Abstract
von Hippel-Lindau (VHL) patients develop multiple central nervous system hemangioblastomas (HB). Some HBs become symptomatic with exponential growth or cyst formation following long periods of quiescence. Understanding the factors underlying growth in hemangioblastoma may lead to better strategies to arrest or prevent tumor growth. In 5 VHL patients, we resected quiescent hemangioblastomas (Q-HB) that were en-route during surgical access to symptomatic hemangioblastomas (S-HB), for matched tumor analysis. Quantitative reverse transcriptase analysis demonstrated a 2-fold increase in EPO expression in all S-HB, while 4/5 showed either Hypoxia Inducible Factor-1α or 2α upregulation. Additionally, all S-HB had increased phosphorylated erythropoietin (EPO) receptor and phosphorylated STAT-5 relative to matched Q-HB, with increased phosphorylated JAK-2 largely confined to the stromal cells in clusters within the tumors. These findings suggest that Q-HB to S-HB conversion may be associated with an erythropoietin-signaling loop. Furthermore, we found that EPO is detectable in cyst fluid from S-HB (n = 14), while absent in CSF (n = 1). Additionally, S-HB presentation or S-HB resection does not result in discernible change in serum EPO or hemoglobin (n = 60). These observations suggest that the altered erythropoietin signaling is focal and suggests that studying modulation of erythropoietin receptor pathway may lead to strategies in preventing HB growth.
Von Hippel-Lindau (VHL) disease is an autosomal dominant neoplastic disorder. Majority (80%) of VHL patients develop central nervous system hemangioblastomas (HBs), with tumor development driven by VHL protein (pVHL) loss, resulting in hypoxia inducible factor (HIF) 1α and HIF-2α accumulation1,2,3. In VHL half of the HBs remain quiescent, but the rest display stochastic growth or cyst formation, leading to significant morbidity and mortality4,5,6,7. HIF signaling and downstream factors such as VEGF have been implicated in initial tumor development in VHL8,9,10,11. HIF-inducible erythropoietin (EPO) and EPO-Receptor (EPO-R) are co-localized in VHL-associated HBs12 and renal-cell carcinoma (RCC)13 suggesting that autocrine/paracrine EPO/EPO-R signaling loop may underlie RCC formation14. But, loss of quiescence in VHL-associated HBs remains unexplained, to a certain extent due to the lack of in-vitro/in-vivo HB models.
In VHL disease, HBs are resected only when these tumors cause neurologic symptoms1,2,6. Therefore, laboratory investigations involving VHL HBs are exclusively performed on symptomatic HBs (S-HB). Autopsy studies of VHL patients have identified microscopic ‘tumorlets’11,15, but, no studies have reported on the differences between S-HB and grossly evident quiescent HBs (Q-HB) in surgical patients. In this study, we analyzed differences in tumors from a rare set of five VHL patients who had simultaneously resected S-HB and ‘en-route’ Q-HB.
Materials and Methods
Population
HB tumor volumes, serum EPO and hemoglobin (Hgb) were measured in VHL subjects with HBs (NCT00005902, NIH 00-N-0140). S-HB tumors were resected (by EHO and PC) in an ongoing clinical trial (NCT00060541, NIH 03-N-0164), for symptomatic tumor growth or cyst formation6. Quiescent HBs (Q-HBs) were resected when encountered en-route during surgical access to S-HB. S-HB with cysts were prospectively selected for intraoperative cyst fluid aspiration and collection prior to surgical resection of HBs. Cyst fluid was immediately frozen at −80 °C for later use. Enzyme-linked immunosorbent assay (ELISA) was used to measure EPO level in S-HB cyst fluid (n = 14) and available paired CSF (n = 1). Clinically reported serum EPO (118 subjects) and hemoglobin values (60 subjects) were analyzed for changes related to S-HB resection. The trials are approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health, Bethesda, MD, USA. The trials were conducted in accordance with the approved guidelines. All subjects provided informed consent for trial participation, and for use of clinical data and samples.
Expression analysis
Primers for HIF-1, HIF-2, glucose transporter 1 (GLUT-1), vascular endothelial growth factor (VEGF) (Qiagen), and EPO (BioRad) were used to perform qRT-PCR on tumor RNA.
Western blot
Anti-STAT5 (Santa Cruz sc-835), anti-phosphorylated STAT5 (Millipore 05-495), anti-EPO-R (Santa Cruz sc-697), anti-phosphorylated EPO-R (Abcam ab79824), and anti-actin (Sigma) probes were used and fluorescently conjugated secondary antibodies used to image on Odyssey CLx (Li-Cor).
Immunohistochemistry
Formalin fixed HBs were stained with anti-phosphorylated JAK2 using Leica BondMax and reviewed by blinded neuropathologist (ARC).
Results
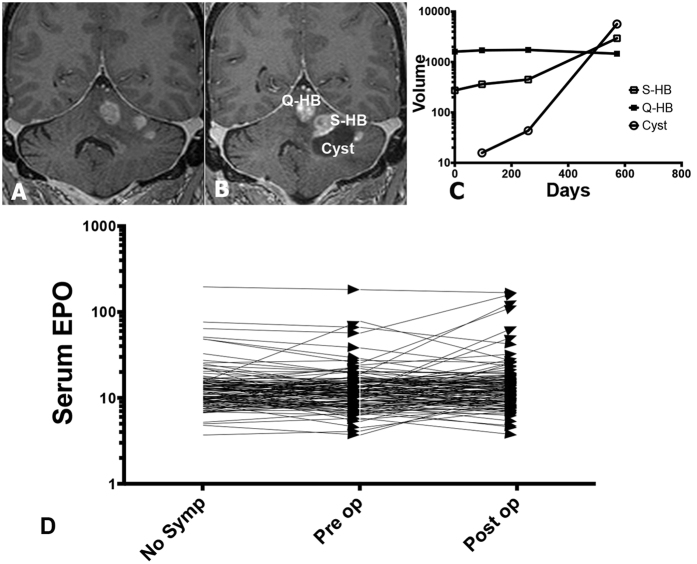
Clinical and imaging characteristics of the 5 patients are summarized in Table 1. In all cases, the location of Q-HB was adjacent to the S-HB, and within the planned surgical approach. The clinical presentation of S-HB depended on its location (Table 1), and was either due to tumor volume increase, cyst formation or both (Fig. 1). In contrast, Q-HBs demonstrated minimal growth, and no cyst formation (Supplementary Figure 1). No significant changes in serum Hemoglobin (Supplementary Figure 2) or serum EPO (Fig. 1) were detected with S-HB presentation or following resection of S-HB.
Table 1. Clinical and radiologic features of the 5 patients included in the current study.
| Patient No. | Sex | Age in years | Surgery Date | Presenting symptoms | S-HB Location | S-HB Volume (mm3) | S-HB Associated Cyst Volume (mm3) | Q- HB Location | Q-HB Volume (mm3) | Q-HB Associated Cyst Volume (mm3) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 57 | 3/19/03 | Progressive Ataxia | Dorsal C-4 | 107 | 7,835 | Dorsal C-3 | 358 | 0 |
| 2 | F | 60 | 8/24/04 | Progressive Ataxia | Ventral C-7 | 545 | 0 | Right C-5 nerve Root | 41 | 0 |
| 3 | M | 26 | 7/3/06 | Headache | Right cerebellar hemisphere | 66 | 18,969 | Dorsal cervico-medullary junction | 3,211 | 0 |
| 4 | M | 49 | 1/28/14 | Altered mental status, left dysmetria | Left cerebellar hemisphere | 2,957 | 5,682 | Superior vermis | 1,463 | 0 |
| 5 | M | 39 | 11/5/14 | Gait imbalance, dysmetria, right arm weakness | Cerebellar vermis | 7,724 | 0 | Right cerebellar hemisphere | 1,477 | 0 |
The spinal locations of the hemangioblastomas are represented by the vertebral levels (C–cervical). Volumes are represented in mm3. Q- HB quiescent hemangioblastoma, S- HB symptomatic hemangioblastoma.
Figure 1. Symptomatic hemangioblastomas are not associated with a change in serum erythropoietin.
Surveillance coronal gadolinium contrast enhanced T1 MRI images of patient 4 obtained 42 months prior to surgery (A) and at symptom presentation (B) demonstrate an increase in tumor volume (S-HB, 2,957 mm3) as well as formation of a new cyst (5,682 mm3). There was no significant change in the volume of the adjacent quiescent hemangioblastoma (Q-HB, 1,463 mm3) (C). In C, y-axis represents volume in cubic millimeters plotted on a log10 scale. The x-axis represents time in days. D: Pre-operative and post-operative serum erythropoietin (EPO) levels (mIU/mL) of 117 VHL patients that underwent surgical resection of symptomatic hemangioblastomas are plotted in D. No trend in the change of serum EPO levels was evident following surgical resection of symptomatic hemangioblastomas. The y-axis represents serum EPO levels plotted on a log10 scale. EPO–erythropoietin, Q-HB–quiescent hemangioblastoma, S-HB–symptomatic hemangioblastoma.
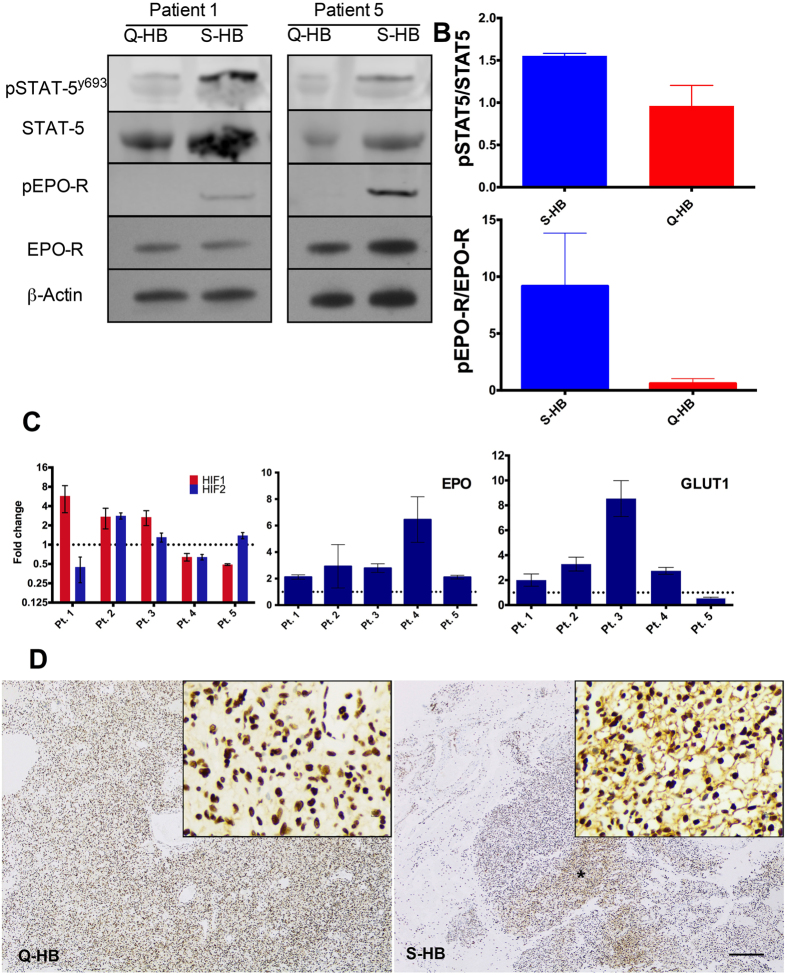
HBs expressed EPO-R variably, but S-HB had consistently elevated tyrosine phosphorylated EPO-R (densitometry mean absorption values: p = 0.11, 95% CI −46.67 to 29.54) (pEPO-R). STAT5 expression was unchanged between S-HBs and Q-HBs, but tyrosine phosphorylated STAT5 (STAT5y694) was elevated in S-HBs (densitometry mean absorption values: p = 0.025, 95% CI −1.18 to 0.00) (Fig. 2). Though a myriad of factors can induce STAT5 tyrosine phosphorylation, the association with increased pEPO-R is suggestive of an EPO-driven mechanism for STAT5 activation to a greater extent in S-HBs than Q-HBs1.
Figure 2. EPO-HIF signaling in Symptomatic and Quiescent Hemangiblastomas.
(A) Increased phosphorylated EPO-receptor (pEPO-R) observed in representative sytmptomatic hemangioblastomas (S-HB) when compared with quiescent hemangioblastomas (Q-HB), indicative of increased EPO-R activation by EPO. Increased tyrosine phosphorylated STAT-5 in symptomatic HBs relative to quiescent HBs indicative of increased transduction of EPO-R activation in S-HBs. (STAT-5 and pSTAT-5 detected within the same experiment. EPO-R and pEPO-R detected within other experiments conducted under similar conditions (Supplementary Data available on request). Images were cropped and presented together for clarity.) (B) Densitometry revealed trend towards increased ratio of both pSTAT5 and pEPO-R in S-HB (n = 5). (C) HIF-1α and HIF-2α variably expressed, with overexpression of HIF-1α or HIF-2α in 4/5 S-HBs in comparison to matched Q-HBs. EPO expression increased in all S-HBs relative to Q-HBs, and GLUT1 expressed at higher levels in 4/5 S-HBs than Q-HBs, suggesting some role of aberrant HIF signaling associated with S-HB growth. Expression values are relative to levels in Q-HB in the same patient. (D) Phosphorylated JAK-2 in focal areas exhibited denser staining in S-HB, as seen here in a representative selection of a focal region from a S-HB and a typical region of Q-HB from Patient 3. Jak-2 phosphorylation transduces the EPO-R signal to STAT-5, completing a signaling loop from EPO expression to EPO-R activation and STAT-5 phosphorylation. The focality of this staining also is consistent with serum EPO findings (as shown in Fig. 1D), suggesting a paracrine/autocrine effect, rather than effects of elevated systemic EPO0. EPO–erythropoietin, EPO-R–erythropoietin receptor, GLUT1–glucose transporter 1, HIF–hypoxia inducible factor, Q-HB–quiescent hemangioblastoma, S-HB–symptomatic hemangioblastoma, STAT5–signal transducer and activator of transcription 5.
IHC probing of phosphorylated-JAK2 demonstrated mild membranous staining in both Q-HBs and S-HBs, with focal areas of strong membranous staining (Fig. 2) more common in S-HBs than Q-HBs. Importantly, the presence of focal stromal cell membranous pJAK2 in localized clusters of tumor cells with increased levels of EPO-R activation suggests paracrine effects of EPO in regions of local activation6.
qRT-PCR was used to elucidate the role of HIF pathway in HB growth. EPO was reliably upregulated (mean fold change 2.11 to 6.45, all p < 0.05) in S-HBs relative to Q-HBs. GLUT-1 expression was increased (mean fold change 2.00 to 8.54, all p < 0.05) in 4 of 5 S-HBs. HIF-1α and HIF-2α expression changes varied between patients; 3/5 patients had increases (mean fold change 2.68 to 5.74) in HIF-1α, and 3 patients had HIF-2α increased expression (mean fold change 1.30 to 2.82) in S-HBs relative to Q-HBs (Fig. 2).
HIF-2α has been demonstrated to be a transcriptional target of STAT5, and STAT5 activation can drive glucose uptake and metabolic supply in hematopoietic cells16. The upregulation of the STAT5 target GLUT1 also suggests STAT5-activation mediating selective HIF cascade activation. HIF-2α, in contrast to HIF-1α, signaling is a significant regulator of EPO17. Thus the increased EPO-R signaling with STAT-5 induced HIF-2α and resultant EPO upregulation and activation seen in focal stromal cells suggests an EPO-HIF-2α signaling loop driving local tumor evolution.
As shown before15,18, S-HB associated cyst fluid samples (n = 14) had detectable levels of EPO (9.98 ± 10.10, range 1.72–33.9 pg/mL). Paired VHL CSF sample had undetectable EPO level (Supplementary Figure 2). We found no change in serum EPO levels leading up to symptomatic clinical presentation (Mean difference −0.514 mU/mL, 95% CI −2.044 to 1.023 mU/mL, p = 0.51) in 117 patients with VHL disease (Fig. 1D). Additionally, surgical removal of S-HB did not result in a significant change in serum EPO levels (Mean difference 1.774 mU/mL, 95% CI −8.931 to 12.48, p = 0.74) (Fig. 1D). The lack of increased serum EPO from S-HBs suggests localized EPO signaling (autocrine/paracrine) driving focal areas of tumor proliferation through induced HIF-2α. Similarly, polyglobulia16 was notably absent with S-HB presentation, and S-HB resection did not result in fall of Hgb (ANOVA, p = 0.11) (Supplementary Figure 2). Taken together, these findings indicate that S-HB may release EPO focally (cyst fluid, but not in CSF), with no discernible systemic effects (change in serum EPO and Hgb).
Discussion
In this study the differences in EPO and HIF mediated signaling pathways were analyzed in a unique cohort of 5 patients with simultaneously resected Q-HBs and S-HBs. All 5 patients showed higher EPO expression in the S-HB relative to the Q-HB, with 4 of 5 showing the same pattern in GLUT1 expression but no other notable changes in HIF signaling cascade observed. EPO upregulation was associated with increased EPO-R signaling, as seen by the increased relative pEPO-R and downstream induction of pSTAT5 in S-HBs relative to Q-HBs. This signaling was confined to the stromal cells, and was focally present in S-HBs as seen through membranous JAK2 phosphorylation on IHC. GLUT1 and STAT5 target genes were also upregulated in S-HBs, indicating active STAT5 nuclear signaling. These results may suggest an EPO-STAT5 autocrine/paracrine loop in HB loss of quiescence with increased activation of EPO-R, increased tyrosine phosphorylation of STAT5 and upregulation of downstream GLUT1 in S-HBs. Increased EPO-signaling activity is also suggested by the increased pEPO-R in S-HBs as the JAK-2 mediated STAT5 phosphorylation would also auto-phosphorylate both JAK2 and EPO-R17. The absence of detectable EPO on patient-wide analysis also suggests that this is an autocrine/paracrine effect. It is important to note that our findings are limited by the inability to assess this mechanism in-vitro, as no viable cell model exists for VHL-associated HBs. Further, due to the scarcity of these paired samples, both the quality and quantity of the analyzable tissue accrued over the past 10 years was extremely variable, and we were limited in our ability to explore more possible pathways in multiple patients in this study by a lack of remaining archived tumor specimen. Further, though our posited mechanism of an EPO and HIF mediated loop would explain variable growth between Q-HBs and S-HBs, and local secretion of EPO from HBs also fits with the focally observed pJAK2 on IHC, we are currently unable to offer concrete evidence of a “trigger” that could drive individual stromal cells (or clusters of stromal cells) into this vicious EPO-mediated cycle. However, our findings are only the first step towards understanding what drives quiescent HBs into growth and may offer novel ways to both detect and prevent growth in VHL-associated hemangioblastomas.
Additional Information
How to cite this article: Feldman, M. J. et al. Loss of Quiescence in von Hippel-Lindau Hemangioblastomas is Associated with Erythropoietin Signaling. Sci. Rep. 6, 35486; doi: 10.1038/srep35486 (2016).
Supplementary Material
Acknowledgments
This work was funded by the Intramural Research Program of the National Institute of Neurological Diseases and Stroke. The study was also supported by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer, Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation at: http://fnih.org/work/education-training-0/medical-research-scholarsprogram.
Footnotes
Author Contributions M.J.F. designed experiments, generated and analyzed data, and drafted the manuscript. S.S. designed experiments and analyzed clinical data. N.A.E. designed experiments, generated and analyzed data. A.R.-C. performed blinded histopathologic analysis. M.J.M. and Z.Z. provided study guidance and interpreted data. E.H.O. provided study oversight and provided tumor specimens. R.R.L. provided study oversight and reviewed data. P.C. designed experiments, analyzed data, revised manuscript, provided study oversight and provided tumor specimens.
References
- Wind J. J. & Lonser R. R. Management of von Hippel–Lindau disease-associated CNS lesions. Expert Review of Neurotherapeutics 11, 1433–1441 (2011). [DOI] [PubMed] [Google Scholar]
- Lonser R. R. et al. Von Hippel-Lindau disease. in Lancet 361, 2059–2067 (2003). [DOI] [PubMed] [Google Scholar]
- Gossage L. & Eisen T M. E. VHL, the story of a tumour suppressor gene Maher ER. Nat. Rev. Cancer 15, 55–64 (2015). [DOI] [PubMed] [Google Scholar]
- Filling-Katz M. R., Choyke Peter L. & Oldfield E. Central nervous system involvement in Von Hippel-Lindau disease. Neurology 41, 41–46 (1991). [DOI] [PubMed] [Google Scholar]
- Slater A., Moore N. R. & Huson S. The Natural History of Cerebellar Hemangioblastomas in von Hippel-Lindau Disease. Am. J. Neuroradiol. 24, 1570–1574 (2003). [PMC free article] [PubMed] [Google Scholar]
- Lonser R. R. et al. Prospective natural history study of central nervous system hemangioblastomas in von Hippel-Lindau disease. J. Neurosurg. 120, 1055–1062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläsker S. Central Nervous System Manifestations in VHL: Genetics, Pathology and Clinical Phenotypic Features. Fam Cancer 4, 37–42 (2005). [DOI] [PubMed] [Google Scholar]
- Park D. M., Zhuang Z. & Chen L. Von Hippel-Lindau Disease-Associated Hemangioblastomas are Derived from Embryologic Multipotent Cells. PLoS Med. 4, e60 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläsker S. et al. Hemangioblastomas share protein expression with embryonal hemangioblast progenitor cell. Cancer Res. 66, 4167–4172 (2006). [DOI] [PubMed] [Google Scholar]
- Zhuang Z. et al. Tumor derived vasculogenesis in von Hippel-Lindau disease-associated tumors. Sci. Rep. 4, 4102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortmeyer A. O. et al. Evolution of VHL tumourigenesis in nerve root tissue. J. Pathol. 210, 374–382 (2006). [DOI] [PubMed] [Google Scholar]
- Vortmeyer A. O. et al. Developmental Arrest of Angioblastic Lineage Initiates Tumorigenesis in von Hippel-Lindau Disease. Cancer Res. 7051–7055 (2003). [PubMed] [Google Scholar]
- Lee Y. et al. Coexpression of Erythropoietin and Erythropoietin Receptor in Von Hippel-Lindau Disease–Associated Renal Cysts and Renal Cell Carcinoma. Clin. Cancer Res. 11, 1059–1064 (2005). [PubMed] [Google Scholar]
- Wu P. et al. The Erythropoietin/Erythropoietin Receptor Signaling Pathway Promotes Growth and Invasion Abilities in Human Renal Carcinoma Cells. PLoS One 7, e45122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys R. V., Napier J. a & Reynolds S. H. Erythropoietin levels in posterior fossa haemangioblastoma. J. Neurol. Neurosurg. Psychiatry 45, 264–266 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläsker S. et al. Hemangioblastomas and neurogenic polyglobulia. Neurosurgery 72, 930–935 (2013). [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A. et al. JAK2 Associates with the Erythropoietin Receptor and Is Tyrosine Phosphotylated and Activated following Stimulation with Etythropoietin. Cell 74, 227–236 (1993). [DOI] [PubMed] [Google Scholar]
- Horton J. C., Harsh G. R., Fisher J. W. & Hoyt W. F. Von Hippel-Lindau disease and erythrocytosis: radioimmunoassay of erythropoietin in cyst fluid from a brainstem hemangioblastoma. Neurology 41, 753–754 (1991). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.