Introduction
Basal cell carcinomas (BCCs) are considered the most common tumors of the white population.1 Even though metastatic BCCs have an average survival rate as low as 3.6 years,2 there is a lack of consensus in the guidelines regarding imaging for BCC follow-up care.3 Fluorodesoxyglucose (18F-FDG) positron emission tomography–computed tomography (PET/CT) imaging is established in the follow-up care of many cancer types but not in BCCs.
Case report
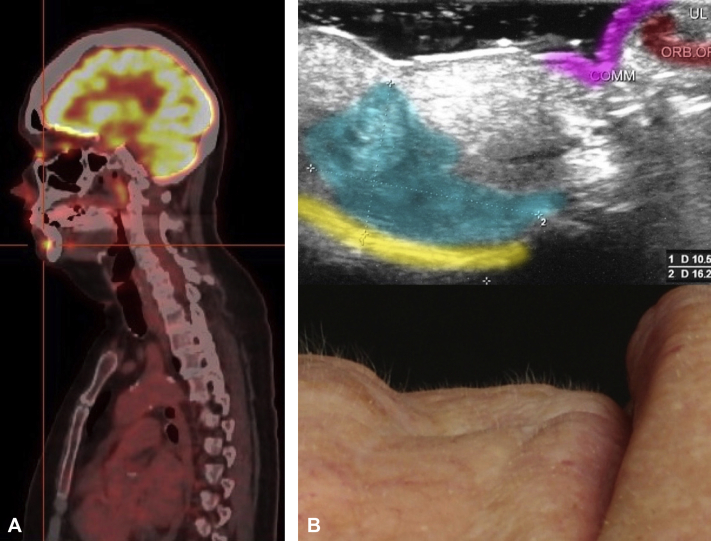
A white woman in her 70s is a melanoma patient in our clinic. Her 18F-FDG PET/CT scan, conducted as part of her melanoma follow-up regimen showed a surprising uptake of 18F-FDG in her chin (Fig 1, A). The patient had suffered from a partly nodular and partly morpheaform BCC at the same location 7 years earlier, which had been excised with narrow tumor margins. Clinical examination of her chin found a discrete subcutaneous lump palpable both in the oral vestibule and transcutaneously at the level of the former labiomental crease. There was no visible epidermal manifestation. Ultrasound scan found a 1.6- × 1.1-cm irregular hypoechogenic lesion corresponding to the palpable tumor (Fig 1, B). Histology of a deep punch biopsy found a BCC with focal nodulocystic growth and partly basosquamous differentiation (Fig 2, A). Immunohistochemistry displayed a diffuse cytoplasmatic staining with BerEP4 supporting the finding that the tumor originated from the basal cell layer. We then performed surgical excision with tumor-free margins (R0).
Fig 1.
A, 18F-FDG PET/CT image shows an increased uptake of 263 MBq 18F-FDG (SUV max, 8.4) in the patient's chin. B, Ultrasound image shows a hypoechogenic lesion of 10.5 × 16.2 mm in the sagittal plane (color marked in cyan), adjacent to the mandible (yellow). The corresponding clinical image can be seen below.
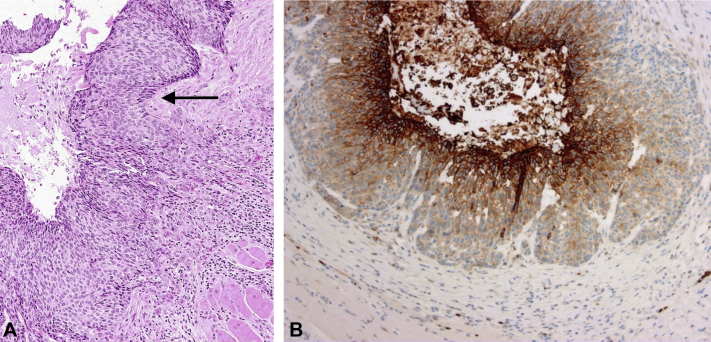
Fig 2.
A, Histology of the BCC shows characteristic histomorphology, such as peripheral palisading (black arrow). B, Immunohistochemistry for glucose transporter GLUT1 displays a focal membranous and cytoplasmatic staining pattern in the center of nodular-cystic areas, whereas rather classic nodular BCC differentiated parts remained negative for GLUT1. (A, hematoxylin-eosin stain; B, GLUT1 stain; original magnifications: A, ×100; B, ×200.)
Discussion
The utility of PET/CT imaging for detecting primary and recurring BCCs has been investigated in a recent literature review.4 There is yet no universal consensus concerning the use of PET/CT imaging, as a systematic benefit analysis is challenging because of the scarcity of metastasizing BCCs. Because of a lack of evidence in support of PET/CT imaging for BCCs, it is currently not thought to be cost effective and is to be used only if a metastasized BCC is suspected.4 Poor performance of PET/CT imaging for detecting tumor metastases has been reported.5 The exact reasons remain elusive. An influence of tumor size and subtype has been suggested.4, 5
Limited attention has been paid to tumor characteristics on a molecular level. The success of PET/CT imaging relies on the tracer uptake by malignant cells. Literature suggests that there is a significant positive correlation between 18F-FDG uptake into cancer cells and glucose transporter-1 (GLUT1) expression, for example, in lung tumors.6 Metastases of pulmonary adenocarcinomas with lower GLUT1 expression show higher false-negative rates in PET/CT imaging compared with those with higher expression.7 Also, GLUT1 expression is an emerging marker for poorer tumor prognosis in oral squamous cell carcinomas.8, 9
A recent study compared GLUT1 expression in BCCs (n = 16) with that in squamous cell carcinomas (n = 16). The authors described a statistically significant higher expression of GLUT1 among squamous cell carcinomas compared with BCCs and that in BCCs, expression was associated with squamous metaplastic areas.10
To further assess the involvement of GLUT1 expression in our patient's basosquamous BCC, we performed a GLUT1 immunochemistry stain. To our expectation, this stain showed a focal expression of GLUT1 in its area of squamous differentiation (Fig 2, B).
In our view, GLUT1 expression is, in our case, the most likely reason the recurrent BCC was incidentally detected by PET/CT imaging and may suggest that GLUT1 expression holds a more important role for PET/CT detection of BCCs than previously assumed. Given that nodular BCCs of larger sizes than in our case had reportedly been missed,5 we conclude that tumor size could be of lesser relevance compared with molecular properties, such as GLUT1 expression or maybe other unknown mechanisms. Because GLUT1 seems to be expressed more likely in squamous cell tumors, we think that PET/CT holds most relevance in detecting metastases of the basosquamous BCC subtype.
A clear association between enhanced detectability by overexpression of GLUT1 and 18F-FDG PET/CT imaging for BCCs has yet to be shown in a larger cohort. However, given that metastatic BCC is exceedingly rare, this association may be difficult to prove. Alternatively, measuring GLUT1 expression in histology and drawing suggestions as to tumor behavior and detectability may be more realistic to attain.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Wong C.S., Strange R.C., Lear J.T. Basal cell carcinoma. BMJ. 2003;327:794–798. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCusker M., Basset-Seguin N., Dummer R. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer. 2014;50:774–783. doi: 10.1016/j.ejca.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Trakatelli M., Morton C., Nagore E. Update of the European guidelines for basal cell carcinoma management. Eur J Dermatol. 2014;24:312–329. doi: 10.1684/ejd.2014.2271. [DOI] [PubMed] [Google Scholar]
- 4.Duncan J.R., Carr D., Kaffenberger B.H. The utility of positron emission tomography with and without computed tomography in patients with nonmelanoma skin cancer. J Am Acad Dermatol. 2016;75:186–196. doi: 10.1016/j.jaad.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Fosko S.W., Hu W., Cook T.F., Lowe V.J. Positron emission tomography for basal cell carcinoma of the head and neck. Arch Dermatol. 2003;139:1141–1146. doi: 10.1001/archderm.139.9.1141. [DOI] [PubMed] [Google Scholar]
- 6.Usuda K., Sagawa M., Aikawa H. Correlation between glucose transporter-1 expression and 18F-fluoro-2-deoxyglucose uptake on positron emission tomography in lung cancer. Gen Thorac Cardiovasc Surg. 2010;58:405–410. doi: 10.1007/s11748-010-0603-1. [DOI] [PubMed] [Google Scholar]
- 7.Chung J.H., Cho K.J., Lee S.S. Overexpression of Glut1 in lymphoid follicles correlates with false-positive (18)F-FDG PET results in lung cancer staging. J Nucl Med. 2004;45:999–1003. [PubMed] [Google Scholar]
- 8.Ohba S., Fujii H., Ito S. Overexpression of GLUT-1 in the invasion front is associated with depth of oral squamous cell carcinoma and prognosis. J Oral Pathol Med. 2010;39:74–78. doi: 10.1111/j.1600-0714.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 9.Azad N., Kumari Maurya M., Kar M. Expression of GLUT-1 in oral squamous cell carcinoma in tobacco and non-tobacco users. J Oral Biol Craniofac Res. 2016;6:24–30. doi: 10.1016/j.jobcr.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdou A.G., Eldien M.M., Elsakka D. GLUT-1 Expression in Cutaneous Basal and Squamous Cell Carcinomas. Int J Surg Pathol. 2015;23:447–453. doi: 10.1177/1066896915589968. [DOI] [PubMed] [Google Scholar]