Abstract
Objective
McCune-Albright syndrome (MAS) is a rare disorder with a spectrum including precocious puberty (PP) due to recurrent estrogen-secreting ovarian cysts. This study evaluates the long-term safety and efficacy of letrozole treatment in large cohort of girls with MAS-associated PP.
Design
Retrospective cohort analysis.
Methods
Clinical data were reviewed, including history and physical, bone age, and pelvic ultrasounds on 28 letrozole-treated girls. Adult height was reviewed for 42 historical controls. Outcomes included rate of skeletal maturation, growth velocity, predicted adult height, and adult height.
Results
Twenty-eight girls received letrozole treatment. Treatment duration was 4.1 ± 2.6 years (mean ± 1SD) (0.5–10.9, range) and mean follow-up was 6.0 ± 3.3 years (range 0.5-15.0), for a total of 135.9 person-years of follow-up. Letrozole was highly effective at decreasing the rate of skeletal maturation, with a decline in change in bone age over change in chronological age (ΔBA/ΔCA) from 1.7 (IQR 2.3) to 0.5 (IQR 0.4) (p<0.0001), and growth velocity Z-scores, which declined from 2.2 ± 2.3 to −0.6 ± 1.6 (p = 0.0004). Predicted adult height Z-scores increased significantly from −2.9 ± 3.2 to −0.8 ± 1.5 for subjects on treatment (p = 0.004). Four subjects who completed treatment reached adult height Z-scores ranging from −1.5 to 1.7 (median −0.6), which was increased in comparison to untreated historical controls (p=0.02). There was no change in uterine size or ovarian volumes, and no adverse events over the treatment period.
Conclusions
In this study with the longest follow-up to date, letrozole treatment resulted in sustained beneficial effects on skeletal maturation, growth velocity and predicted adult height.
INTRODUCTION
McCune-Albright syndrome (MAS) is a rare disorder arising from somatic activating mutations in GNAS, resulting in a clinical spectrum that includes fibrous dysplasia of bone, café-au-lait macules, and hyperfunctioning endocrinopathies 1-3. Gonadotropin-independent precocious puberty (PP) is a hallmark feature in girls, and is frequently the presenting symptom 4. MAS-associated PP is the result of recurrent, autonomously functioning ovarian cysts leading to intermittent estrogen production. Girls typically present with episodic signs of estrogen exposure, including breast development and vaginal bleeding, resulting in growth acceleration and skeletal maturation. Clinical evaluation may show elevated estradiol levels, suppressed gonadotropins, and ovarian cysts on pelvic ultrasound; however, between episodes these findings are typically absent. While some may experience a mild course with few cysts, the disease is more often progressive, and if untreated can lead to skeletal advancement, central PP, and compromised adult height. 5.
Treatment goals in MAS-associated PP are to prevent disabling short stature in adulthood, and to mitigate psychosocial consequences of early sexual development. The preferred method of treatment has not been established. Aromatase inhibitors (AI) have been advocated as a potential therapy to decrease serum estradiol and mitigate its effects on skeletal maturation; however studies in most formulations have been disappointing. Early reports of the first and second generation AIs testolactone and fadrozole demonstrated no sustained beneficial effects on skeletal growth or maturation 6-8. Findings from a more recent study in the third generation AI anastrozole demonstrated a similar lack of efficacy 9. In contrast, a small pilot study of the potent third generation AI letrozole demonstrated beneficial effects, including reductions in skeletal maturation, growth velocity and vaginal bleeding episodes 10. One subject with unrecognized comorbid central PP developed ovarian torsion while on letrozole, which raised concerns about a potential treatment-related adverse event. However given the established risk of torsion in MAS and other conditions with ovarian enlargement 11, 12, the relationship between letrozole treatment and this event is unclear. While findings from this pilot study were overall promising, the routine use of letrozole in MAS has been limited by small subject numbers reported in the literature and potential safety concerns 13.
The purpose of this study is to retrospectively evaluate the long-term safety and efficacy of letrozole treatment in a well-characterized cohort of subjects with MAS.
SUBJECTS AND METHODS
Study Design
This is a single center, retrospective analysis of subjects seen at the NIH as part of a long-standing FD/MAS natural history study (Clinical Trials Number NCT00001727). All subjects and/or their guardians gave informed assent/consent, and the study was approved by the NIDCR Institutional Review Board. Clinical documents, laboratory data, and radiology studies were reviewed. Subjects underwent serial clinical evaluation including history and physical examination, biochemical testing, bone age, and transabdominal pelvic ultrasound. Prior to and between NIH visits, clinical care was provided at the discretion of the patients’ local endocrinologists, and available outside bones ages, pelvic ultrasounds, laboratory studies and height measurements were obtained for review. Pre- and on-treatment safety and efficacy measures were evaluated using paired t-tests, Wilcoxon matched-pairs signed rank tests, and Mann-Whitney tests. Statistical analyses were performed and figures prepared using GraphPad Prism© 6 for Windows, Version 6.02. Data are presented as mean ± standard deviation, or median (interquartile range), as appropriate depending on the normality of distribution.
Height and Skeletal Maturation
Height measurements at the NIH Clinical Center were performed using a stadiometer and determined as the average of three serial morning values. Bone ages were determined using the Greulich and Pyle method 14. For subjects with a skeletal age of 7 years or greater, predicted adult height (PAH) was calculated according to the Bayley-Pinneau method 15. Mid-parental target height (MPH) was calculated using reported parental heights. The bone age obtained just prior to letrozole initiation was used as the baseline study. When available, pre-letrozole bone age and height measurements were used to determine pre-treatment skeletal maturation rates and growth velocities. Height and growth velocity Z-scores were determined from normative reference data 16-18. On-treatment studies were defined as the bone age at the time of letrozole discontinuation for subjects who completed treatment, and the most recent bone age for those remaining on-treatment.
Pelvic Ultrasound
Transabdominal pelvic ultrasounds were performed at each NIH evaluation. The pelvic ultrasound just prior to letrozole initiation was used as the baseline study. On-treatment studies were defined as the ultrasound just prior to letrozole discontinuation for subjects who completed treatment, and the most recent ultrasound for those remaining on-treatment. Ovarian and uterine volumes were calculated using the formula volume = length × width × thickness × 0.52 19. Mean ovarian volumes were calculated as the mean of the right and left ovarian volume. If ovaries were not visualized due to small size, or absent due to surgical resection, a standard pre-pubertal volume of 0.7 mL was assigned for statistical purposes based on normative pediatric ultrasonography data 19.
RESULTS
Clinical Characteristics at Baseline
Twenty-eight girls received letrozole treatment for a minimum of 6 months and were included in the analysis. This group included the 9 children originally reported by Feuillan et al 10, in addition to subsequently collected follow-up data in these subjects. The majority of patients were diagnosed with MAS based on clinical grounds (two or more of the classic features of MAS) according to previously published criteria 3. If there was doubt about the diagnosis, mutation testing for typical mutations in GNAS was performed.
Baseline individual clinical characteristics of the 28 subjects are summarized in Table 1. Subjects had overall advanced skeletal maturation (mean BA/CA 1.6 ± 0.4) and accelerated linear growth (mean growth velocity Z-score 2.2 ± 2.3)]. Subjects initially developed signs of PP at 2.2 ± 1.3 years (0.1-4.5). The symptom prompting evaluation was vaginal bleeding in 21 subjects and breast development in 7 subjects. All subjects received treatment for additional endocrinopathies according to previously published guidelines 3.
Table 1. Baseline clinical and treatment characteristics.
| Subject | Age at PP symptoms (y) |
Age at letrozole start (y) |
BA/CA | Tanner stage (B/PH) |
Letrozole dose (mg/day) |
Other endocrine |
Other PP treatment |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 2.0 | - | 2.5 | FD, HT | Medroxyprogesterone ᵵ Leuprolide* |
| 2 | 4.5 | 5.9 | 1.4 | 2/1 | 2.5 | FD | - |
| 3 | 2.5 | 2.4 | 1 | 4/1 | 2.5 | FD | - |
| 4 | 2.8 | 8.3 | 1.4 | 2/2 | 1.9 | FD, PW | - |
| 5 | 3.5 | 6 | 1.2 | 4/3 | 1.5 | FD | - |
| 6 | 2 | 3.8 | 2.0 | - | 1 | FD, HT | Anastrazoleᵵ |
| 7 | 4.2 | 5.8 | 1.4 | 2/2 | 2.5 | FD | - |
| 8 | 1.3 | 4.9 | 1.4 | 4/1 | 1.5 | FD, PW | - |
| 9 | 0.2 | 3.3 | 2.3 | 2/3 | 2.5 | FD, GH, HT, CS |
Cyproteroneᵵ Anastrazoleᵵ Tamoxifen* Leuprolide* |
| 10 | 0.3 | 2 | 2.5 | 4/3 | 1 | FD | - |
| 11 | 4 | 4.7 | 1.8 | 3/1 | 1 | FD, HT | - |
| 12 | 1.7 | 4.6 | 1.5 | 2/1 | 2.5 | FD | - |
| 13 | 3.1 | 6.1 | 1.5 | 3/2 | 1.25 | - | - |
| 14 | 2 | 4.8 | 2.1 | 4/3 | 2.5 | - | Leuprolide* |
| 15 | 0.9 | 3.4 | 1.5 | 4/2 | 1.25 | FD | - |
| 16 | 1.8 | 2.2 | 1.6 | 2/1 | 2.5 | FD | - |
| 17 | 3.1 | 4.3 | 1.7 | 2/1 | 2.5 | FD, GH | - |
| 18 | 3.7 | 7.9 | 1.5 | 2/1 | 2.5 | FD | - |
| 19 | 2.0 | 5.4 | 1.7 | 2/1 | 2.5 | FD | - |
| 20 | 2.9 | 4.3 | 1.9 | 4/1 | 1 | FD | Leuprolide* |
| 21 | 0.5 | 2 | 2.7 | 4/4 | 2.5 | FD, HT, CS | Anastrazoleᵵ |
| 22 | 2.0 | 6 | 1.5 | 2/3 | 2.5 | FD, GH, HT, PW, CS |
Testolactoneᵵ Leuprolide* |
| 23 | 4.8 | 4.8 | 1.2 | 3/1 | 2.5 | FD, HT, PW | - |
| 24 | 2.3 | 4.8 | 1.2 | 3/2 | 2.5 | FD | - |
| 25 | 2.2 | 6.7 | 1.4 | 3/3 | 2.5 | FD, HT | Tamoxifenᵵ |
| 26 | 0.8 | 3.3 | 1.5 | 3/1 | 2.5 | FD, HT | Leuprolideᵵ |
| 27 | 1.5 | 1.5 | 1 | 2/1 | 2.5 | FD, HT | - |
| 28 | 0.1 | 3.9 | 2 | 3/3 | 2.5 | FD, GH, HT, CS |
Tamoxifenᵵ |
PP = precocious puberty, CA = chronologic age, BA = bone age, SD = standard deviation B = breast, PH = pubic hair, y = years, mg = milligrams, FD = fibrous dysplasia, GH = growth hormone excess, HT = hyperthyroidism, PW = phosphate wasting, CS = Cushing syndrome.
Treatment discontinued prior to letrozole.
Treatment given concomitantly with letrozole.
Subjects 6, 9 and 21 had received previous treatment for PP prior to starting letrozole, during which they had continued to progress in skeletal advancement (Table 1). These therapies were discontinued at or shortly after letrozole initiation. Tamoxifen was added as adjuvant therapy for Subject 9 due to persistent linear growth acceleration after initiating letrozole.
Letrozole Initiation and Treatment Duration
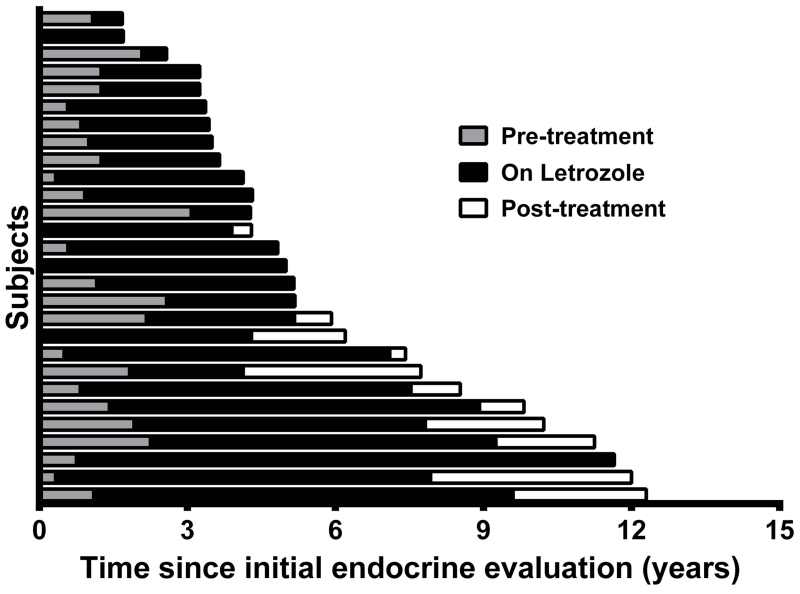
The mean age at letrozole initiation was 4.6 ± 1.7 years (range 1.5-8.3). Letrozole dosing was variable between subjects. Nine subjects from the initial letrozole pilot were dosed according to the parameters of that study, which included a target of 1.5 mg/m2/day in two divided doses, with the option to increase to 2 mg/m2/day in subjects with pubertal progression 10. Based in part on the finding in the pilot study demonstrating increased metabolism of letrozole in children, clinical practice shifted to administering a single 2.5 mg dose once daily. Individual dosing parameters for each subject are included in Table 1. The mean duration of treatment was 4.1 ± 2.6 years (range 0.5-10.9) and mean follow-up after letrozole initiation was 6.0 ± 3.3 years (range 0.5-15.0), for a total of 135.9 person-years of follow-up (Figure 1). Of the 11 subjects who completed letrozole therapy at the time of the analysis, the mean age at discontinuation was 10.5 ± 1.4 years (range 7.0-12.3). Subject 14 discontinued letrozole after developing ovarian torsion as described in the previous pilot study 10, and the remainder discontinued therapy to allow puberty to progress.
Figure 1. Duration of observation and treatment periods.
Horizontal bars indicate individual subjects, with gray bars representing the length of observation prior to initiating letrozole treatment, black bars representing the length of time on letrozole treatment, and white bars representing the on-treatment observation period following letrozole discontinuation.
Skeletal Advancement and Growth Velocity
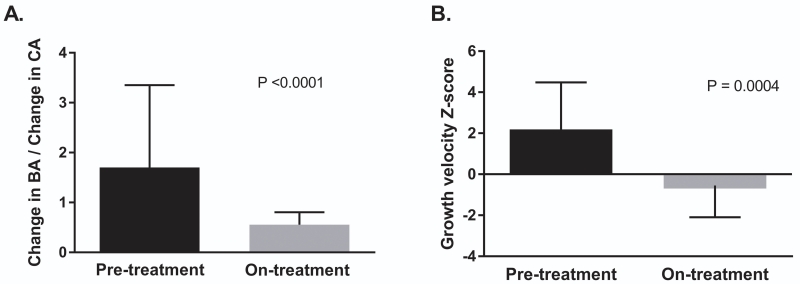
Skeletal advancement was defined by change in bone age over change in chronological age (ΔBA/ΔCA). Pre-treatment, baseline start of therapy, and on-treatment bone ages were available to determine pre- and on-treatment ΔBA/ΔCA for 24 subjects. The mean pre-treatment observation period was 1.3±0.7 years. There was a significant decrease in ΔBA/ΔCA after initiating letrozole, from a pre-treatment median of 1.7 (IQR 2.3) to 0.5 (IQR 0.4) on-treatment (p < 0.0001) (Figure 2A).
Figure 2. Letrozole effects on rate of skeletal maturation and growth velocity.
There was a significant decrease in the ratio of change in bone age to change in chronologic age (ΔBA/ΔCA) (panel A) and growth velocity Z-scores (panel B) over the course of the treatment period. Error bars represent the median and interquartile range in panel A, and the mean and one standard deviation in panel B.
There was a significant decrease in mean growth velocity Z-score following letrozole treatment, from a mean at baseline of 2.2 ± 2.3 to −0.6 ± 1.6 on-treatment (p = 0.0004) (Figure 2B). This effect was preserved when subjects with additional MAS-associated endocrinopathies were excluded from the analysis (p = 0.01).
Predicted Adult Height and Adult Height
Predicted adult height Z-scores were determined for 18 subjects with pre- and on-treatment skeletal ages ≥ 7 years, according to previously described methods 14. There was a significant increase over the treatment period from −2.9 ± 3.2 at baseline to −0.8 ± 1.5 on-treatment (p = 0.004). The mean mid-parental height (MPH) Z-score was 0.4 ± 0.9 for the group of subjects with available PAH data. Over the treatment period, the difference between PAH and MPH increased from −3.1 ± 3.1 at baseline to −1.1 ± 1.8 post-treatment (p=0.003).
Four subjects who completed letrozole therapy reached adult height. Subject 1 reached an adult height Z-score of −0.8 with MPH Z-score −0.3 (0.5 SD below MPH). Subject 3 reached an adult height Z-score of −1.5 with MPH Z-score 0.9 (2.4 SD below MPH). Subject 6 reached an adult height Z-score 0.5 with MPH Z-score 1.1 (0.6 SD below MPH). Subject 22 reached an adult height Z-score 1.7 with MPH Z-score 0.7 (1 SD above MPH). Subjects 1, 6, and 22 had BA exams performed just prior to letrozole discontinuation, allowing for comparison between PAH at treatment completion and adult height. In all three subjects, adult height was shorter than PAH at treatment completion (−1.1, −6.4, and −5.4 cm respectively).
Adult height data were analyzed in a historical control group of 62 women with MAS in the natural history study. Twenty women (30%) had no history of PP, and 42 (70%) had a history of PP that was either untreated or treated with therapies subsequently determined to be ineffective, including progestin, leuprolide monotherapy and/or testolactone. Within the historical control group the women with PP were significantly shorter than those without PP [adult height Z-scores −3.0 ± 2.7 for the PP group versus 0.5 ± 1.2 for the non-PP group, respectively] (p<0.0001). MPH data were available for 14 subjects with PP and 4 subjects without PP. Subjects with PP had adult height Z-scores that were significantly lower than MPH [−3.5 ± 3.1 SD from MPH] compared to the non-PP group (0.3 ± 0.5 SD from MPH) (p=0.001).
Adult heights for subjects treated with letrozole were compared to adult heights for subjects from the historical control group with untreated or inadequately treated PP. The mean adult height Z-score was significantly higher in the four letrozole-treated subjects (−0.1 ± 1.4) compared to the historical control group (−3.0 ± 2.7) (p = 0.04). Subjects treated with letrozole had adult height Z-scores that were closer to MPH (−0.7 ± 1.4 SD from MPH) compared to the historical control group (−3.5 ± 3.1 SD from MPH), however this did not reach statistical significance (p = 0.1).
Uterine and Ovarian Volumes
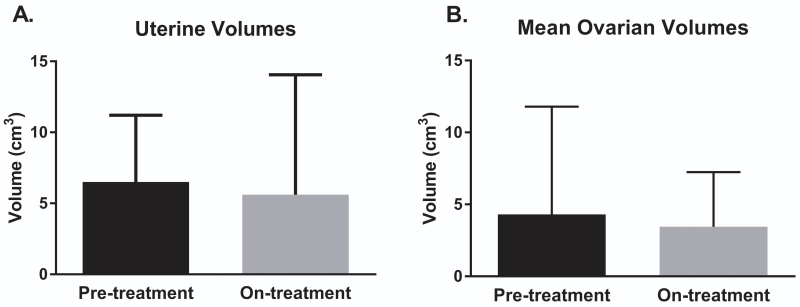
Pre- and on-treatment pelvic ultrasounds were available for 26 subjects. There was no change in median uterine or ovarian volumes over the treatment period (Figure 3). Pre- and on-treatment uterine volumes were 5.4 cm3 (IQR 5.6) and 3.3 cm3 (IQR 2.5) (p = 0.2), and pre- and on-treatment ovarian volumes were 2.0 cm3 (IQR 3.7) and 1.5 cm3 (IQR 4.6) (p = 0.8), respectively.
Figure 3. Uterine and mean ovarian volumes before and after letrozole treatment.
Pre- and on-treatment uterine volumes (panel A) and mean ovarian volumes (panel B) as determined by transabdominal pelvic ultrasound. There was no significant change in mean volumes for either parameter during the treatment period. Error bars represent the mean and one standard deviation. cm3 = centimeters cubed.
Hormone Levels
Pre- and on-treatment estradiol levels were available for 24 subjects. As expected, given the episodic nature of estradiol production in MAS, pre-treatment values were variable ranging from undetectable (9 subjects) to 1523 pg/mL (median 30.4, IQR 139). On-treatment levels were reduced, ranging from undetectable (16 subjects) to 39 pg/mL (median 0.0, IQR 20.2)(p=0.006). Pre- and on-treatment testosterone levels were available for 19 subjects, drawn concomitantly with estradiol levels. Pre-treatment levels ranged from undetectable (14 subjects) to 17 ng/dL (median 0.0, IQR 10), and on-treatment levels were unchanged (p = ns).
Clinical Characteristics at Follow-up
Pre- and on-treatment vaginal bleeding frequency data were available for 19 subjects. Prior to letrozole treatment, vaginal bleeding occurred at a median frequency of 3 episodes per year (IQR 4.6)(range 0-10). This declined significantly on letrozole, with 14 subjects experiencing no bleeding episodes, and 5 subjects experiencing between 0.2 and 0.5 bleeding episodes per year (median for the letrozole-treated group=0.0, IQR 0.2)(p<0.0001).
Pubertal stages of breasts and pubic hair stabilized during the treatment period (II–IV and I–IV, respectively, before treatment versus I–IV and I–V after treatment). Subjects were monitored for central PP, which is a typical development in children with treated and untreated peripheral PP. No subjects were in central PP at baseline; subjects 1, 9, 14, 20 and 22 subsequently entered central puberty while on letrozole. The mean chronologic age and bone age for entrance into central puberty were 8.9 ± 1.9 years (7.0-12.3) and 11.5 ± 1.1 years (10.0-13.0), respectively. Subjects with central puberty were treated with gonadotropin releasing hormone analog therapy, with the exception of Subject 7 who at age 12.3 years discontinued letrozole to allow puberty to commence.
There were no additional cases of ovarian torsion and no suspected treatment-related adverse events.
DISCUSSION
In this retrospective cohort study, letrozole treatment had beneficial effects on skeletal maturation, growth velocity, and predicted adult height in girls with MAS-associated PP. These findings demonstrate that unlike previous studies in other AI formulations, the third-generation AI letrozole is an effective treatment for MAS-associated PP. Letrozole treatment was well-tolerated with no adverse events, no increase in uterine or ovarian volumes, and no additional cases of torsion during the treatment or follow-up period. These findings provide supportive evidence that letrozole treatment is likely safe, and unlikely to be associated with increased risk of ovarian torsion.
The ultimate outcome of interest in the treatment of PP is adult height, which requires an extended length of follow-up and is only rarely reported, particularly in rare disorders such as MAS. This study is the first to report adult height for any intervention in girls with MAS, representing a significant novel contribution. The four letrozole-treated subjects who completed skeletal growth achieved normal adult heights. The availability of historical controls with and without PP provides a unique opportunity to evaluate adult height outcomes in this rare disorder. Despite the small subject numbers, letrozole treatment resulted in a statistically significant increase in adult height, further supporting the efficacy of this therapy.
Determining the preferred treatment for MAS-associated PP has been limited by multiple challenges, including evolving understanding of disease pathophysiology, safety concerns, and owing to the rarity of the disease small numbers of subjects. Similar AI’s, anti-estrogen treatment is another approach which was originally developed for the treatment of breast cancer 20, and was subsequently adapted for the treatment of MAS. The first prospective trial of anti-estrogen treatment for MAS involved the use of the estrogen receptor modulator tamoxifen 21. This study found promising short-term efficacy, however of concern was an increase in uterine volumes, which is consistent with the agonistic effect of tamoxifen on endometrial stroma. In adults, tamoxifen treatment has been associated with an increased risk of endometrial lesions, including hyperplasia, polyps, and cancer 22. This is particularly concerning given that activating GNAS mutations are oncogenic, and may place patients with MAS at higher risk of malignant transformation in affected tissues 23. In light of these potential malignancy risks, and the likely need for long-term therapy in girls with PP, it is the authors’ opinion that tamoxifen should be used with caution in MAS. Fulvestrant is a pure estrogen receptor antagonist that was evaluated in a recent prospective trial of girls with MAS-associated PP, and was well-tolerated with no adverse events or changes in uterine size 24. While there was a decrease in skeletal maturation, there was no effect on growth velocity or predicted adult height, calling into question the efficacy of fulvestrant monotherapy. Letrozole is therefore the only available treatment for MAS-associated PP that demonstrates long-term efficacy without stimulatory effects on the uterus.
Strengths of this investigation include the uniquely large number of subject given the disease rarity, detailed clinical phenotyping, and extensive length of follow-up. This report represents the longest follow-up for any treatment for MAS-associated PP. Limitations arise primarily due to the retrospective design. This was a 9-year observational extension of a previously published 3-year prospective study. As a result there was heterogeneity in letrozole dosing, duration of the pre- and on-treatment periods, and inconsistent collection of certain data elements such as bleeding frequency. Growth outcomes in MAS are complex and may be impacted by multiple confounders. These include skeletal deformities and other endocrinopathies, specifically hyperthyroidism and growth hormone excess which may accelerate growth, as well as FGF23-mediated hypophosphatemia and Cushing syndrome which may lead to growth deceleration. In the current study all potential comorbid endocrinopathies were carefully assessed and managed medically. The beneficial effects of letrozole were preserved when subjects without additional endocrinopathies were analyzed separately. Future studies with additional patients who have completed treatment and reached skeletal maturity are needed to confirm the effect of letrozole on adult height.
Acknowledgments
Funding: This research was supported in part by the Division of Intramural Research of the NIDCR, NICHD, of the Intramural Research Program of the NIH, DHHS, and the Bone Health Program, Division of Orthopaedics and Sports Medicine, Children’s National Health System.
Footnotes
Declaration of Interest: The authors have nothing to disclose.
REFERENCES
- 1.Albright FBA, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas, of pigmentation, and endocrine dysfunction, with precocious puberty in females: report of 5 cases. N Engl J Med. 1937;216:727–746. [Google Scholar]
- 2.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 3.Boyce AM, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews(R) University of Washington, Seattle; Seattle WA: 1993. [PubMed] [Google Scholar]
- 4.Collins MT, S F, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;24(Suppl 1):S4. doi: 10.1186/1750-1172-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eugster EA. Aromatase inhibitors in precocious puberty: rationale and experience to date. Treat Endocrinol. 2004;3:141–151. doi: 10.2165/00024677-200403030-00002. [DOI] [PubMed] [Google Scholar]
- 6.Feuillan PP, Foster CM, Pescovitz OH, Hench KD, Shawker T, Dwyer A, Malley JD, Barnes K, Loriaux DL, Cutler GB., Jr. Treatment of precocious puberty in the McCune-Albright syndrome with the aromatase inhibitor testolactone. N Engl J Med. 1986;315:1115–1119. doi: 10.1056/NEJM198610303151802. [DOI] [PubMed] [Google Scholar]
- 7.Feuillan PP, Jones J, Cutler GB., Jr. Long-term testolactone therapy for precocious puberty in girls with the McCune-Albright syndrome. J Clin Endocrinol Metab. 1993;77:647–651. doi: 10.1210/jcem.77.3.8370686. [DOI] [PubMed] [Google Scholar]
- 8.Nunez SB, Calis K, Cutler GB, Jr., Jones J, Feuillan PP. Lack of efficacy of fadrozole in treating precocious puberty in girls with the McCune-Albright syndrome. J Clin Endocrinol Metab. 2003;88:5730–5733. doi: 10.1210/jc.2003-030864. [DOI] [PubMed] [Google Scholar]
- 9.Mieszczak J, Lowe ES, Plourde P, Eugster EA. The aromatase inhibitor anastrozole is ineffective in the treatment of precocious puberty in girls with McCune-Albright syndrome. J Clin Endocrinol Metab. 2008;93:2751–2754. doi: 10.1210/jc.2007-2090. [DOI] [PubMed] [Google Scholar]
- 10.Feuillan P, Calis K, Hill S, Shawker T, Robey PG, Collins MT. Letrozole treatment of precocious puberty in girls with the McCune-Albright syndrome: a pilot study. J Clin Endocrinol Metab. 2007;92:2100–2106. doi: 10.1210/jc.2006-2350. [DOI] [PubMed] [Google Scholar]
- 11.Clark TJ, Tan BK, Kennedy CR. Asynchronous ovarian torsion in a patient with McCune-Albright syndrome. J Obstet Gynaecol. 2000;20:204. doi: 10.1080/01443610063165. [DOI] [PubMed] [Google Scholar]
- 12.Geimanaite L, Trainavicius K. Ovarian torsion in children: management and outcomes. J Pediatr Surg. 2013;48:1946–1953. doi: 10.1016/j.jpedsurg.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Wit JM, Hero M, Nunez SB. Aromatase inhibitors in pediatrics. Nat Rev Endocrinol. 2012;8:135–147. doi: 10.1038/nrendo.2011.161. [DOI] [PubMed] [Google Scholar]
- 14.Greulich WW, P S. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford University Press; Stanford, CA: 1950. [Google Scholar]
- 15.Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40:423–441. doi: 10.1016/s0022-3476(52)80205-7. [DOI] [PubMed] [Google Scholar]
- 16.Kelly A, Winer KK, Kalkwarf H, Oberfield SE, Lappe J, Gilsanz V, Zemel BS. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99:2104–2112. doi: 10.1210/jc.2013-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0-59 months in the United States. MMWR Recomm Rep. 2010;59:1–15. [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 19.Goldberg BB, M J. Atlas of Ultrasound Measurements. 2nd ed. Mosby Elsevier; Philadelphia, PA: 2006. [Google Scholar]
- 20.Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. 2014;143:207–222. doi: 10.1016/j.jsbmb.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Eugster EA, Rubin SD, Reiter EO, Plourde P, Jou HC, Pescovitz OH. Tamoxifen treatment for precocious puberty in McCune-Albright syndrome: a multicenter trial. J Pediatr. 2003;143:60–66. doi: 10.1016/S0022-3476(03)00128-8. [DOI] [PubMed] [Google Scholar]
- 22.Hu R, Hilakivi-Clarke L, Clarke R. Molecular mechanisms of tamoxifen-associated endometrial cancer (Review) Oncol Lett. 2015;9:1495–1501. doi: 10.3892/ol.2015.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 24.Sims EK, Garnett S, Guzman F, Paris F, Sultan C, Eugster EA. Fulvestrant treatment of precocious puberty in girls with McCune-Albright syndrome. Int J Pediatr Endocrinol. 2012;2012:26. doi: 10.1186/1687-9856-2012-26. [DOI] [PMC free article] [PubMed] [Google Scholar]