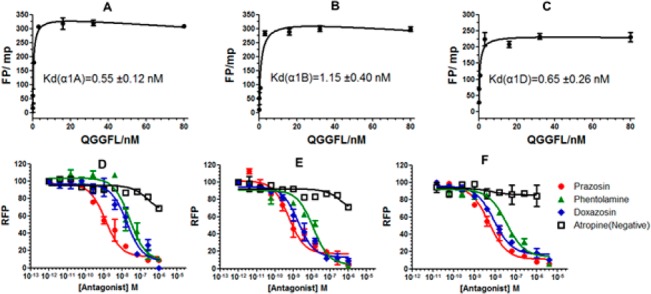
Saturation of increasing concentrations of compound 3 (QGGFL) binding to α1A-AR (A), α1B-AR (B), and α1D-AR (C) proteins (final conc. 0.4 mg/mL). Competition curves of prazosin, doxazosin, phentolamine, and atropine to three α1-AR subtypes, including α1A-AR (D), α1B-AR (E), and α1D-AR (F) using the established “ELISA-like” FP assay.
We further investigated whether this “ELISA-like” FP strategy could be used in HTS for α1-AR antagonists. Competitive binding experiments were carried out in the presence of 16 nM QGGFL and α1-AR membrane proteins (625 ng of α1A-AR and α1B-AR, 1.25 μg of α1D-AR, respectively, Table S1). As known competitive antagonists, prazosin, doxazosin, and phentolamine could reduce FP in a concentration-dependent manner, which gave three anticipated sigmoidal response curves (Figure 2) and convincing IC50 and Ki values to three α1-AR subtypes with the similar values acquired from radioligand binding assay (Table S2). The difference of Ki values in the two assays may be caused by the different systems: the unbound ligands in radioligand assay were washed away, while the FP values were recorded in the homogeneous reaction system. FP was almost enacted on the muscarinic receptor antagonist atropine, which could serve as the negative group for atropine do not bind to α1-AR. Comparing with the radioligand competitive binding assay, the safer fluorescent ligands were employed and fewer membrane proteins (>10 fold) were consumed in the FP binding assay (Table S1). According to these results, we also concluded that the new fluorescent antagonist QGGFL could serve as a competitive antagonist.
In conclusion, developing analytical methods to identify compounds interacting with GPCRs or to find new drugs targeting GPCRs is drawing more and more attention from scientists and researchers. Although FP assay can portray important roles in the HTS of the chemical library for GPCRs, it is restricted in many cases due to the lack of FP probes, the narrow measurement window, and low S/N ratio. To overcome these limitations in α1-ARs, we first discovered and synthesized an ideal FP probe QGGFL, compound 3, for α1-ARs, which has improved quantum yield, high receptor affinity, and the ability of receptor imaging specifically in living cells. Moreover, an “ELISA-like” strategy was designed in which proteins are confined to the solid phase in order to amplify the FP signal and increase the measurement window and S/N ratio. Based on the FP probe QGGFL and the ELISA strategy, we established a safe and affordable FP-based screening method for α1-ARs for the first time, which could provide convincing data of binding affinity to three α1-AR subtypes of known antagonists, such as prazosin, doxazosin, and phentolamine, with the classical radioligand binding assay. To the best of our knowledge, this research is the first report that the ELISA strategy is applied in a small-molecule probe-based FP assay. It is our expectation that this strategy would find broad applications in the FP-based HTS for other target proteins to help address their signal issue.
Acknowledgments
The present work was supported by grants from the Qilu Young Professorship of Shandong University, the International Postdoctoral Exchange Fellowship Program, the Fok Ying Tong Education Foundation (No. 122036), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13028), the Major Project of Science and Technology of Shandong Province (No. 2015ZDJS04001), the Shandong Key Research & Development Project (No. 2015GSF118166), and the Fundamental Research Funds of Shandong University (No. 2014JC008). Our cell imaging work was performed at the Microscopy Characterization Facility, Shandong University.
Glossary
ABBREVIATIONS
- GPCRs
G protein-coupled receptors
- HTS
high-throughput screening
- FP
fluorescence polarization
- S/N
signal-to-noise;α1-ARs, α1-adrenergic receptors
- BPH
benign prostatic hyperplasia
- LUTS
lower urinary tract symptoms
- GABA
γ-aminobutyric acid
- ELISA
enzyme linked immunosorbent assay
- EDCI
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride
- HOBT
1-hydroxybenzotriazole
- FITC
fluorescein isothiocyanate
- TFA
trifluoroacetic acid
- BMX
bone marrow X kinase
- ALK
anaplastic lymphoma kinase
- KIT
v-kit Hardy–Zuckerman 4-feline sarcoma viral oncogene homologue
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00048.
Full experimental procedures, analytical and spectral characterization data of all compounds, and results of cell imaging and FP assay (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Dedication
This article is dedicated to Professor Lin Xia on the occasion of her 80th birthday.
Supplementary Material
References
- Ke N.; Nguyen K.; Irelan J.; Abassi Y. A.. Multidimensional GPCR profiling and screening using impedance-based label-free and real-time assay. In G Protein-Coupled Receptor Screening Assays; Springer: New York, 2015; pp 215–226. [DOI] [PubMed] [Google Scholar]
- Sengupta S.; Ye K.; Scott A. D.; Niu B.; Bailey M. H.; McLellan M. D.; Wendl M. C.; Wyczalkowski M. A.; Ding L. Sequence and structure-guided approach to identify functional mutations in G-protein coupled receptors. Cancer Res. 2015, 75, 61–61. 10.1158/1538-7445.AM2015-61. [DOI] [Google Scholar]
- Oueslati N.; Hounsou C.; Belhocine A.; Rodriguez T.; Dupuis E.; Zwier J. M.; Trinquet E.; Pin J.-P.; Durroux T.. Time-resolved FRET strategy to screen GPCR ligand library. In G Protein-Coupled Receptor Screening Assays; Springer: New York, 2015; pp 23–36. [DOI] [PubMed] [Google Scholar]
- Smith D. S.; Eremin S. A. Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal. Bioanal. Chem. 2008, 391, 1499–1507. 10.1007/s00216-008-1897-z. [DOI] [PubMed] [Google Scholar]
- Allen M.; Reeves J.; Mellor G. High throughput fluorescence polarization: a homogeneous alternative to radioligand binding for cell surface receptors. J. Biomol. Screening 2000, 5, 63–69. 10.1177/108705710000500202. [DOI] [PubMed] [Google Scholar]
- Leopoldo M.; Lacivita E.; Berardi F.; Perrone R. Developments in fluorescent probes for receptor research. Drug Discovery Today 2009, 14, 706–712. 10.1016/j.drudis.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Banks P.; Gosselin M.; Prystay L. Fluorescence polarization assays for high throughput screening of G protein-coupled receptors. J. Biomol. Screening 2000, 5, 159–167. 10.1177/108705710000500308. [DOI] [PubMed] [Google Scholar]
- Jones J. W.; Greene T. A.; Grygon C. A.; Doranz B. J.; Brown M. P. Cell-free assay of G-protein-coupled receptors using fluorescence polarization. J. Biomol. Screening 2008, 13, 424–429. 10.1177/1087057108318332. [DOI] [PubMed] [Google Scholar]
- Jia T.; Fu C.; Huang C.; Yang H.; Jia N. A highly sensitive naphthalimide-based fluorescence polarization probe for detecting cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 10013–10021. 10.1021/acsami.5b02429. [DOI] [PubMed] [Google Scholar]
- Owicki J. C. Fluorescence polarization and anisotropy in high throughput screening: perspectives and primer. J. Biomol. Screening 2000, 5, 297–306. 10.1177/108705710000500501. [DOI] [PubMed] [Google Scholar]
- Ma Z.; Du L.; Li M. Toward fluorescent probes for G-protein-coupled receptors (GPCRs). J. Med. Chem. 2014, 57, 8187–8203. 10.1021/jm401823z. [DOI] [PubMed] [Google Scholar]
- Vernall A. J.; Hill S. J.; Kellam B. The evolving small-molecule fluorescent-conjugate toolbox for class A GPCRs. Br. J. Pharmacol. 2014, 171, 1073–1084. 10.1111/bph.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottet M.; Faklaris O.; Zwier J. M.; Trinquet E.; Pin J.-P.; Durroux T. Original fluorescent ligand-based assays open new perspectives in G-protein coupled receptor drug screening. Pharmaceuticals 2011, 4, 202–214. 10.3390/ph4010202. [DOI] [Google Scholar]
- Piascik M. T.; Perez D. M. α1-Adrenergic receptors: new insights and directions. J. Pharmacol. Exp. Ther. 2001, 298, 403–410. [PubMed] [Google Scholar]
- Kyprianou N.; Benning C. M. Suppression of human prostate cancer cell growth by α1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000, 60, 4550–4555. [PubMed] [Google Scholar]
- Partin J.; Anglin I.; Kyprianou N. Quinazoline-based α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via TGF-β signalling and IκBα induction. Br. J. Cancer 2003, 88, 1615–1621. 10.1038/sj.bjc.6600961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatomo T.; Tsuchihashi H.; Sasaki S.; Nakagawa Y.; Nakahara H.; Imai S. Displacement by α1-adrenergic agonists and antagonists of 3H-prazosin bound to the α1-adrenoceptors of the dog aorta and the rat brain. Jpn. J. Pharmacol. 1985, 37, 181–187. 10.1254/jjp.37.181. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. F.; Daly C. J.; Pediani J. D.; McGrath J. C. Quantitative imaging in live human cells reveals intracellular α1-adrenoceptor ligand-binding sites. J. Pharmacol. Exp. Ther. 2000, 294, 434–443. [PubMed] [Google Scholar]
- Ma J.; Hou Z.; Song Y.; Wang L.; Guo E. Visual and quantitative screening of α1-adrenoceptor antagonists in living cells using quantum dots. ACS Comb. Sci. 2014, 16, 155–159. 10.1021/co5000135. [DOI] [PubMed] [Google Scholar]
- Morishima S.; Suzuki F.; Nishimune A.; Yoshiki H.; Akino H.; Yokoyama O.; Muramatsu I. Visualization and tissue distribution of α1L-adrenoceptor in human prostate by the fluorescently labeled ligand Alexa-488-silodosin. J. Urol. 2010, 183, 812–819. 10.1016/j.juro.2009.09.078. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Ma Z.; Li W.; Li G.; Chen L.; Liu Z.; Du L.; Li M. Discovery of quinazoline-based fluorescent probes to α1-adrenergic receptors. ACS Med. Chem. Lett. 2015, 6, 502–506. 10.1021/ml5004298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.; Lin Y.; Cheng Y.; Wu W.; Cai R.; Chen S.; Shi B.; Han B.; Shi X.; Zhou Y.; Du L.; Li M. Discovery of the first environment-sensitive near-infrared (NIR) fluorogenic ligand for α1-adrenergic receptors imaging in vivo. J. Med. Chem. 2016, 59, 2151–2162. 10.1021/acs.jmedchem.5b01843. [DOI] [PubMed] [Google Scholar]
- Elder P.; Lewis J. An enzyme-linked immunosorbent assay (ELISA) for plasma testosterone. J. Steroid Biochem. 1985, 22, 635–638. 10.1016/0022-4731(85)90217-1. [DOI] [PubMed] [Google Scholar]
- de Camargo Z. P.; Guesdon J.; Drouhet P. E.; Improvisi L. Enzyme-linked immunosorbent assay (ELISA) in the paracoccidioidomycosis. Mycopathologia 1984, 88, 31–37. 10.1007/BF00439292. [DOI] [PubMed] [Google Scholar]
- Marholz L. J.; Wang W.; Zheng Y.; Wang X. A fluorescence polarization biophysical assay for the naegleria DNA hydroxylase Tet1. ACS Med. Chem. Lett. 2016, 7, 167–171. 10.1021/acsmedchemlett.5b00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson I.; Spence M. T.. The Molecular Probes Handbook, A Guide to Fluorescent Probes and Labeling Technologies; Life Technologies: Eugene, OR, 2010. [Google Scholar]
- Dewey S. L.; Brodie J. D.; Ashby C. R. Jr.. By administering a drug that increases central nervous system GABA (4-aminobutyric acid) levels; for example gabapentin, valproic acid, progabide, gamma-hydroxybutyric acid, fengabine, cetylGABA, topiramate, tiagabine, or acamprosate. US Patent US6906099 2015, 2005.
- Chalothorn D.; McCune D. F.; Edelmann S. E.; García-Cazarín M. L.; Tsujimoto G.; Piascik M. T. Differences in the cellular localization and agonist-mediated internalization properties of the α1-adrenoceptor subtypes. Mol. Pharmacol. 2002, 61, 1008–1016. 10.1124/mol.61.5.1008. [DOI] [PubMed] [Google Scholar]
- Ravez S.; Castillo-Aguilera O.; Depreux P.; Goossens L. Quinazoline derivatives as anticancer drugs: a patent review (2011 - present). Expert Opin. Ther. Pat. 2015, 25, 789–804. 10.1517/13543776.2015.1039512. [DOI] [PubMed] [Google Scholar]
- Zhang J.-H.; Chung T. D.; Oldenburg K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screening 1999, 4, 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Lei Y.; Hu T.; Wu X.; Wu Y.; Bao Q.; Zhang L.; Xiang H.; Sun H.; You Q.; Zhang X. Affinity-based fluorescence polarization assay for high-throughput screening of polyl hydroxylase 2 inhibitors. ACS Med. Chem. Lett. 2015, 6, 1236–1240. 10.1021/acsmedchemlett.5b00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



