Abstract
Molecular nitrogen exhibits one of the strongest known interatomic bonds, while xenon possesses a closed-shell electronic structure: a direct consequence of which renders both chemically unreactive. Through a series of optical spectroscopy and x-ray diffraction experiments, we demonstrate the formation of a novel van der Waals compound formed from binary Xe-N2 mixtures at pressures as low as 5 GPa. At 300 K and 5 GPa Xe(N2)2-I is synthesised, and if further compressed, undergoes a transition to a tetragonal Xe(N2)2-II phase at 14 GPa; this phase appears to be unexpectedly stable at least up to 180 GPa even after heating to above 2000 K. Raman spectroscopy measurements indicate a distinct weakening of the intramolecular bond of the nitrogen molecule above 60 GPa, while transmission measurements in the visible and mid-infrared regime suggest the metallisation of the compound at ~100 GPa.
Nitrogen is the most abundant element in the terrestrial atmosphere, existing as a diatomic molecule with one of the strongest known triple bonds and as a result is unreactive at ambient conditions. Under high compression, molecular nitrogen exhibits a rich polymorphism1,2,3,4,5,6,7 and significant overlap of thermodynamically competing phases, dependent on formation conditions8. The application of high pressure can also provide new synthesis routes, initiating chemical processes that would not happen otherwise, such as N2 becoming reactive with the noble metals, as in the formation of platinum or iridium nitrides9,10. Xenon, an archetypical inert gas due to its closed shell system, has long been known to form stable halide and oxide compounds through chemical synthesis11,12. The reactivity can also be fundamentally altered with the application of high pressure, the process which has produced van der Waals compounds composed of Xe-H213 and Xe-O214, as well as a Xe-H2O clathrate15. Direct reactions have also been observed such as that between xenon and ice16 and the recently reported stable oxides, Xe2O5 and Xe3O217. Xenon has also been shown to be inserted into both quartz18, and a small-pore zeolite at high pressure and temperature19. Theoretical studies also suggest the increased reactivity of xenon at high pressures with the formation of binary solids Xe-O20,21, Xe-Fe/Ni22, and Xe-Mg23 synthesised solely from their constituent elements. Such studies on the reactivity of xenon, especially with terrestrially abundant elements, could provide an explanation into the significant under-abundance of xenon detectable in the Earth’s atmosphere.
The direct reaction of N2 and Xe would seem unlikely due to the relative inertness of both materials. Nevertheless, a recent theoretical study predicts the formation of novel xenon nitride compounds above 146 GPa with stoichiometry - XeN624. Possible interactions between Xe and N2 have been explored experimentally at low pressures investigating mutual solubility25,26. Through Raman spectroscopic measurements those studies inferred the formation of an orientationally disordered van der Waals compound but were limited up to pressures of 13 GPa at 408 K with no structural investigation.
It is known that at high pressures both xenon and nitrogen exhibit (semi-)conducting phases. Xenon has been shown to transform to metallic state at pressures between 130–150 GPa, giving it the lowest pressure of metallisation amongst the rare gas solids27,28,29 and nitrogen becomes semiconducting with band gap of 0.4 eV at 240 GPa2,3. Previous studies have claimed that by doping Xe with O2, the metallisation pressure is drastically reduced30. Therefore it is of significant interest to investigate pressure-induced electronic effects of any formed Xe-N2 compound. Here, we report the synthesis and characterisation of a Xe-N2 van der Waals compound through x-ray diffraction, Raman and transmission spectroscopies. We show that two inert condensed gases form a Xe(N2)2 compound at pressures as low as 5 GPa at room temperature. When the novel compound is formed in a xenon medium, it becomes metallic at around 100 GPa, whilst Xe(N2)2 with an abundance of nitrogen demonstrates metallic behaviour above ~140 GPa.
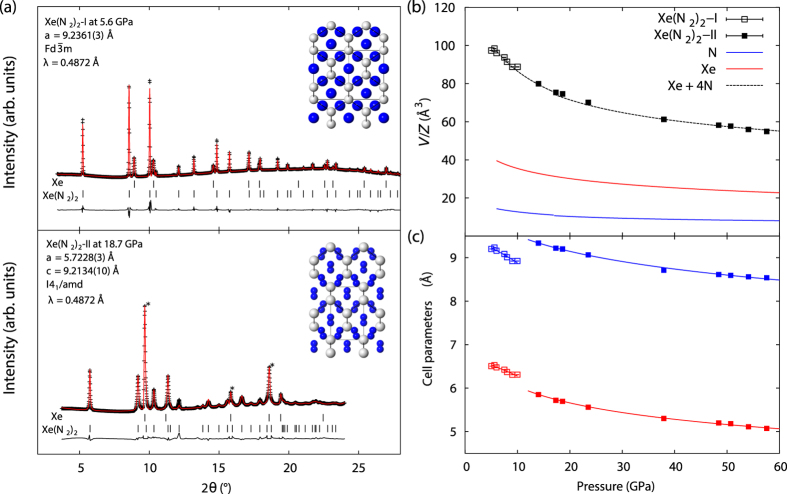
Mixtures of Xe-N2 at various concentration were loaded into diamond-anvil cells (DAC) using a combination of cryogenic and high-pressure gas-loading techniques (see Methods section). Compressing the mixture above 2 GPa leads to the formation of a xenon single crystal surrounded by liquid N2 as seen visually and in x-ray diffraction measurements (see Figs S1 and S2). At pressures above 5 GPa we observe the formation of a N2-rich compound in the media surrounding the xenon single crystal (Fig. S2). Through x-ray powder diffraction analysis we have identified this phase as having a fcc structure, with a = 9.2361(3) Å at 5.6 GPa (Fig. 1), indexing with space group 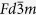 or
or 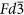 accounts for all observed Bragg peaks. Several patterns were of sufficient quality to allow for Rietveld refinement, otherwise Le Bail fitting was used to extract unit-cell dimensions. Solution of the structure by charge-flipping suggests space group
accounts for all observed Bragg peaks. Several patterns were of sufficient quality to allow for Rietveld refinement, otherwise Le Bail fitting was used to extract unit-cell dimensions. Solution of the structure by charge-flipping suggests space group 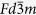 . Two atomic sites could be refined; Xe(0, 0, 0) and N
. Two atomic sites could be refined; Xe(0, 0, 0) and N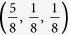 resulting in a cubic Laves Cu2Mg-type structure (Fig. 1(a)).
resulting in a cubic Laves Cu2Mg-type structure (Fig. 1(a)).
Figure 1.
(a) Powder X-ray diffraction patterns at 5.6 and 18.7 GPa used for Rietveld refinement. Below 14 GPa, Xe(N2)2 adopts a face-centered cubic structure, space group 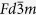 , a = 9.2361(3) Å denoted Xe(N2)2-I. At 14 GPa and above Xe(N2)2 undergoes a transition to a body-centered tetragonal structure, I41/amd, with unit-cell dimensions a = 5.7228(3), c = 9.2134(10) Å at 18.7 GPa. Peaks corresponding to Xe (marked with *) were excluded from the profile used in the refinement. Insets show crystal-structure projections for both phases, phase I is rotated to view down the face diagonal 〈110〉 highlighting structural similarity to phase II. Freely rotating N2 molecules in phase I are represented by blue spheres, whilst in phase II blue spheres represent atoms in aligned N2 molecules; (b) Equation-of-state data for Xe(N2)2 compounds. Pressure-volume per Z data for Xe phases is indicated by red lines32, N2 phases by blue lines6. Black squares indicate volume per Z data for Xe(N2)2 phases I and II, dashed black line indicates the calculated volume for stoichiometry Xe + 4N from atomic volume data; (c) Response of unit-cell dimensions to applied pressure for Xe(N2)2, phase I data are shown for unit-cell length a (blue open squares) and d〈110〉 (red open squares), phase II data is plotted for unit-cell lengths a (red closed squares) and c (blue closed squares). Solid lines indicate fitted linear Birch-Murnaghan linear equations of state?
, a = 9.2361(3) Å denoted Xe(N2)2-I. At 14 GPa and above Xe(N2)2 undergoes a transition to a body-centered tetragonal structure, I41/amd, with unit-cell dimensions a = 5.7228(3), c = 9.2134(10) Å at 18.7 GPa. Peaks corresponding to Xe (marked with *) were excluded from the profile used in the refinement. Insets show crystal-structure projections for both phases, phase I is rotated to view down the face diagonal 〈110〉 highlighting structural similarity to phase II. Freely rotating N2 molecules in phase I are represented by blue spheres, whilst in phase II blue spheres represent atoms in aligned N2 molecules; (b) Equation-of-state data for Xe(N2)2 compounds. Pressure-volume per Z data for Xe phases is indicated by red lines32, N2 phases by blue lines6. Black squares indicate volume per Z data for Xe(N2)2 phases I and II, dashed black line indicates the calculated volume for stoichiometry Xe + 4N from atomic volume data; (c) Response of unit-cell dimensions to applied pressure for Xe(N2)2, phase I data are shown for unit-cell length a (blue open squares) and d〈110〉 (red open squares), phase II data is plotted for unit-cell lengths a (red closed squares) and c (blue closed squares). Solid lines indicate fitted linear Birch-Murnaghan linear equations of state?
From both the structure type and unit-cell dimensions we determine the stoichiometry as Xe(N2)2, designated Xe(N2)2-I herein, which is in excellent agreement with the calculated equation-of-state data for Xe + 4N (Fig. 1(b), see also below). Both the structure type and the stoichiometry are identical to that proposed for oxygen-rich xenon mixtures14. The N-N site distances of 3.2655(1) Å are clearly too long to be bonded, these sites therefore represent scattering from disordered N2 molecules. N2 molecules have been found to adopt both spherical and disk-like rotational disordering in the solid state31, and refinement of both disorder types was attempted, with a spherical disorder model (i.e. with the N-site occupancy equal to 2) resulting in the best fit to the data (see table in SM for more details on the structure refinement). The structure of this phase can be considered as a diamond-type host lattice of Xe atoms with four rotationally disordered N2 molecules forming a tetrahedron within each vacancy. The N-N site distance of 3.2655(1) Å implies a N…N closest-contact distance of 2.1655(1) Å.
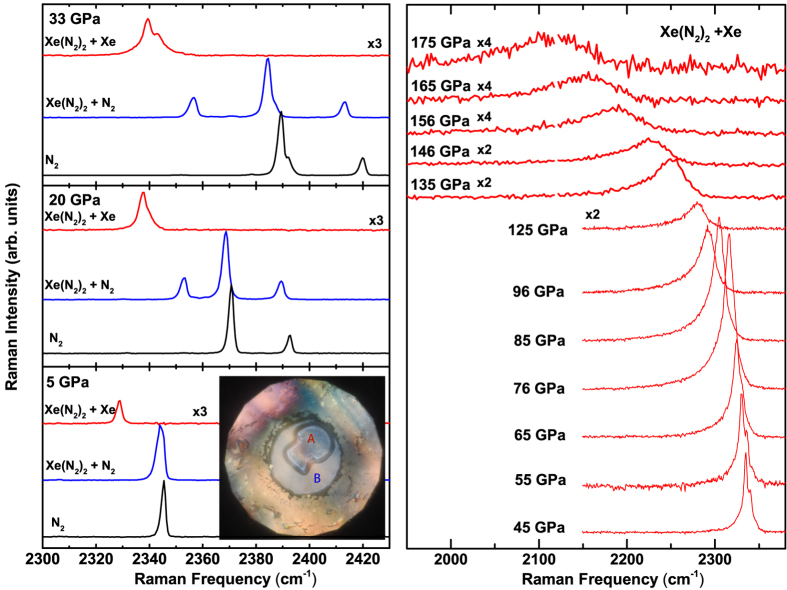
Raman spectroscopy measurements of the formed single crystal at 2 GPa reveals the appearance of a weak vibrational mode, which is lower in frequency than the fluid N2 vibrational mode by 10 cm−1 (compare red and black spectra in Fig. 2). This mode has been observed in a previous high-temperature study and attributed to fluid N2 dissolved in the Xe crystal lattice26. By contrast, in xenon-rich samples (ca. 4:1 concentration), we observe the complete transformation of the sample, evident through only the low-frequency vibrational mode and no evidence of excess N2 (see SM).
Figure 2.
(a) Representative vibrational Raman spectra of the Xe-N2 compound at 5, 20 and 33 GPa. Red spectra are from the formed compound in Xe media, whilst blue spectra show the compound formed in N2 media. As a comparison, vibrational spectra of pure N2 are shown in black. Inset: Photomicrograph of sample at 5 GPa. Red spectra were taken at position A in the single crystal and blue spectra were taken in the surrounding medium at position B. (b) Representative vibrational Raman spectra of Xe(N2)2 in a Xe matrix to pressures of 175 GPa.
In Raman measurements of the surrounding media (see blue spectra in Fig. 2), we observe a broad N2 mode at 5 GPa, which consists of overlapping modes of Xe(N2)2-I, as determined by x-ray diffraction, and pure N2 that increasingly separate in frequency space at higher pressure. The vibrational mode of Xe(N2)2-I (blue spectra in Fig. 2) and the vibrational mode attributed to N2 in Xe (red spectra in Fig. 2), exhibit identical behaviour with pressure (see red and blue points in Fig. 3a), suggesting that the latter is most likely due to small crystallites of Xe(N2)2-I that form within a Xe matrix. It should be noted that the prescence of this N-containing dopant does not significantly affect the measured unit-cell volume which agrees with the literature to within experimental error32.
Figure 3.
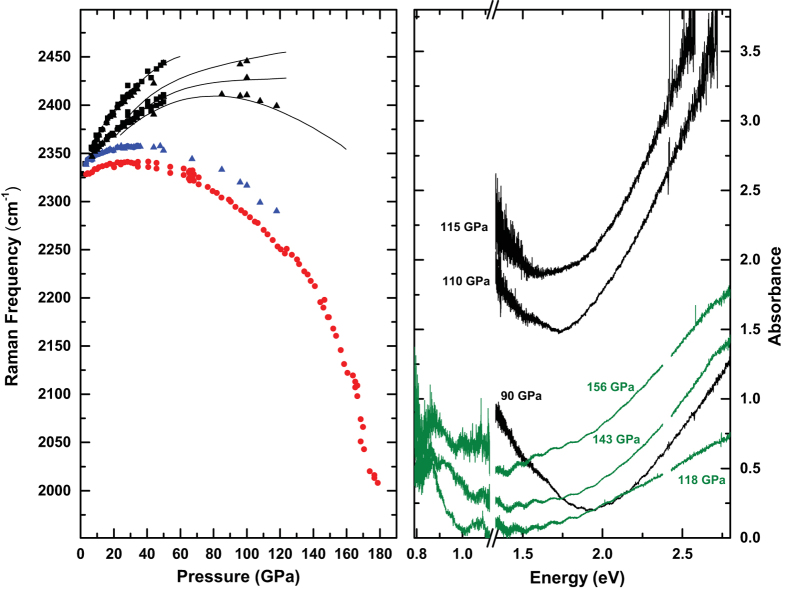
Left Panel: Frequencies of the vibrational modes as a function of pressure. Xe(N2)2 Raman frequencies in Xe medium are shown in red, Xe(N2)2 Raman frequencies in N2 medium are shown in blue and black points are the Raman frequencies of the excess N2. Black lines are taken from a study on pure N21. Right Panel: Optical absorption as a function of energy for Xe-rich (black) and N2-rich (green) samples. The reference spectra were taken at 50 GPa in both experiments.
Above pressures of 14 GPa, we observe a phase transition from the low-pressure Xe(N2)2-I to a high-pressure Xe(N2)2-II phase. This transition pressure corresponds approximately to the critical pressure of the δ to  transition in pure molecular N2. Xe(N2)2-II adopts a body-centered tetragonal cell with a = 5.7228(3), c = 9.2134(10) Å at 18.7 GPa (Fig. 1). Systematic-absence analysis unambiguously confirm space-group symmetry I41/amd. Again Xe is located at the origin, with one N position refined to (0.5, 0.721(2), 0.179(1)). This position lies displaced by 0.52(2) Å from an inversion centre resulting in four ordered N2 molecules aligned along the c-axis. Final Rietveld agreement factors are Rp = 0.015 and R = 0.094.
transition in pure molecular N2. Xe(N2)2-II adopts a body-centered tetragonal cell with a = 5.7228(3), c = 9.2134(10) Å at 18.7 GPa (Fig. 1). Systematic-absence analysis unambiguously confirm space-group symmetry I41/amd. Again Xe is located at the origin, with one N position refined to (0.5, 0.721(2), 0.179(1)). This position lies displaced by 0.52(2) Å from an inversion centre resulting in four ordered N2 molecules aligned along the c-axis. Final Rietveld agreement factors are Rp = 0.015 and R = 0.094.
The origin of this transition lies in the ordered orientation of N2 molecules within the vacancy, corroborated by the poorer fit to the data (R = 0.1672) with a spherically-disordered N2 molecule model. Shortest N…N interatomic distances are now 2.5238(1) Å and 2.610(12) Å at 18.7 GPa. Recalling that the shortest N…N interatomic distances at 5.6 GPa were 2.1655 (1) Å in phase I, the alignment of N2 molecules relieves unfavourable close N…N contacts while maintaing the same coordination number for each N2 molecule.
Over the I-II phase transition the unit cell undergoes a tetragonal distortion elongating by 0.323(3) Å (+3.6%) along c accompanied by a reduction of −0.519(1) Å (−8.1%) along tetragonal a, corresponding to 〈110〉 in phase I (see table in SM for more details on the structure refinement). We tracked unit-cell dimensions for Xe(N2)2-II up to 58 GPa (Fig. 1(c)), confirming again the stoichiometry of the compound (Fig. 1(a)) and allowing the determination of equation-of-state parameters for both Xe(N2)2 phases (see methods section). At pressures of 38 GPa and above there were clear signs of the incipient high-pressure hcp phase of Xe accompanied by strong diffuse scattering and increased background at d-spacings overlapping with a significant number of Xe(N2)2 reflections and above 58 GPa unit-cell dimensions could not be reliably extracted from the data. However the low-angle (101) reflection could be observed up to 103 GPa (see Fig. S4).
Above 40 GPa, the frequency dependence with pressure of the vibrational mode of Xe(N2)2 deviates greatly from that of pure N2 (Fig. 3). The maximum in the vibrational frequency vs. pressure is shifted from 80 GPa in pure N2 to 30 GPa. In the sample in Xe matrix, we observe splitting of the vibrational band (see Fig. 2) up to 70 GPa, after which the splitting is not distinguishable due to the enhanced broadening of the modes. At 140 GPa the N2 vibrational frequencies of Xe(N2)2 are 2161 cm−1 and 2212 cm−1, considerably lower frequencies than either those of κ-N2 (2376 cm−1) or λ-N2 (2320 cm−1, 2400 cm−1). Interestingly, at 178 GPa, we observe the persistence of molecular nitrogen, which is above the pressure at which pure N2 is claimed to become non-molecular (η-N2)1,2,3. We note that although we observe a much softer N2 molecular mode than that just before ζ transforms to the non-molecular amorphous η phase in pure N2, there is no evidence that the N2 molecules in Xe(N2)2 dissociate to form Xe-N bonding. However, there is a clear reduction in intensity (see Fig. 2) together with a marked increase in the FWHH (see Fig. S5) indicating that the molecular N-N bond is weakening. Up to highest pressure studied (180 GPa) we see no evidence of Xe-N bonded compounds predicted by theory24. In an attempt to promote synthesis of such compounds, we performed laser heating of the sample to temperatures of 3000 K at 120, 150, 160 and 180 GPa but no transition was observed in either Raman spectroscopy or x-ray diffraction. It is remarkable that a van der Waals solid, the components of which are inert materials, can remain stable to such extreme conditions.
Figure 3(b) shows the transmission spectra collected from two samples with different initial ratio of Xe and N2. The spectra were collected with both visible and mid infrared light sources which allow the coverage of energy region between ~3 to 0.6 eV. The samples in a Xe matrix (black), appear to exhibit metallic behaviour evident by the sharp rise in the absorption in the near-IR, which shifts with pressure. By 120 GPa, no detectable transmission was observed in the visible, the sample appearance became shiny and reflected red laser light (see photomicrographs in Fig. S6). Samples of Xe(N2)2 with higher N2 concentrations do exhibit absorption (Fig. 3 green) but not to the same extent as in the Xe matrix, which could be due to the excess of N2. Pure xenon has been shown to be conductive at above 135 GPa through both absorption/reflectivity and electrical measurements27,28,29. The mechanism of conductivity is an indirect overlap of the 5p valence and 5d conduction bands. Although determining the mechanism was beyond the scope of this study, our results indicate that by doping Xe with N2, or Xe(N2)2, we are able to tune the conductive properties of Xe and lower the pressure of metallisation.
Our results demonstrate that xenon can form compounds not only with chemically reactive gases such as hydrogen or oxygen, but also with unreactive nitrogen. That such a compound forms at low pressure, exhibits metallic properties, and stable to both high-pressure and high-temperature conditions will no doubt stimulate further research in the reactivity of xenon, an element which now appears to be substantially less inert than previously thought.
Methods
We have studied the formation conditions and stability of xenon and nitrogen compounds up to pressures of 180 GPa in a diamond anvil cell (DAC). In total 12 DAC loadings were performed. 200 μm culet flat diamonds were used for experiments under 50 GPa, while 60 μm and 150 μm culets were used for higher pressure experiments. In all experiments rhenium foil was used as the gasket material.
The loading of the Xe-N2 consisted of two stages. Solid Xe (99.9% purity) was initially cryogenically loaded into a DAC under a N2 atmosphere. Loading was confirmed initially through comparisons of white light transmission spectra due to the change in refractive index between the empty sample chamber and loaded sample. Thorough mapping of the sample with Raman spectroscopy was carried out to ensure no impurities were present in the sample and further confirmation was obtained through x-ray diffraction analysis.
N2 gas (99.9% purity) was then loaded into the cell at a pressure of 20 MPa using a high-pressure gas loading system, displacing some of the pre-existing Xe gas. The volume ratio was estimated by the phase separation of Xe and N2 in the fluid state. Using a combination of varying pressure and temperature, single crystals of the Xe rich mixture were grown.
We have used 514 and 647 nm as excitation wavelengths in the Raman spectroscopy measurements using a custom-built micro-focussed Raman system. Pressure was determined through both ruby fluorescence (P < 100 GPa) and the Raman edge of the stressed diamond33.
Powder x-ray diffraction data were collected at several beamlines: BL10XU at SPring-8 (Japan), IDB PETRA-III (Germany), ID09 at the European Synchrotron Radiation Facility (France), and ID-BMD of HPCAT at APS (USA). Incident beam energies in the range 25–30 keV were used. Intensity vs. 2θ plots were obtained by integrating image plate data in various formats using DIOPTAS34. Indexing was carried out in GSAS-II35, Le Bail and Rietveld refinements were carried out in Jana2000636. Equation of state data were determined using EosFIT 737. Fitted equation of state parameters for Xe(N2)2-I: V0 = 873(90) Å3, K0 = 47(56) GPa, K′ = 1(5). Equation-of-state parameters for Xe(N2)2-II: V0 = 526(158) Å3, K0 = 9(14) GPa, K′ = 4.5(11), K″ = −0.51131 GPa−1.
Additional Information
How to cite this article: Howie, R. T. et al. Formation of xenon-nitrogen compounds at high pressure. Sci. Rep. 6, 34896; doi: 10.1038/srep34896 (2016).
Supplementary Material
Acknowledgments
Synchrotron radiation experiments were performed at the BL10XU of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2016A1041), the authors would like to acknowledge Saori Imada and Naohisa Hirao for assistance with experiments. We acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities and we would like to thank Micheal Hanfland for assistance in using beamline ID09. Parts of this research were carried out at the light source PETRA III at DESY, a member of the Helmholtz Association (HGF). We would like to thank Konstantin Glazyrin for assistance in using beamline IDB. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Part of this work were performed at HPCAT (Sector 16), Advanced Photon Source (APS), Argonne National Laboratory (ANL), we would like to thank Changyong Park for assistance with experiments. This work was supported by a research grant from the U.K. EPSRC Leadership Fellowship grant EP/J003999/1.
Footnotes
Author Contributions R.T.H. conceived and designed the project, carried out the experiments, analysed the data and wrote the paper. R.T. and J.B. carried out the experiments, analysed the data and wrote the paper. M.F. and P.D.-S. carried out the experiments. E.G. analysed the data and wrote the paper.
References
- Goncharov A., Gregoryanz E., Mao H., Liu Z. & Hemley R. Optical evidence for a non-molecular phase of nitrogen above 150 gpa. Phys. Rev. Lett. 85, 1262–1265 (2000). [DOI] [PubMed] [Google Scholar]
- Eremets M. I., Hemley R. J., Mao H.-k. & Gregoryanz E. Semiconducting non-molecular nitrogen up to 240 gpa and its low-pressure stability. Nature 411, 170–174 (2001). [DOI] [PubMed] [Google Scholar]
- Gregoryanz E., Goncharov A. F., Hemley R. J. & Mao H.-k. High-pressure amorphous nitrogen. Phys. Rev. B 64, 052103 (2001). [Google Scholar]
- Gregoryanz E. et al. Raman, infrared, and x-ray evidence for new phases of nitrogen at high pressures and temperatures. Phys. Rev. B 66, 224108 (2002). [Google Scholar]
- Eremets M. I., Gavriliuk A. G., Trojan I. A., Dzivenko D. A. & Boehler R. Single-bonded cubic form of nitrogen. Nat. Mater. 3, 558–563 (2004). [DOI] [PubMed] [Google Scholar]
- Gregoryanz E. et al. High p-t transformations of nitrogen to170 gpa. J. Chem. Phys. 126, 184505 (2007). [DOI] [PubMed] [Google Scholar]
- Tomasino D., Kim M., Smith J. & Yoo C.-S. Pressure-induced symmetry-lowering transition in dense nitrogen to layered polymeric nitrogen (lp-n) with colossal raman intensity. Phys. Rev. Lett. 113, 205502 (2014). [DOI] [PubMed] [Google Scholar]
- Frost M., Howie R. T., Dalladay-Simpson P., Goncharov A. F. & Gregoryanz E. Novel high-pressure nitrogen phase formed by compression at low temperature. Phys. Rev. B 93, 024113 (2016). [Google Scholar]
- Gregoryanz E. et al. Synthesis and characterization of a binary noble metal nitride. Nat. Mater. 3, 294–297 (2004). [DOI] [PubMed] [Google Scholar]
- Young A. et al. Synthesis of novel transition metal nitrides IrN2 and OsN2. Phys. Rev. Lett. 96, 155501 (2006). [DOI] [PubMed] [Google Scholar]
- Bartlett N. Xenon hexafluoroplatinate(v) xe + [ptf6]. Proc. Chem. Soc. 218 (1962). [Google Scholar]
- Classen H. H., Selig H. & Malm J. G. Xenon tetrafluoride. J. Am. Phys. Soc. 84, 3593–3593 (1962). [Google Scholar]
- Somayazulu M. et al. Pressure-induced bonding and compound formation in xenon-hydrogen solids. Nat. Chem. 2, 50–53 (2009). [DOI] [PubMed] [Google Scholar]
- Weck G., Dewaele A. & Loubeyre P. Oxygen/noble gas binary phase diagrams at 296 K and high pressures. Phys. Rev. B 82, 014112 (2010). [Google Scholar]
- Sanloup C., Mao H.-k. & Hemley R. J. High-pressure transformations in xenon hydrates. P. Natl. Acad. Sci. 99, 25–28 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanloup C., Bonev S. A., Hochlaf M. & Maynard-Casely H. E. Reactivity of xenon with ice at planetary conditions. Phys. Rev. Lett. 110, 1–5 (2013). [DOI] [PubMed] [Google Scholar]
- Dewaele A. et al. Synthesis and stability of xenon oxides Xe2O5 and Xe3O2 under pressure. Nat. Chem. advance online publication (2016). [DOI] [PubMed] [Google Scholar]
- Sanloup C. et al. Retention of xenon in quartz and earth’s missing xenon. Science 310, 1174–1177 (2005). [DOI] [PubMed] [Google Scholar]
- Seoung S. et al. Irreversible xenon insertion into a small-pore zeolite at moderate pressures and temperatures. Nat. Chem. 6, 835 (2014). [DOI] [PubMed] [Google Scholar]
- Brock D. S. & Schrobilgen G. J. Synthesis of the missing oxide of xenon, XeO2, and its implications for earth’s missing xenon. J. Am. Chem. Soc. 133, 6265–6269 (2011). [DOI] [PubMed] [Google Scholar]
- Zhu Q. et al. Stability of xenon oxides at high pressures. Nat. Chem. 5, 61–65 (2012). [DOI] [PubMed] [Google Scholar]
- Zhu L., Liu H., Pickard C. J., Zou G. & Ma Y. Reactions of xenon with iron and nickel are predicted in the earth’s inner core. Nat. Chem. 6, 644–648 (2014). [DOI] [PubMed] [Google Scholar]
- Miao M.-s. et al. Anionic chemistry of noble gases: Formation of mg-ng (ng = xe, kr, ar) compounds under pressure. J. Am. Chem. Soc. 137, 14122–14128 (2015). [DOI] [PubMed] [Google Scholar]
- Peng F., Wang Y., Wang H., Zhang Y. & Ma Y. Stable xenon nitride at high pressures. Phys. Rev. B 92, 094104 (2015). [Google Scholar]
- Kooi M. E. & Schouten J. A. High-pressure raman investigation of mutual solubility and compound formation in Xe N2 and Ne N2. Phys. Rev. B 60, 12635–12643 (1999). [Google Scholar]
- Kooi M. E., Michels J. P. J. & Schouten J. A. Negative vibrational shift of nitrogen diluted in xenon at the fluidsolid transition. J. Chem. Phys. 110, 3023–3025 (1999). [Google Scholar]
- Reichlin R. et al. Evidence for the insulator-metal transition in xenon from optical, x-ray, and band-structure studies to 170 gpa. Phys. Rev. Lett. 62, 669–672 (1989). [DOI] [PubMed] [Google Scholar]
- Goettel K. A., Eggert J. H., Silvera I. F. & Moss W. C. Optical evidence for the metallization of xenon at 132(5) gpa. Phys. Rev. Lett. 62, 665–668 (1989). [DOI] [PubMed] [Google Scholar]
- Eremets M. et al. Electrical conductivity of xenon at megabar pressures. Phys. Rev. Lett. 85, 2797–2800 (2000). [DOI] [PubMed] [Google Scholar]
- Dewaele A., Loubeyre P., Dumas P. & Mezouar M. Oxygen impurities reduce the metallization pressure of xenon. Phys. Rev. B 86, 014103 (2012). [Google Scholar]
- Hanfland M. et al. Structures of molecular nitrogen at high pressures. The Review of High Pressure Science and Technology 7, 787–789 (1998). [Google Scholar]
- Cynn H. et al. Martensitic fcc-to-hcp transformation observed in xenon at high pressure. Phys. Rev. Lett. 86, 4552–4555 (2001). [DOI] [PubMed] [Google Scholar]
- Akahama Y. & Kawamura H. Pressure calibration of diamond anvil raman gauge to 410 gpa. J. Phys.: Conf. Ser. 215, 012195 (2010). [Google Scholar]
- Prescher C. & Prakapenka V. B. Dioptas: a program for reduction of two-dimensional x-ray diffraction data and data exploration. High Pressure Res. 35, 223–230 (2015). [Google Scholar]
- Toby B. H. & Von Dreele R. B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013). [Google Scholar]
- Petřček V., Dušek M. & Palatinus L. Crystallographic computing system jana2006: general features. Z. Kristallogr. 229, 345–352 (2014). [Google Scholar]
- Gonzalez-Platas J., Alvaro M., Nestola F. & Angel R. EosFit7-GUI: a new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Crystallogr. 49, 1377–1382 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.