Abstract
The aim of this study was to investigate the effects of focused low-intensity pulsed ultrasound (FLIPUS) therapy on the functional and health status of patients with knee osteoarthritis (KOA). A total of 106 subjects with bilateral KOA were randomized sequentially into two groups. Group I received FLIPUS + diclofenac sodium, and group II received sham FLIPUS + diclofenac sodium. The therapeutic effects of the interventions were evaluated by measuring changes in VAS pain, the WOMAC scores, and the LI scores after 10 days of treatment as well as changes in LI and VAS at follow-up, 4 and 12 weeks later. In addition, changes in the range of motion, ambulation speed, and the SF-36 in each group were recorded after 10 days of treatment. Compared with those in group II, patients in group Ishowed significant improvements in VAS, WOMAC, LI, ambulation speed, and most items in the SF-36 after 10 days of treatment. In addition, patients in group I showed significant improvements in LI and VAS at follow-up. There were no FLIPUS-related adverse events during and after the interventions. In conclusion, FLIPUS is a safe and effective treatment modality for relieving pain and improving the functions and quality of life of patients with KOA.
Osteoarthritis (OA), the most common of all arthritic conditions, is characterized by joint pain and stiffness. It is a common and significant chronic musculoskeletal disease that reduces mobility and has a considerable impact on quality of life. Over 50% of people over the age of 65 years have radiological evidence of the disease, and approximately 10% of males and 18% of females have symptomatic knee OA1. Therefore, knee OA is likely to become the fourth most important global cause of disability in females and the eighth most important cause in males2.
Although various management techniques are available for the treatment of OA, there are presently no therapies that modify the onset or progression of OA-induced structural damage3,4. The aims of managing knee OA are to relieve pain, delay complications, and prevent disease progression. Clinical guidelines for managing knee OA, which have been published by the American College of Rheumatology (ACR), the American Academy of Orthopedic Surgeons (AAOS), and the European League Against Rheumatism (EULAR), recommend conservative treatments (i.e., self-management programs, strengthening, low-impact aerobic exercises, weight loss, and neuromuscular education) as well as pharmacologic treatments (i.e., nonsteroidal anti-inflammatory drugs and tramadol)5,6. Surgery is reserved for patients whose symptoms have not responded to other treatments. These recommended methods are not perfect and have many disadvantages: they often are expensive and invasive if surgery is involved, and the adverse effects of NSAIDs are cause for concern. Therefore, innovative and cost-effective approaches that can prevent the development and progression of OA are urgently needed.
Ultrasound (US) treatment has been used as a non-invasive modality for the management of OA for more than 60 years because of its reputed ability to relieve pain7, reduce edema, increase the range of motion, and accelerate tissue repair8 via thermal and non-thermal mechanisms (mechanical effects). US can be administered in either a continuous or a pulsed mode. Pulsed US produces non-thermal effects and is beneficial for cartilage health9,10,11,12,13,14, whereas continuous US aims to generate thermal effects that could enhance fibrous tissue extensibility, increase tissue metabolism, promote capillary permeability, and elevate the pain threshold15,16,17,18. A recent systematic review and meta-analysis suggested that pulsed US is the preferred treatment mode both in terms of more effective pain relief and improved function without significant adverse effects in clinical trials19. In addition, US can be administered in either an unfocused or a focused mode. The basic differences between FLIPUS and traditional US are that the main biological effect of FLIPUS is a mechanical effect and the targeted tissue is cartilage, while the biological effect of traditional US is a thermal effect and the targeted tissues are periarticular soft tissue lesions. The results of a number of studies have suggested that unfocused therapeutic US may be useful for reducing the pain and disability associated with knee OA20,21,22,23,24. However, few studies of focused low-intensity pulsed US(FLIPUS) have been published that describe knee OA rehabilitation.
Therefore, the purpose of this study was to elucidate the effects of FLIPUS for the management of knee OA. The aim of this prospective randomized double-blind placebo-controlled trial was to evaluate the short-term effectiveness of FLIPUS therapy on pain, physical function, ambulation activity, and health-related quality of life (HRQoL) in patients with knee OA.
Subjects and Methods
Subjects
This prospective randomized placebo-controlled clinical trial was approved by the Institutional Review Board and Hospital Research Ethics Committee of Chongqing Medical University, on April 3, 2014 (approval no. 2014005), and was conducted at the Department of Rehabilitation Medicine, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, China between Feb 2015 and Feb 2016. The Chinese Clinical Trial Registry (a non-profit organization, established according to both the WHO International Clinical Trials Register Platform Standard and Ottawa Group Standard)granted full approval of the study protocol, recruitment materials, and consent form (http://www.chictr.org.cn; Registration NO. ChiCTR-IPR- 14005748; Date of registration.2014-12-26). All methods were carried out in accordance with the approved ethical guidelines. After the study had been completely described to the participants, they all signed written informed consent forms.
Each participant was initially interviewed and evaluated by an attending orthopedist, and the evaluation was then confirmed by a well-trained and experienced research team physiatrician. The inclusion criteria were as follows: age ≥ 40 years, knee OA fulfilling the ACR classification criteria25, Kellgren & Lawrence class rating of II, and III26, knee pain, and limitation on most days within the past 6 months. The exclusion criteria were as follows: rheumatoid arthritis, gouty arthritis, infectious arthritis, a history of knee joint replacement on the study knee, current or past (within 6 months) oral or intra-articular corticosteroid use, physiotherapy, acupuncture treatment, the use of exercises specifically for the knee within the past 6 months, a medical condition that precludes safe exercise (such as uncontrolled hypertension, a heart condition, hematological diseases coagulopathy, gastrointestinal ulcers, or a hemorrhage), a history of taking NSAIDs or symptomatic slow-acting drugs for OA (diacerein, hyaluronic acid) within the previous 30 days, or theinability to complete the study.
Study Design
This pilot study was designed according to the CONSORT 2010 statement27. The study procedure is outlined in Fig. 1. All participants who fulfilled the study design criteria were assessed. Specifically, a detailed medical history was taken, a detailed physical examination was performed, and patients were questioned about their age, sex, weight, height, and duration of knee OA. Participants were assigned into group I (FLIPUS + diclofenac sodium sustained-release tablets) or group II (sham FLIPUS + diclofenac sodium sustained-release tablets) at a 1:1 ratio in a random and double-blinded manner. Randomization was performed using a computer generated list of random numbers, and was stratified by gender to ensure equal numbers of males and females in each group. A statistician who was unaware of the enrollment status assigned participants consecutively to treatment codes that corresponded to labels on otherwise identical concealed containers. Participants, investigators, and outcome assessors were blinded to the treatment for the duration of the study. Treatment assignments were not revealed prior to data collection and analysis.
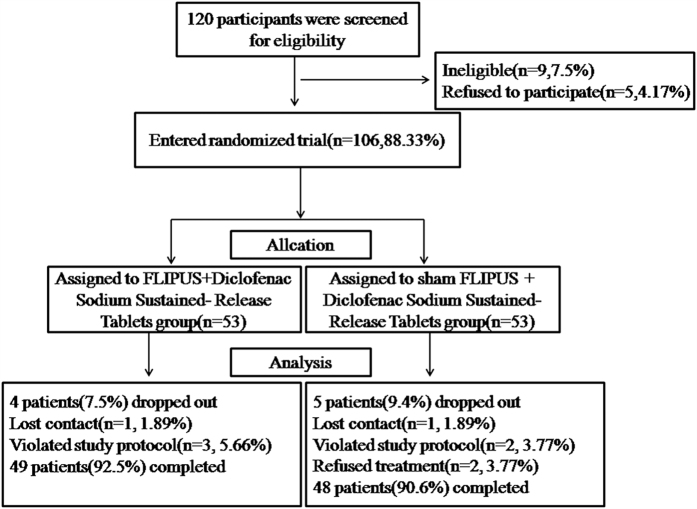
Figure 1. CONSORT diagram showing the disposition of patients in the study.
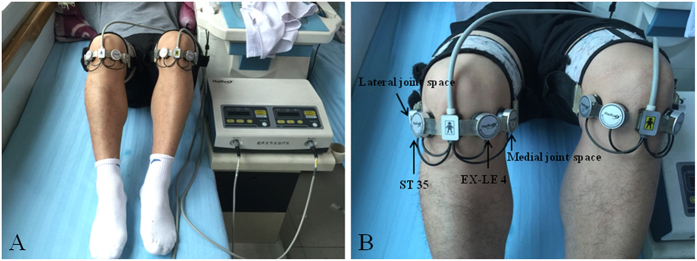
No physiotherapy and pharmacotherapy were given prior to US treatment to either of the groups. US treatment was applied to both sides of the knee. Group I received FLIPUS for 20 min once daily fora total treatment duration of 10 days. Group II received a sham treatment (without energy output) for 20 min for the same treatment period. In low-intensity pulsed US mode, energy output or not, patients wouldn’t feel heat or any sensation. Patients and the US device were separated from each other by a curtain so that patients couldn’t see the power switch position. These measures were intended to enhanceblinding of the patients. All treatments were standardized using a device that placed the participant in a supine position, and the knee was angled ~90° at the flexion position. The four US probes were close to the surface skin of the ST 35 acupoint (located in the depression lateral to the patellar ligament when the knee is flexed), EX-LE 4 acupoint (located in the depression medial to the patellar ligament when the knee is flexed), and interior and lateral knee joint spaces, respectively (Fig. 2). The cartilage of lateral and medial femoral condyle was the tissue being targeted. The Model CZG200 Ultrasound Therapeutic Device for Arthritis used (Chongqing Haifu Medical Technology Co. Ltd., China) had an ultrasonic transducer diameter of 25 mm, a radius-of-curvature of 28 mm, a frequency of 0.6 MHz, a pulse repetition frequency of 300 Hz, a spatial and temporal average intensity (Ista) of 120 mW/cm2, and a duty cycle of 20%28,29. The ellipsoid-shaped acoustic focus was 0.25 mm in diameter and 0.54 mm in length, measured at the full width at half-maximum of the acoustic intensity30. At the same time, US therapy patients in Groups I and II received 75 mg oral sustained-release diclofenac sodium tablets (Beijing Novartis Pharma Ltd., China) once daily for the entire 10 day treatment period31.
Figure 2. Procedure used for FLIPUS.
The ST-35, EX-LE 4, interior, and lateral knee joint spaces have been marked and the heads have been fixed to ST 35, EX-LE 4, and the knee joint space.
Outcome Measures
Clinical assessments of the participants were performed at baseline, after 10 days of treatment, and at follow-up after 4 and 12 weeks. The primary outcome was knee pain on movement for 5 minutes, as assessed using the visual analogue scale (VAS). The VAS instrument consisted of a 10-cm horizontal or vertical lines, a score of 0 cm indicated no pain, whereas 10 cm indicated very severe pain32. The secondary study outcomes were kneefunctional ability (assessed using the Chinese version of the Western Ontario and McMaster Universities Osteoarthritis Index WOMAC score), disability (assessed using Lequesne index LI), ambulation activity (ambulation speed AS), joint motion (active range of motion ROM), and HRQoL (assessed using the Medical Outcomes Study 36 Short-Form Health Survey SF-36).
The active ROM was measured with plastic goniometer with 25-cm movable double arms, marked in 1-degree increments. This device is reportedly reliable if the patient remains in one position for all measurements33. Measurement of knee flexion was performed in the supine position by simultaneously flexing the hip and knee, with the foot on the measured side resting on the table as far as possible. The fully extended knee was considered zero position, and the degrees of maximum flexion, maximum extension, and extension deficit, were recorded. A negative ROM score for extension indicated that the patient was unable to reach the zero position. The angle between maximum flexion and maximum extension was described as the excursion range22.
The ambulation time required to walk a distance 50 meters as fast as possible was measured with a stopwatch and recorded in minutes.
Sample size
Using an open preliminary study on 20 usual consecutive patients with knee OA we calculated that 95% could be improved in the group I and an estimated 75% could improve in the group II. Thus, if the agreed alpha risk is 5% and the beta risk 20%, 50 patients per group or 53 allowing for 5% loss to follow-up was required for this analysis.
Statistical Analyses
Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as means ± SDs (standard deviation). The calculations were based on detecting a mean difference of a 2 cm minimally clinically important difference (MCID) on a 10 cm VAS assuming a standard deviation of 2 cm, a 2-tailed test, an alpha level of 0.05 and a desired power of 80%34. The sociodemographic characteristics of the groups were evaluated using Chi-square tests. Prior to comparisons we tested whether the data were normally distributed and the variances were equal. If so, paired t-sample t-tests were used to compare the pre- and post-treatment changes in each group. Student’s t-tests were used to compare the two groups. Independent samples t-tests were used forcomparisons of the before and after treatment changes between groups, including the mean differences between groups, with 95% confidence intervals (CIs). If the data were not normally distributed non-parametric, Wilcoxon and Mann-Whitney U tests were applied. P-values < 0.05 were considered to be statistically significant.
Results
Patient Characteristics
One-hundred-and-twenty knee OA patients were screened for eligibility (see Fig. 1 for the CONSORT flow diagram). Nine of those (7.5%) screened declined to participate (failed to complete the evaluation and were not interested in treatment). Finally, 106 (recruitment rates: 88.3%) knee OA participants entered the study and underwent randomization. The patients were assigned randomly to group I (n = 53) or group II (n = 53). Ninety-seven (91.5%) of the 106 patients completed the double-blind phase, and nine (8.5%) dropped out (group I, n = 4; 7.5%; group II, n = 5; 9.4%). Their reasons for discontinuation were; loss to follow-up for an unknown reason (group I, n = 1; group II, n = 1), refused treatment (group I, n = 0; group II, n = 2), and violation of the study protocol (group I, n = 3; group II, n = 2). No FLIPUS-related adverse events were reported in either treatment group. There were no significant differences between the group I and group II with respect to age, gender, body mass index (BMI), duration of knee OA, blood pressure,glucose, and Kellgren & Lawrence class rating at baseline (p > 0.05; Table 1).
Table 1. Baseline Demographic and Clinical Characteristics.
| Variable | FLIPUS + NSAIDs | Sham FLIPUS + NSAIDs | P-value |
|---|---|---|---|
| n | 53 | 53 | |
| Sex (M/F) | 14/39 | 16/37 | 0.666 |
| Age, y | 63.42 ± 9.73 | 61.34 ± 10.25 | 0.774 |
| BMI, kg/m2 | 25.79 ± 3.46 | 26.17 ± 5.92 | 0.568 |
| Duration of knee OA, month | 140.36 ± 87.32 | 137.45 ± 92.48 | 0.532 |
| SBP, mmHg | 125.65 ± 8.25 | 126.35 ± 9.79 | 0.676 |
| DBP, mmHg | 75.45 ± 7.85 | 76.38 ± 7.78 | 0.725 |
| Glucose, mmol/L | 9.59 ± 8.24 | 9.69 ± 7.87 | 0.458 |
| Kellgren & Lawrenceclass rating | |||
| Grade II | 46(86.79%) | 44(83.02%) | 0.587 |
| Grade III | 7(13.21%) | 9(16.98%) | |
NOTE: Data expressed as mean ± SD
Abbreviations: BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure.
Primary Outcomes
106 knee OA participants were included in the outcomes analyses and 97 patients completed follow-up. According to intention to treat analyses (ITT), minimally clinically important improvement (reduction in VAS scores more than 2 cm) was observed in 97 patients (91.5% in total, 92.5% in group I and 90.6% in group II respectively). There were no significant differences between groups with respect to VAS at baseline. The VAS measurements improved significantly in both groups after intervention (p = 0.000). However, the VAS reduction in group I was greater than in group II after 10 days of intervention (p = 0.000; Table 2).
Table 2. Primary and Secondary Outcomes Mean Differences in Change From Baseline.
| Variable | Baseline | Endpoint | Between-Group Difference | |||
|---|---|---|---|---|---|---|
| Group I | Group II | Group I | Group II | Mean* 95%CI | p | |
| n | 53 | 53 | 49 | 48 | ||
| Primary Outcome | ||||||
| VAS | 6.98 ± 1.06 | 6.76 ± 1.02 | 1.54 ± 0.81 | 2.28 ± 1.01 | 0.000 | |
| VAS mean change from baseline 95%CI | 5.44 ± 0.84 5.05 to 5.79 | 4.48 ± 0.84 4.08 to 4.88 | 0.96 0.63 to1.29 | 0.000 | ||
| Secondary Outcome | ||||||
| WOMAC | 44.34 ± 10.79 | 42.42 ± 9.39 | 10.92 ± 8.57 | 15.88 ± 5.26 | 0.001 | |
| WOMAC mean change from baseline 95%CI | 33.42 ± 7.99 29.55 to 37.29 | 26.54 ± 5.85 23.52 to 29.56 | 6.88 4.10 to 9.66 | 0.000 | ||
| LI | 7.56 ± 2.73 | 7.10 ± 2.12 | 1.82 ± 1.44 | 2.66 ± 1.12 | 0.000 | |
| LI mean change from baseline 95%CI | 5.74 ± 1.99 4.87 to 6.61 | 4.44 ± 1.39 3.57 to 5.02 | 1.30 0.62 to 1.98 | 0.000 | ||
| ROM,degree | 127.42 ± 6.36 | 127.68 ± 6.75 | 130.78 ± 5.20 | 129.14 ± 6.27 | 0.066 | |
| ROM mean change from baseline 95%CI | −3.36 ± 2.95 −5.56 to−1.05 | −1.46 ± 1.97 −4.05 to 1.13 | 1.90 0.90 to 2.90 | 0.001 | ||
| AS, m/min | 0.61 ± 0.05 | 0.65 ± 0.29 | 0.91 ± 0.26 | 0.74 ± 0.32 | 0.006 | |
| AS mean change from baseline 95%CI | −0.30 ± 0.16 −0.39 to −0.21 | −0.12 ± 0.12 −0.32 to 0.01 | 0.18 0.13 to 0.24 | 0.000 | ||
| SF-36: GH | 40.64 ± 13.58 | 43.14 ± 17.12 | 57.22 ± 10.37 | 45.60 ± 16.55 | 0.007 | |
| GH mean change from baseline 95%CI | 16.58 ± 9.29 −18.39 to −2.21) | 2.46 ± 5.68 3.09 to −1.51 | 14.12 8.38to19.89 | 0.000 | ||
| SF-36: PF | 54.30 ± 12.12 | 57.60 ± 14.75 | 81.20 ± 11.50 | 73.00 ± 11.56 | 0.000 | |
| PF mean change from baseline 95%CI | −26.90 ± 13.32 −29.89 to −6.31 | −15.40 ± 12.32 −18.37to −4.68 | 11.50 6.41 to16.59 | 0.000 | ||
| SF-36: RP | 38.50 ± 29.11 | 42.90 ± 29.90 | 51.50 ± 26.37 | 55.23 ± 19.72 | 0.873 | |
| RP mean change from baseline 95%CI | −13.00 ± 16.24 −8.36 to −19.36 | −12.33 ± 17.69 −7.76to −18.82 | 0.67 0.15 to13.64 | 0.631 | ||
| SF-36: RE | 48.67 ± 22.14 | 43.33 ± 13.88 | 63.20 ± 33.16 | 58.21 ± 25.52 | 0.485 | |
| RE mean change from baseline 95%CI | −14.53 ± 12.13 −19.36 to −10.36 | −14.88 ± 25.03 −20.16 to −12.64 | −0.35 −1.07 to 8.10 | 0.803 | ||
| SF-36: SF | 54.75 ± 14.70 | 51.75 ± 13.83 | 81.00 ± 15.00 | 71.25 ± 14.34 | 0.001 | |
| SF mean change from baseline 95%CI | −26.25 ± 17.34 −32.14 to −20.36 | −19.50 ± 15.19 −25.09 to−13.91 | 6.75 0.28 to 13.22 | 0.047 | ||
| SF-36: BP | 31.30 ± 13.03 | 34.46 ± 13.11 | 68.31 ± 11.56 | 55.22 ± 11.32 | 0.000 | |
| BP mean change from baseline 95%CI | −37.01 ± 14.44 −41.90 to−32.12 | −20.76 ± 9.49 −31.43 to−12.52 | 16.25 11.39 to21.11 | 0.000 | ||
| SF-36:Vitality | 44.00 ± 15.12 | 40.80 ± 11.44 | 65.62 ± 11.39 | 56.70 ± 10.86 | 0.000 | |
| Vitality mean change from baseline 95%CI | −21.62 ± 12.35 −26.93− to −16.31 | −15.90 ± 9.41 −20.33 to− 11.47 | 5.72 1.36 to 10.07 | 0.024 | ||
| SF-36: MH | 42.64 ± 13.51 | 41.36 ± 10.74 | 68.06 ± 10.45 | 59.60 ± 10.39 | 0.000 | |
| MH mean change from baseline 95%CI | −23.64 ± 12.42 −31.65 to−20.11 | −18.66 ± 7.55 -22.48 to−14.84 | 4.98 0.90 to 9.06 | 0.007 | ||
*Mean difference between groups in change from baseline scores.
NOTE. Values are mean ± SD Abbreviations: VAS = visual analog scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; LI = Lequesne index; ROM = range of motion; AS = ambulation speed; SF-36 = short form 36 item general health questionnaire; GH = general health; PF = physical function; RP = role physical; RE = role emotional; SF = social function; BP = bodily pain; MH = mental health.
Secondary Outcomes
There were no significant differences between groups I and group II with respect to WOMAC score, LI, AS, ROM, and SF-36 subscale scoresat baseline. Most secondary measures improved from baseline after both interventions (p < 0.05). However, the reduction in WOMAC and LI scores was greater in group I than group II after 10 days of intervention (p = 0.001 and 0.000, respectively). There was a significantly greater improvement in the ambulation speed in group I compared with group II (p = 0.006). Although there was no difference between the two groups with respect to range of knee motion (p = 0.066) after 10 days of intervention, the total mean increment of range of motion was greater in group I than group II (p = 0.001). There was a significantly greater improvement in the SF-36 subscale scores in group I compared with group II (p < 0.05),except for the physical role and emotional role subscale scores (p = 0.066; Table 2).
Change at Follow-up
The movement pain (VAS scores) and disability (LI scores) measures remained significantly lower at the 12-week follow-up compared with baseline in group I, but were higher than at baseline in group II (p = 0.000). The significantly greater improvements in VAS and LI scores were evident in group I compared with group II at both 4 and 12 weeks follow-up (p < 0.05; Table 3).
Table 3. VAS and LI score measures after 4 and 12 weeks of follow-up.
| VAS scores | LI sores | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4 weeks | 12 weeks | P-value | Baseline | 4 weeks | 12 weeks | P-value | |
| FLIPUS + NSAIDs | 6.98 ± 1.06 | 2.36 ± 1.22 | 6.42 ± 1.57 | 0.000 | 7.56 ± 2.73 | 2.76 ± 1.71 | 6.78 ± 2.48 | 0.000 |
| Sham FLIPUS + NSAIDs | 6.76 ± 1.02 | 4.12 ± 0.75 | 7.18 ± 0.94 | 0.000 | 7.10 ± 2.12 | 3.96 ± 0.90 | 7.84 ± 1.56 | 0.000 |
| P-value | 0.396 | 0.000 | 0.007 | 0.460 | 0.000 | 0.006 | ||
NOTE. Values are mean ± SD. Abbreviations: VAS = visual analog scale; LI = Lequesne index.
Adverse Events
Minor and short lived NSAID (diclofenac sodium)-related adverse events were reported during the interventions. In group I these included headache and dizziness (2%), vertigo (2%), and gastrointestinal symptoms (13%). In group II these included vertigo (2%), transaminase elevation (2%), and gastrointestinal symptoms (15%). There were no significant differences between the two groups with respect to NSAID (diclofenac sodium)-related adverse, and no FLIPUS-related adverse events were found during and after interventions in either group.
Discussion
OA is caused by bone breakdown and cartilage degeneration, including fibrosis, cracks, ulcers, and even loss of the full thickness of the articular cartilage28. Compared with drugs or surgery, US is preferred for its non-invasiveness, minimal adverse effects, and cost-effectiveness. Despite the popularity of US therapy for the treatment of knee OA, its clinical efficacy is somewhat controversial35.
The biological actions of US may vary widely depending on the physical parameters of US. In previous studies continuous ultrasonic waves with frequencies of 1 or 1.5 MHz20,23,36,37,38,39 and 1–2.5 W/cm2 20,23,36,37,38,39 were applied. Knee OA not only affects the articular cartilage, but also involves the entire joint including the subchondral bone, synovial membrane, ligaments, joint capsule, and periarticular muscles. Therefore, previous studies selected the tendons and muscles around the knee joint for unfocused US application, including locations of tendinopathy and enthesopathy23,37,39. The biological effects of unfocused US are mainly considered to be thermal, which alleviates various muscles and tendons spasm38. However, the thermal effects of US for alleviating muscle and tendon spasms are limited for knee OA38. Muscle does not absorb energy well because of its homogeneity, high water content, and low collagen content, and heating muscles and tendons involves treating a larger area than unfocused US can heat effectively38. Although cartilage degeneration is the primary problem in knee OA, in clinical practice few studies have focused US therapy on articular cartilage directly. Although the pressurewaves propagated by US transfer mechanical energy into tissue40,41, a traditional unfocused US therapeutic regimen using higher frequency (≥1 MHz) ultrasonic waves barely propagates through the bone and fails to deliver US energy into the articular cartilage. In addition, energy from unfocused US can diffuse and destroy adjacentstructures42.
Interestingly, our previous in vivo experiment29 demonstrated that the main biological effects of FLIPUS (using a spatial and temporal mean intensity of 120 mW/cm2, a frequency of 0.6 MHz, a pulse repetition frequency of 300 Hz, and a 20% duty cycle) are mechanical, which could be quite different from the biological effects of US reported in other previous studies (thermal effects). We also revealed that FLIPUS at 0.6 MHz could propagate through the patella andsoft tissue to stimulate the cartilage directly, and also protect cartilage by decreasing the joint effusion volume, pro-inflammatory mediators, cell apoptosis, and also inducing cell proliferation29. Recently, pulsed US applied using a low intensity (<1 W/cm2) and low frequency (<1 MHz) had a positive effect on patients with knee OA, including alleviating joint symptoms, relieving joint swelling, increasing joint mobility, and reducing inflammation28. However, in clinical practice fewinvestigations have been conducted into the application of FLIPUS to the degenerated cartilage of patients with knee OA. Therefore, the current randomized, double-blind, placebo-controlled trial was conducted to evaluate the effectiveness of FLIPUS for the treatment of patients with symptomatic knee OA. This is the first study investigating the use of FLIPUS for degenerative cartilage in a clinical trial setting. Because of the controversial clinical efficacy of US, we used NSAIDs in both groups to avoid any potential nonintervention since the AAOS recommends NSAIDs for patients with symptomatic knee OA.
In addition to the physical parameters of FLIPUS, selecting the location for ultrasonic energy application is another key consideration with treatment. Previous studies have suggested that ST35 is a common point for treating pain in the knee joints and knee-related disorders43,44,45,46, whereas EX-LE4 is used for the treatment of both knee pain and knee arthritis47. The interior and lateral knee joint space affords an adequate acoustic channel that allows ultrasonic energy to be transferred to the surface of the intra-articular degenerative cartilage. In the current study both pain and joint function improved, after total treatment duration and follow-up after 3 months, in only patients that received FLIPUS + diclofenac sodium sustained-release tablets. Compared with sham, patients receiving FLIPUS treatment showed statistically significant improvements in all pain, knee functional ability, disability, ambulation activity, and HRQoL measurements. There were no FLIPUS-related adverse events during or after interventions. Based on these results, we conclude that FLIPUS therapy is a safe and effective treatment modality for pain relief and improving functions and HRQoL in patients with symptomatic knee OA.
Individuals with knee OA often complain of joint pain, stiffness, and difficulty with purposeful movement. Pain is not only the predominant symptom of knee OA, but also the main reason for medical consultation. The pain could be caused by several conditions, including loss of articular cartilage, capsular distension by effusion that leads to mechanical pain48, patellar and associated syndromes such as anserine bursitis or prepatellar bursitis due to inflammatory mediators and inflammatory pain49, and micro fractures and subchondral fractures22. In the current study the most striking effect of FLIPUS was pain reduction, which is consistent with previous studies21,23,24,50. In the current study the VAS pain scores improved in both groups. However, the VAS reduction was greater in group I than group II both after 10 days of intervention and during the follow-up period. In our results, small differences were found between groups (5.44 ± 0.84 vs 4.48 ± 0.84) because minor changes of VAS scores were detected when patients feel low pain after treatment51 (VAS ≤ 3/10, VAS scores were 1.54 ± 0.81 vs 2.28 ± 1.01 at endpoint respectively). The lower the severity of patient pain, the less obvious changes of VAS scores are found. We believe FLIPUS + NSAIDs could relieve pain more intensely than NSAIDs alone from the clinical significance we found, though small differences were found between groups at endpoint. We believe a number of ultrasonic effects can relieve pain. First, FLIPUS significantly increases extracellular matrix (ECM) production by downregulating chondrocyte apoptosis and upregulating cell proliferation, which both improve ECM preservation29. Second, FLIPUS can reduce effusion volumes to relieve mechanical pain29. Finally, FLIPUS might attenuate the release of inflammatory mediators (prostaglandin E2 and nitric oxide) and keeping them low over time, which could relieve inflammatory pain29.
Ambulation speed and joint ROM are important indicators of functional performance39, and restricted flexion of the knee appears to be an important determinant of disability in patients with OA52. The soft tissue around the knee OA may become fibrotic, contracted, or shortened when subjected to immobilization or inactivity due to joint pain, thereby decreasing the ROM and decreasing the patient’s ability to walk22. A previous study found strong correlations between knee joint ROM and disability53. Furthermore, restricted flexion of the knees was a strong risk factor for locomotor disability during activities including walking and climbing stairs54. Previous studies suggested the use of WOMAC for assessing the functional ability of the knee in patients with knee OA55,56, and the culturally and linguistically validated Chinese version of the WOMAC for mainland China was psychometrically robust in its validity, reliability, and sensitivity to change for patients with knee OA57.
The LI has been validated and is used for assessing kneedisability in patients with knee OA58. In the current study, the improvement in functional and disability status according to WOMAC and LI scores was significantly different in both groups after treatment compared with at baseline. However, the reduction in WOMAC and LI scores was greater in group I compared with group II both after 10 days of intervention and during follow-up. We also found that significantly greater improvements in ambulation speed and increment range of motion were evident in group I compared with group II. We hypothesize that the improved function and disability were attributable to the ability of ultrasonic waves to relieve pain. Therefore, the combination of FLIPUS and NSAIDs had better analgesia effects than NSAIDs alone.
Because of pain, loss of joint function, anddeformities, patients with knee OA have been reported to gradually reduce their physical activity, which consequently worsens their quality of life59,60. In the current study, HRQoL was assessed in knee OA patients using SF-36. The SF-36 was originally developed as an instrument for health surveying, and it has been used widely as a sensitive health status measure for clinical evaluation61. The SF-36 contains eight domains: the first four (physical function, physical role, bodily pain, and general health perceptions) assess physical health, and the last four (vitality, social function, emotional role, and mental health) assess mental health62. Several previous studies have revealed that pain severity, disability, and loss of joint function are negatively associated with quality of life in patients with OA61,62. In the current study there were significant differences between the two groups in six domains (general health, physical function, social function, bodily pain, vitality, and mental health) in SF-36. This suggests that FLIPUS and NSAIDs could improve HRQoL in patients with knee OA significantly compared with NSAIDs alone. Improvements in the HRQoL of patients with knee OA are due to pain relief and improved functional ability after the application of FLIPUS. In addition, the current data showed that there were no differences between the two groups with respect to the physical role and emotional role sub-scale scores because, even in the presence of severe joint pain and disability, the patients maintained their work activities and domestic chores during the study period.
No adverse effects have been reported with US in previous trials, and the current data suggest that US therapy is safe20,35,50. Similarly, no adverse events occurred during or after the FLIPUS treatment in the current study; therefore FLIPUS can be used safely in patients with knee OA.
In the current study, FLIPUS proved to be a safe and effective treatment modality for relieving pain, however,it has become clear that one cannot conclude a pain treatment is clinically significant based strictlyon statistical significance63. As such, the minimal clinically important difference (MCID) of VAS has been accounted for. The minimal detectable change (MDC) scores for the VAS in the group I was very high at the end of the treatment and 4 weeks later; the scores were greater than 4.5 cm. This change was greater than that of the minimal clinically important difference of 2.0 cm64 and our results are clinically significant. However, MDC scores for the VAS in both groups were lower than 2 cm 12 weeks later. These results indicated pain reduction of FLIPUS lasted for about 4 weeks after treatment.
The present study has some limitations that should be discussed. First, all participants included this study were enrolled from a single center, and there was a relatively small sample size. Therefore, future studies with larger populations and a multi-center clinical trial are needed. Second, the long-term effectiveness of FLIPUS should also be assessed in a continuing study. Third, we compared effectiveness between combination of FLIPUS + NSAIDs and NSAIDs in current study. The difference effectiveness between FLIPUS and NSAIDs on the functional status of patients with KOA is not clear which will be investigated in our future continuing study. Finally, the physical parameters of FLIPUS used in this trial were based on our previous in vivo experiment37, and an important direction for future research is elucidating the optimum acoustic exposure parameters of FLIPUS in patients.
In conclusion, the current study revealed that FLIPUS is a safe and effective treatment modality that causes pain relief and improves function and HRQoL in patients with knee OA. This study makes a contribution that may have important implications for future US research, particularly in terms of managing knee pain, stiffness, physical function, and HRQoL in knee OA.
Additional Information
How to cite this article: Jia, L. et al. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: a randomized, double blind, placebo-controlled trial. Sci. Rep. 6, 35453; doi: 10.1038/srep35453 (2016).
Acknowledgments
We gratefully acknowledge the financial support provided by the National Basic Research Program 973 of China (Grant No. 2012 CB722402 and Grant No. 2011 CB707900), the National Natural Science Foundation of China (Grant No. 81127901, 30830040, 11274404, 11574039, 31000435 and 30970827), and the Medical Scientific Research Projects Foundation of ChongQing (Grant No. 2012-2-064).
Footnotes
Author Contributions L.J., J.C. and W.C. conceived and designed the experiments. L.J. and J.C. performed the experiments and analyzed the data. L.J.and J.C. wrote the main manuscript text. Y.W. prepared all figures and tables. All authors reviewed the manuscript.
References
- Woolf A. & Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 81, 646–656 (2003). [PMC free article] [PubMed] [Google Scholar]
- Murra C. J. L. & Lopez A. D. The Global Burden of Disease. vol 1.Cambridge, Harvard University Press, 1996.
- Matthews G. L. & Hunter D. J. Emerging drugs for osteoarthritis. Expert Opin Emerg Drugs. 16, 479–491 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. et al. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 18, 476–499 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 15, 981–1000 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 16, 137–162 (2008). [DOI] [PubMed] [Google Scholar]
- Ter Haar G. Therapeutic ultrasound. Eur J Ultrasound. 9, 3–9 (1999). [DOI] [PubMed] [Google Scholar]
- van der Windt D. A. et al. Ultrasound therapy for musculoskeletal disorders: a systematic review. Pain. 81, 257–271 (1999). [DOI] [PubMed] [Google Scholar]
- Cook S. D. et al. Improved cartilage repair after treatment with low intensity pulsed ultrasound. Clin Orthop Relat Res. 391, (Suppl).S231–S243 (2001). [DOI] [PubMed] [Google Scholar]
- Cui J. H., Park K., Park S. R. & Min B. H. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: an in vivo study. Tissue Eng. 12, 75–82 (2006). [DOI] [PubMed] [Google Scholar]
- Huang M. H., Ding H. J., Chai C. Y., Huang Y. F. & Yang R. C. Effects of sonication on articular cartilage in experimental osteoarthritis. J Rheumatol. 24, 1978–1984 (1997). [PubMed] [Google Scholar]
- Huang M. H., Yang R. C., Ding H. J. & Chai C. Y. Ultrasound effect on level of stress proteins and arthritic histology in experimental arthritis. Arch Phys Med Rehabil. 80, 551–556 (1999). [DOI] [PubMed] [Google Scholar]
- Gurkan I. et al. Modification of osteoarthritis in the guinea pig with pulsed low-intensity ultrasound treatment. Osteoarthritis Cartilage. 18, 724–733 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K. et al. Low-intensity pulsed ultrasound (LIPUS) increases the articular cartilage type II collagen in a rat osteoarthritis model. J Orthop Res. 28, 361–369 (2010). [DOI] [PubMed] [Google Scholar]
- Baker K. G., Robertson V. J. & Duck F. A. A review of therapeutic ultrasound: biophysical effects. Phys Ther. 81, 1351–1358 (2001). [PubMed] [Google Scholar]
- Srbely J. Z. et al. Stimulation of myofascial trigger points with ultrasound induces segmental antinociceptive effects: a randomized controlled study. Pain. 139, 260–266 (2008). [DOI] [PubMed] [Google Scholar]
- Rutjes A. W., Nüesch E., Sterchi R. & Jüni P. Therapeutic ultrasound for osteoarthritis of the knee or hip (Review). Cochrane Database Syst Rev. 20, CD003132 (2010). [DOI] [PubMed] [Google Scholar]
- Johns L. D. Nonthermal effects of therapeutic ultrasound:the frequency resonance hypothesis. J Athl Train. 37, 293–299 (2002). [PMC free article] [PubMed] [Google Scholar]
- Zeng C. et al. Effectiveness of continuous and pulsed ultrasound for the management of knee osteoarthritis: Systematic review and network meta-analysis. Osteoarthritis Cartilage. 22, 1090–1099 (2014). [DOI] [PubMed] [Google Scholar]
- Cetin N., Aytar A., Atalay A. & Akman M. N. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 87, 443–451 (2008). [DOI] [PubMed] [Google Scholar]
- Kozanoglu E., Basaran S., Guzel R. & Guler-Uysal F. Short term efficacy of ibuprofen phonophoresis versus continuous ultrasound therapy in knee osteoarthritis. Swiss Med Wkly. 133, 333–338 (2003). [DOI] [PubMed] [Google Scholar]
- Huang M. H., Yang R. C., Lee C. L., Chen T. W. & Wang M. C. Preliminary results of integrated therapy for patients with knee osteoarthritis. Arthritis Rheum. 53, 812–820 (2005). [DOI] [PubMed] [Google Scholar]
- Huang M. H., Lin Y. S., Lee C. L. & Yang R. C. Use of ultrasound to increase effectiveness of isokinetic exercise for knee osteoarthritis. Arch Phys Med Rehabil. 86, 1545–1551 (2005). [DOI] [PubMed] [Google Scholar]
- Ozgonenel L., Aytekin E. & Durmusoglu G. A double-blind trial of clinical effects of therapeutic ultrasound in knee osteoarthritis. Ultrasound Med Biol. 35, 44–49 (2009). [DOI] [PubMed] [Google Scholar]
- Hochberg M. C. et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of knee. American College of Rheumatology. Arthritis Rheum. 38, 1541–1546 (1995). [DOI] [PubMed] [Google Scholar]
- Kellgren J. H. & Lawrence J. S. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 16, 494–502 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D. et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ.340,c869 (2010). [DOI] [PMC free article] [PubMed]
- Yang P. F. et al. Efficacy of ultrasound in the treatment of osteoarthritis of the knee. Orthop Surg. 3, 181–187 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Chen J., Wang Y., Zhang Y. & Chen W. Focused low-intensity pulsed ultrasound affects extracellular matrix degradation via decreasing chondrocyte apoptosis and inflammatory mediators in a surgically induced osteoarthritic rabbit model. Ultrasound Med Biol. 42, 208–219 (2015). [DOI] [PubMed] [Google Scholar]
- Yoo S. S. et al. Focused ultrasound modulates region-specific brain activity. Neuroimage. 56, 1267–1275 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M. C. et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 64, 465–74 (2012). [DOI] [PubMed] [Google Scholar]
- Carlsson A. M. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analog scale. Pain. 16, 87–101 (1983). [DOI] [PubMed] [Google Scholar]
- Rothstein J. M., Miller P. J. & Roettger R. F. Goniometric reliability in a clinical setting: elbow and knee measurements. Phys Ther. 63, 1611–1615 (1983). [DOI] [PubMed] [Google Scholar]
- Pillastrini P. et al. Effectiveness of neuromuscular taping on painful hemiplegic shoulder: a randomised clinical trial. Disabil Rehabil. 38, 1603–1609 (2016). [DOI] [PubMed] [Google Scholar]
- Ozgönenel L., Aytekin E. & Durmuşoglu G. A double-blind trial of clinical effects of therapeutic ultrasound in knee osteoarthritis. Ultrasound Med Biol. 35, 44–49 (2009). [DOI] [PubMed] [Google Scholar]
- Park K., Hoffmeister B., Han D. K. & Hasty K. Therapeutic ultrasound effects on interleukin-1beta stimulated cartilage construct in vitro. Ultrasound Med Biol. 33, 286–295 (2007). [DOI] [PubMed] [Google Scholar]
- Cakir S. et al. Efficacy of therapeutic ultrasound for the management of knee osteoarthritis: a randomized, controlled, and double-blind study. Am J Phys Med Rehabil. 93, 405–412 (2014). [DOI] [PubMed] [Google Scholar]
- Falconer J., Hayes K. W. & Chang R. W. Effect of ultrasound on mobility in osteoarthritis of the knee. A randomized clinical trial. Arthritis Care Res. 5, 29–35 (1992). [DOI] [PubMed] [Google Scholar]
- Ulus Y. et al. Therapeutic ultrasound versus sham ultrasound for the management of patients with knee osteoarthritis: a randomized double-blind controlled clinical study. Int J Rheum Dis. 15, 197–206 (2012). [DOI] [PubMed] [Google Scholar]
- Gleizal A., Li S., Pialat J. B. & Beziat J. L. Transcriptional expression of calvarial bone after treatment with low-intensity ultrasound: An in vitro study. Ultrasound Med Biol. 32, 1569–1574 (2006). [DOI] [PubMed] [Google Scholar]
- Reher P., Elbeshir el-NI., Harvey W., Meghji S. & Harris M. The stimulation of bone formation in vitro by therapeutic ultrasound. Ultrasound Med Biol. 23, 1251–1258 (1997). [DOI] [PubMed] [Google Scholar]
- Jung Y. J. et al. Focused low-intensity pulsed ultrasound enhances bone regeneration in rat calvarial bone defect through enhancement of cell proliferation. Ultrasound Med Biol. 41, 999–1007 (2015). [DOI] [PubMed] [Google Scholar]
- Berman B. M. et al. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med. 141, 901–910 (2004). [DOI] [PubMed] [Google Scholar]
- Tukmachi E., Jubb R., Dempsey E. & Jones P. The effect of acupuncture on the symptoms of knee osteoarthritis-an open randomised controlled study. Acupunct Med. 22, 14–22 (2004). [DOI] [PubMed] [Google Scholar]
- Sangdee C. et al. Electroacupuncture versus diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. BMC Complement Altern Med. 2, 3 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X. et al. Effect of combined laser acupuncture on knee osteoarthritis: a pilot study. Laser Med Sci. 24, 129–136 (2009). [DOI] [PubMed] [Google Scholar]
- Taechaarpornkul W., Suvapan D., Theppanom C., Chanthipwaree C. & Chirawatkul A. Comparison of the effectiveness of six and two acupuncture point regimens in osteoarthritis of the knee: a randomised trial. Acupunct Med. 27, 3–8 (2009). [DOI] [PubMed] [Google Scholar]
- Chan K. K., Sit R. W., Wu R. W. & Ngai A. H. Clinical, radiological and ultrasonographic findings related to knee pain in osteoarthritis. PLoS One. 9, e92901 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible H. G. & Schmidt R. F. Excitation and sensitization of fine articular afferents from cat’s knee joint by prostaglandin E2. J Physiol. 403, 91–104 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tascioglu F., Kuzgun S., Armagan O. & Ogutler G. Short term effectiveness of ultrasound therapy in knee osteoarthritis. J Int Med Res. 38, 1233–1242 (2010). [DOI] [PubMed] [Google Scholar]
- Farrar J. T., Pritchett Y. L. & Robinson M. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain. 11, 109–118 (2010). [DOI] [PubMed] [Google Scholar]
- Steultjens M. P., Dekker J., van Baar M. E., Oostendorp R. A. & Bijlsma J. W. Range of joint motion and disability in patients with osteoarthritis of the knee or hip. Rheumatology (Oxford). 39, 955–961 (2000). [DOI] [PubMed] [Google Scholar]
- Bergstrom G. et al. Functional consequences of joint impairment at age 79. Scand J Rehabil Med. 17, 183–190 (1985). [PubMed] [Google Scholar]
- Odding E. et al. The association of abnormalities on physical examination of the hip and knee with locomotor disability in the Rotterdam study. Br J Rheumatol. 35, 884–890 (1996). [DOI] [PubMed] [Google Scholar]
- Rejeski W. J. et al. The evaluation of pain in patients with knee osteoarthritis: the knee pain scale. J Rheumatol. 22, 1124–1129 (1995). [PubMed] [Google Scholar]
- Roos E. M., Klassbo M. & Lohmander L. S. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and McMaster Universities. Scand J Rheumatol. 28, 210–215 (1999). [DOI] [PubMed] [Google Scholar]
- Symonds T., Hughes B., Liao S., Ang Q. & Bellamy N. Validation of the Chinese Western Ontario and McMaster Universities Osteoarthritis Index in Patients From Mainland China With Osteoarthritis of the Knee. Arthritis Care Res (Hoboken). 67, 1553–1560 (2015). [DOI] [PubMed] [Google Scholar]
- Lequesne M. G., Mery C., Samson M. & Gerard P. Indexes of severity for osteoarthritis of the hip and knee. Validation value in comparison with other assessment tests. Scand J Rheumatol Suppl. 65, 85–89 (1987). [DOI] [PubMed] [Google Scholar]
- Krasnokutsky S., Samuels J. & Abramson S. B. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis. 65, 222–228 (2007). [PubMed] [Google Scholar]
- Cook C., Pietrobon R. & Hegedus E. Osteoarthritis and the impact onquality of life health indicators. Rheumatol Int. 27, 315–321 (2007). [DOI] [PubMed] [Google Scholar]
- Alkan B. M., Fidan F., Tosun A. & Ardıçoğlu O. Quality of life and self-reported disability in patients with knee osteoarthritis. Mod Rheumatol. 24, 166–171 (2014). [DOI] [PubMed] [Google Scholar]
- Figueiredo Neto E. M., Queluz T. T. & Freire B. F. Physical activity and its association with quality of life in patients with osteoarthritis. Rev Bras Reumatol. 51, 544–549 (2011). [PubMed] [Google Scholar]
- Gatchel R. J. & Mayer T. G. Testing minimal clinically important difference: Consensus or conundrum. Spine J. 10, 321–327 (2010). [DOI] [PubMed] [Google Scholar]
- Villafañe J. H., Cleland J. A. & Fernández-de-Las-Peñas C. The effectiveness of a manual therapy and exercise protocol in patients with thumb carpometacarpal osteoarthritis: a randomized controlled trial. J Orthop Sports Phys Ther. 43, 204–213 (2013). [DOI] [PubMed] [Google Scholar]