Abstract
Recurrent spontaneous abortion (RSA) is a common health problem that affects women of reproductive age. Recent studies have indicated that microRNAs are important factors in miscarriage. This study investigated the role of miR-16 in regulating vascular endothelial growth factor (VEGF) expression and the pathogenesis of RSA. In this report, clinical samples revealed that miR-16 expression was significantly elevated in the villi and decidua of RSA patients. In vitro, miR-16 upregulation inhibited human umbilical vein endothelial cell proliferation, migration and tube formation. Conversely, the downregulation of miR-16 reversed these effects. In vivo, we demonstrated that abnormal miR-16 levels affect the weights of the placenta and embryo and the number of progeny and microvascular density, as well as cause recurrent abortions by controlling VEGF expression in pregnant mice. VEGF, a potential target gene of miR-16, was inversely correlated with miR-16 expression in the decidua of clinical samples. Furthermore, the luciferase reporter system demonstrated that miR-16 was found to directly downregulate the expression of VEGF by binding a specific sequence of its 3′-untranslated region (3′UTR). Collectively, these data strongly suggest that miR-16 regulates placental angiogenesis and development by targeting VEGF expression and is involved in the pathogenesis of RSA.
Spontaneous abortion, a common complication of pregnancy, accounts for approximately 15% of clinically recognized pregnancies1. Recurrent spontaneous abortion (RSA) has been defined as the unintentional termination of two or more consecutive pregnancies before 20 weeks of gestation2. The World Health Organization (WHO) has estimated that 1–5% of all women suffer from recurrent pregnancy loss during reproductive age3. The risk of a spontaneous abortion was 8.9% in women aged 20–24 years and 74.7% in those aged 45 years or older4. The causes of RSA have been generally recognized to include anatomical issues, genetics and hormonal abnormalities, exposure to environmental factors, infection, smoking and alcohol consumption, psychological trauma and stressful life events and immune regulatory protein defects5,6. However, for half of the patients with RSA, a definitive cause for recurrent miscarriages cannot be identified7. Thus, it is urgent that the factors involved in RSA are identified and that we gain a clearer understanding of its causes.
The placenta, a maternal-fetal barrier, provides the developing fetus with all of the nutrients necessary for its development8. To effectively exchange nutrients and waste between mother and fetus throughout gestation, the placenta needs to maintain its own circulation and metabolism via angiogenesis9. Angiogenesis involves the formation of new branches from pre-existing vessels and the remodeling of an existing vascular network10. The three steps of angiogenesis—initiation, proliferation-invasion, and maturation-differentiation—are all critical for normal uteroplacental development11. Abnormal angiogenesis is considered one of the most important causes of RSA. Identifying a possible mechanism for the regulation of placental angiogenesis might lead to a treatment to combat RSA.
miRNAs are a class of conservative, 19- to 24-nucleotide non-protein-coding RNAs that can regulate gene expression by targeting messenger RNAs (mRNAs) via binding to specific sites in the 3′UTR, resulting in translational repression, cleavage or destabilization12,13. miRNAs elicit critical changes in gene expression programs that underlie diverse aspects of biology, such as embryogenesis and regulation of cellular differentiation, metabolism, proliferation and apoptosis14. An increasing number of studies have demonstrated that miRNAs are expressed abundantly in the human placenta, and dysregulation of miRNAs has been associated with RSA pathogenesis7,15.
The objectives of the current study were to investigate the expression level of miR-16 in RSA, to evaluate the role of miR-16 on placental angiogenesis both in vitro and in vivo, to provide evidence for the regulation of VEGF expression by miR-16 and to reveal the role this regulation plays in the pathogenesis of RSA (see Supplementary Fig. S1).
Results
The abundance of mir-16 in the villi and deciduais elevated in RSA patients
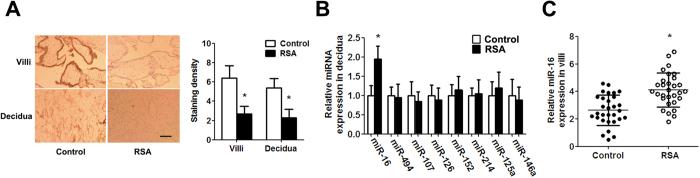
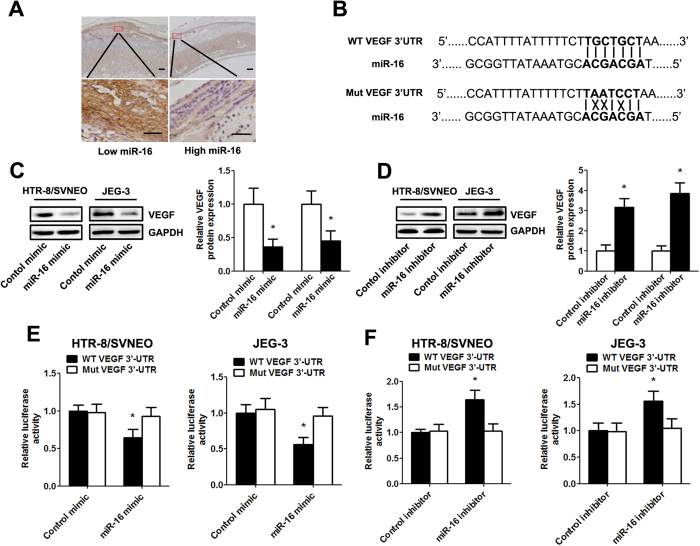
Immunohistochemistry revealed that VEGF expression was remarkably lower in RSA patients compared with control pregnant women (Fig. 1A). To investigate whether a specific miRNA regulates VEGF expression in RSA, we detected a total of 8 potential miRNAs that targeted the VEGF gene. The expression of these 8 miRNAs was demonstrated by qPCR. We found that the expression of miR-16 in the decidua of the RSA group was significantly greater than that in the decidua of the control group (Fig. 1B). The higher expression of miR-16 was confirmed in the villi of RSA cases (Fig. 1C). Therefore, these results suggested that miR-16 is overexpressed in RSA.
Figure 1. Aberrantly high miR-16 expression in RSA cases.
(A) Expression of VEGF in the villi and decidua as revealed by immunohistochemistry (scale bar = 50 μm). The immunostaining intensity of VEGF was evaluated according to IRS. (B) Mature miRNA expression was determined by TaqMan qPCR in the decidua. (C) miR-16 expression was determined by TaqMan qPCR in villi. Data are expressed as the mean ± SD. *P < 0.05 vs. control group, n = 30.
mir-16 inhibits placentation and angiogenesis in vitro and in vivo
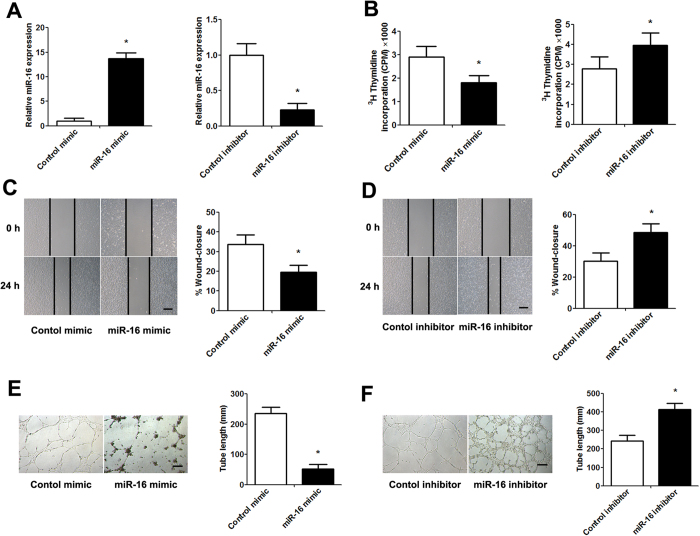
Considering the inhibitory effect of miR-16 on VEGF expression, which is related to placental angiogenesis, maintenance and stabilization, we assessed the anti-angiogenic activity of miR-16 by transfecting Human Umbilical Vein Endothelial Cells (HUVECs) with a miR-16 mimic/inhibitor in vitro. qPCR analysis demonstrated that transfecting HUVECs with the miR-16 mimic increased the miR-16 levels by approximately 14-fold over the endogenous transcription level. In contrast, transfecting HUVECs with a miR-16 inhibitor significantly decreased the miR-16 levels (Fig. 2A). It is known that angiogenesis demands the proliferation and migration of endothelial cells. The effects of miR-16 on the VEGF-induced proliferation and migration of HUVECs were evaluated. The overexpression of miR-16 in HUVECs significantly inhibited HUVEC proliferation and migration, whereas the reduction of miR-16 expression promoted HUVEC proliferation and migration (Fig. 2B–D). In tube formation assays, the length of formed tubules was calculated using inverted phase contrast microscopy, which directly revealed the ability of the HUVECs to form tubular structures. Moreover, we discovered that miR-16 overexpression significantly repressed HUVEC tube formation, whereas transfection with the miR-16 inhibitor increased tube formation (Fig. 2E,F).
Figure 2. The effect of miR-16 on HUVEC proliferation, migration and tube formation.
HUVECs were transfected with control or miR-16 mimic/inhibitor for 48 h. (A) qPCR analysis was performed to examine miR-16 levels. (B) The level of 3H-thymidine incorporation was measured after transfection. (C,D) Representative images were taken at 0 and 24 h to assess the cell migration into the open space (scale bar = 50 μm). Quantification of the percentage of the distance migrated was achieved by measuring these distances in 5 high-power fields. (E,F) Representative images of tube formation (scale bar = 50 μm). Quantification of the vascular tube structure was conducted by measuring the total tubule length in 5 high-power fields. Data are expressed as the mean ± SD. *P < 0.05 vs. control mimic/inhibitor group, n = 5.
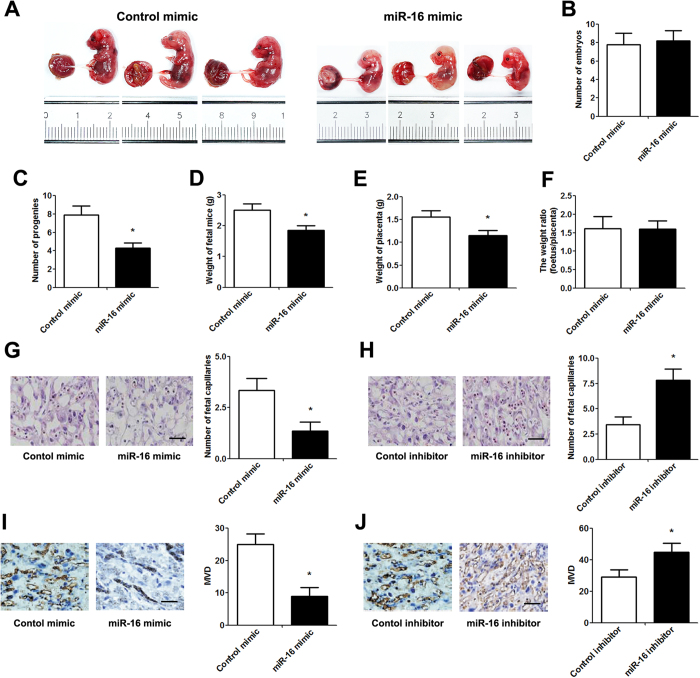
To identify the effect of miR-16 on placental angiogenesis in vivo, cholesterol-conjugated miRNAs were locally injected into the placenta using a percutaneous ultrasound-guided approach on day 7.5 of pregnancy. Mice fetuses and placentas were collected 7 days after injection (Fig. 3A). Although miR-16 overexpression did not affect the number of embryos, the number of progeny was significantly reduced after miR-16 injection (Fig. 3B,C). Moreover, the weights of the placenta and embryo were significantly decreased in the miR-16 overexpression group compared with the control group (Fig. 3D,E). However, the weight ratio of fetus/placenta at 14.5 days of embryogenesis did not differ between the two groups (Fig. 3F). Subsequent histologic analysis revealed that the total placental vasculature and the number of capillaries were significantly inhibited by miR-16 mimic injection but significantly promoted by miR-16 inhibitor injection (Fig. 3G,H, Table 1). Immunohistochemistry also showed similar results in which miR-16 overexpression inhibited placental angiogenesis, whereas the inhibition of miR-16 expression induced placental angiogenesis (Fig. 3I,J).
Figure 3. Impact of miR-16 on placentation and angiogenesis.
Cholesterol-conjugated miRNAs (10 nmol) were locally injected into the placenta using apercutaneous ultrasound-guided approach at 7.5-days of embryogenesis. (A) Mouse fetuses and placentas at 14.5days of embryogenesis. (B) The number of embryos at pregnancy. (C) Average litter size after birth. (D) The weight of fetal mice.(E) The weight of placenta. (F) The weight ratio (fetus/placenta). (G,H) Histological analysis of decidua from different groups using hematoxylin and eosin staining (scale bar = 50 μm). The capillaries were manually counted in 5 high-power fields. (I,J) CD34 staining by immunochemistry in decidua (scale bar = 50 μm). MVD was assessedusingthe Weidner method. Data are expressed as the mean ± SD. *P < 0.05 vs. control mimic/inhibitor group, n = 5.
Table 1. Comparison of placental morphological parameters.
| Morphological parameters | Control mimic (n = 5) | miR-16 mimic (n = 5) | Control inhibitor (n = 5) | miR-16 inhibitor (n = 5) |
|---|---|---|---|---|
| Proportion of spongiotrophoblast | 0.206 ± 0.047 | 0.208 ± 0.021 | 0.205 ± 0.026 | 0.207 ± 0.028 |
| Proportion of labyrinth | 0.788 ± 0.057 | 0.791 ± 0.066 | 0.781 ± 0.049 | 0.790 ± 0.061 |
| Total volume of spongiotrophoblast (mm3) | 249 ± 78.6 | 148 ± 43.4* | 254 ± 68.8 | 343 ± 72.6# |
| Total volume of labyrinth (mm3) | 609 ± 102 | 449 ± 78.1* | 599 ± 108 | 849 ± 126# |
| Total volume of fetal capillaries (mm3) | 72.3 ± 15.6 | 41.6 ± 10.2* | 70.8 ± 14.3 | 102.7 ± 18.8# |
| Exchange barrier (μm) | 2.02 ± 0.056 | 5.13 ± 0.176* | 1.98 ± 0.069 | 1.06 ± 0.044# |
*P < 0.05 vs. control mimic; #P < 0.05 vs. control inhibitor.
VEGF is a direct target of mir-16
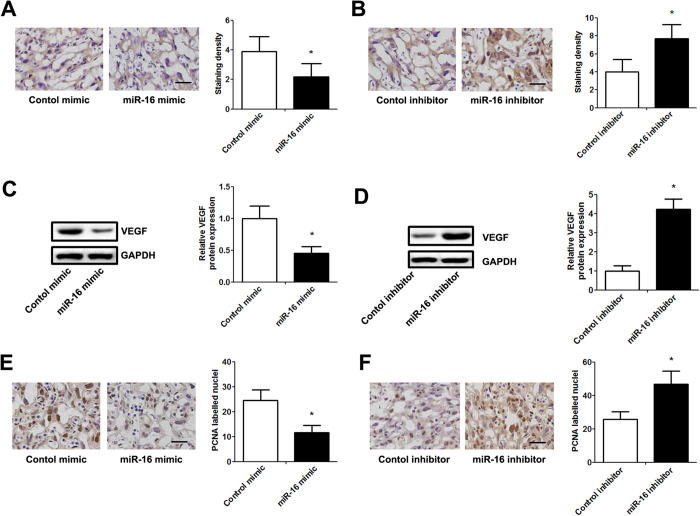
miRNAs exert their function mainly by targeting the 3′UTR of target mRNAs. To demonstrate whether miR-16 affects the expression of VEGF, we first evaluated the correlation between VEGF expression and the miR-16 level in placentas of pregnant mice. Immunohistochemistry and western blotting confirmed that the VEGF protein expression level was significantly decreased by miR-16 mimic injection but significantly elevated by miR-16 inhibitor injection (Fig. 4A–D). In addition, overexpression due to injection of the placenta with miR-16 mimics significantly decreased the protein expression of Proliferating Cell Nuclear Antigen (PCNA). However, injection with miR-16 inhibitors significantly upregulated the PCNA protein levels (Fig. 4E,F).
Figure 4. miR-16 regulates VEGF expression and placental proliferation in vivo.
Cholesterol-conjugated miRNAs (10 nmol) were locally injected into the placentas of pregnant mice for 7 days. (A,B) Representative images of immunostaining for VEGF in decidua from different groups (scale bar = 50 μm). The immunostaining intensity was evaluated according to IRS. (C,D) The expression of VEGF protein was analyzed by western blot. The relative amount of VEGF was normalized to the internal protein GAPDH. (E,F) Representative images of immunolabeling for PCNA in decidua from different groups (scale bar = 50 μm). The intensity of PCNA expression was evaluated in a manner corresponding to positively labelled nuclei in 5 high-power fields. Data are expressed as the mean ± SD. *P < 0.05 vs. control mimic/inhibitor group, n = 5.
In the clinical specimens, the VEGF levels in the decidua with high miR-16 expression were significantly lower than those in the decidua with low miR-16 expression (Fig. 5A). To verify whether miR-16 directly suppresses VEGF expression, we analyzed the potential miR-16 seed sequence in the 3′UTR of VEGF using the online publically available algorithms (TargetScan andmiRanda) and cloned a wild-type or mutant fragment containing the binding sequence (376 bp) into the luciferase reporter gene system (Fig. 5B). Western blot analysis revealed that VEGF expression was significantly downregulated by miR-16 mimic transfection in HTR-8/SVNEO and JEG-3 cells. However, the inhibition of miR-16 upregulated VEGF levels (Fig. 5C,D). The miR-16 mimic/inhibitor and the redesigned luciferase reporter plasmid were then co-transfected into HTR-8/SVNEO and JEG-3 cells. The results showed that overexpression of miR-16 in both cell lines led to significantly reduced luciferase activity for the wild-type, whereas the miR-16 knockdown increased wild-type luciferase activity. In contrast, the activity of the luciferase reporter gene linked to the 3′UTR of mutant VEGF did not change in the presence of the miR-16 mimic/inhibitor (Fig. 5E,F).
Figure 5. miR-16 directly targeted VEGF 3′UTR and down-regulates its expression.
(A) An inverse relationship between the expression of miR-16 and VEGF was found in clinical decidual specimens of RSA patients (scale bar = 50 μm). (B)Predicted miR-16 target sequences in the 3′UTR of VEGF are shown (solid lines indicate matching base pairs and crosses represent non-matching base pairs). (C,D) Western blotting analysis of HTR-8/SVNEO and JEG-3 cells following miRNA mimic/inhibitor transfection for 48 h. (E,F) HTR-8/SVNEO and JEG-3 cells were co-transfected with the luciferase reporter plasmid carrying the wild type/mutant binding sequences of VEGF and the miRNA mimic/inhibitor. A dual-luciferase reporter system analysis was performed. Data are expressed as the mean ± SD. *P < 0.05 vs. control mimic/inhibitor group, n = 5.
Discussion
The identification and validation of novel biomarkers for RSA is a high priority not only for the diagnosis and clinical follow-up of RSA but also for defining novel therapeutic strategies. During the past few years, although there have been substantial advances in the awareness of RSA, major limitations still exist in managing RSA. Endometrial angiogenesis and decidualization are prerequisites for a successful implantation and a good outcome of pregnancy. In the uterus during pregnancy, critical angiogenic signals likely are produced by the decidualizing endometrial cells acting on the endothelial cells to promote their proliferation, migration and differentiation16. Accumulating evidence shows that placental angiodysplasia and endothelial dysfunction may contribute to cases of RSA17,18. A majority of pregnancies that result in miscarriage are believed to be caused by defective placentation associated with an absence of physiological changes in maternal spiral arteries19.
miRNAs are believed to play important roles in a variety of gynecological reproductive diseases including leiomyoma, endometriosis, and RSA20. Indeed, there is a differential expression of miRNAs in the villi and decidua of RSA patients, and all significantly differentially expressed miRNAs affect pregnancy by regulating adhesion. Furthermore, a few of the miRNAs can lead to RSA by regulating the expression of angiogenesis-related genes. All of these miRNAs act together to regulate a variety of physiological functions, leading to RSA. One study suggested that miR-16 inhibits angiogenesis, trophoblast cell proliferation, and migration, and inhibits angiogenesis through VEGF suppression21. These data imply that miR-16 may play a critical role in placental angiogenesis.
In the present study, we initially detected the expression level of miR-16 in samples of RSA patients and control women. Importantly, quantification of the data indicated that miR-16 was expressed at significantly higher levels in the villi and decidua of the RSA patients than that in the controls. These results indicate that the status of miR-16 expression should be determined in RSA patients. In vitro, miR-16 overexpression in HUVECs suppresses proliferation, migration and tube formation, suggesting that miR-16 may be a novel suppressor of and may play a critical role in angiogenesis. Moreover, injection with miR-16 in the placenta of pregnant mice caused remarkably decreased placental vasculature and microvascular density, leading to aberrant placentation and spontaneous abortion. A recent study indicated that miR-16 inhibits the proliferation, migration and angiogenesis-regulating potential of mesenchymal stem cells22. Our data are consistent with a previous study demonstrating that miR-16 exerts inhibitory biological features during placental angiogenesis.
In addition, we validated VEGF as a direct target gene for miR-16, and we found that miR-16 negatively regulates VEGF expression by directly targeting the 3′UTR of VEGF mRNA in HTR-8/SVNEO and JEG-3 cells. VEGF, acting as a key angiogenesis promoter, plays a significant role in physiological and pathophysiological vascular development and maintenance23. It has been shown that VEGF expression is found in the decidua cells of early pregnancy, and it participates in physiological placental angiogenesis during embryogenesis and reproductive functions during the initial stages of pregnancy24. Dysregulation of VEGF expression has been linked to the onset of placental pathologies including preeclampsia, early pregnancy loss and intrauterine growth restriction and is deeply implicated in multiple steps of RSA occurrence and development25,26. Previous studies have indicated that early pregnancy loss is associated with low VEGF expression and that altered VEGF expression is likely to contribute to the etiology of RSA27,28. In this study, we demonstrated that miR-16 mediates the reduction of VEGF and found that normal VEGF expression is a notable feature and may be one of the many critical events that occur in placental angiogenesis. Aberrant angiogenesis may be partially attributed to overexpressed miR-16 in the placenta of RSA patients.
Overall, the current study found that miR-16 expression is upregulated in the villi and decidua of RSA patients. Our data revealed that the important molecular mechanism by which miR-16 exerts its negative effects on placental angiogenesis occurs via VEGF suppression. However, our work is merely a starting point for the study of miRNAs in RSA, and the exact regulatory mechanism and related signaling pathways of miR-16in RSA still require more experimental evidence to verify. Taken together, the identification of miR-16 provides a potential diagnostic marker and therapeutic target for RSA patients.
Materials and Methods
Patients and Tissue Samples
A prospective case-control study was conducted between October 2013 and November 2014 in the Key Laboratory of Fertility Preservation and Maintenance of Ministry of Education, Yinchuan, China. A total of 30 women aged 25–31 years with a history of three or more spontaneous abortions and 30 fertile women aged 24–31 years with one or more children and no history of spontaneous abortions were enrolled in this study between 2013 and 2014 in the Department of Obstetrics and Gynecology, General Hospital of Ningxia Medical University. Baseline characteristics of all women were recorded (Table 2). Placental villi and decidua tissue were also obtained from cases of induced abortion between 6 and 12 gestational weeks. Medical abortion was performed as previously described29. All samples were examined to exclude chromosomal, anatomical, hormonal and infectious pathologies. All tissue samples were snap-frozen in liquid nitrogen for real-time polymerase chain reaction (qPCR) analyses or were fixed in 10% formalin and embedded in paraffin for immunohistochemical analyses. The institutional ethics committee of the Ningxia Medical University approved the study protocol, and written informed consent was obtained from participants. The human experiments were performed in accordance with the relevant guidelines, including any relevant details.
Table 2. Clinical characteristics of subjects.
| Characteristics | Control (n = 30) | RSA (n = 30) | P value |
|---|---|---|---|
| Age (years) | 30.3 ± 1.8 | 30.8 ± 2.2 | NS |
| Gestational age (weeks) | 11.9 ± 2.1 | 12.6 ± 2.6 | NS |
| Smoking | none | none | NS |
| Pregnancy time (weeks) | 10.8 ± 1.2 | 11.2 ± 1.9 | NS |
| Median maternal weight (kg) | 52.8 ± 4.5 | 53.4 ± 5.3 | NS |
| Median maternal BMI (kg/m2) | 22.3 ± 2.5 | 21.6 ± 1.9 | NS |
miRNA expression assay
Total RNAs were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized from total mRNA (5 μg) with specific reverse transcription primers. The expression of miRNAs was quantified using a miRNA-specific TaqMan miRNA Assay Kit (Applied Biosystems, Foster City, CA, USA). qPCR was performed with a FastStart Universal SYBR Green Master (Roche Diagnostics, Mannheim, Germany). Quantitative PCR reactions utilized TaqMan Universal PCR Master Mix and TaqMan miRNA Assays (ABI, Foster City, CA, USA) according to the manufacturer’s directions. The relative quantification of miRNAs was calculated using the 2−△△Ct method. The miRNA levels were normalized using U6 small nuclear RNA as an internal control and measured relative to a calibrator sample as the external control.
Cell Culture and Transfection
The choriocarcinoma cell line JEG-3 and the immortalized human trophoblast cell line HTR-8/SVNEO were obtained from the American Type Culture Collection and grown in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA) and 100 U/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in humidified air at 37 °C and 5% CO2. The miR-16 mimics and control mimics were purchased from Ambion (Austin, TX, USA). For miRNA transfection, cells were plated in 6-well plates and cultured to 50–70% confluence prior to transfection of the mimic or inhibitor using Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. All further analyses were performed 48 hours after transfection.
3H-thymidine incorporation assay
For the 3H-thymidine incorporation assays, HUVECs (1 × 105 cells/well) were plated in 24-well plates and cultured until 60% confluence. Cells were pretreated with VEGF (Peprotech, Offenbach, Germany) for 24 h and serum-starved overnight, followed by the addition of serum containing 3H-thymidine (2 Ci/mM) for 6 h. The cells were then fixed in 0.3 ml of 10% trichloroacetic acid (TCA) and lysed in 100 μl of 0.2 M NaOH/0.2% SDS. 3H-thymidine incorporation was measured by scintillation counting in a Packard Scintillation Analyzer (Covina, USA).
Migration assay
For the migration assay, monolayer HUVECs were wounded by scratching the plate with a sterile 200-μl pipette tip. The cells were then washed several times with PBS to remove cell debris, and the culture was continued in fresh medium. Images of the cells were obtained with an inverted phase contrast microscope (Olympus, Tokyo, Japan) at 0 h and 24 h after scratching. The migration capacity was calculated as the percentage of wound closure with the initial wound width defined as 100%.
Tube formation assay
The formation of HUVECs into capillary-like structures was conducted as described previously30. At least 30 min before the experiment, 96-well plates were coated with Matrigel (BD, Biosciences, Bedford, MA, USA). HUVECs (5 × 103 cells/well) were cultured in Ham’s F12K basal medium (Gibco, Carlsbad, CA, USA) with 200 ml tumor cell-conditioned medium. Tumor cell-conditioned medium was prepared from 1 × 106 JEG-3 cells transfected with a miR-16 mimic or inhibitor, which were cultured in the same conditions for 48 h. Images showing the formation of capillary-like structures were obtained after 12 h with an inverted microscope (Olympus, Tokyo, Japan) at 50 × magnification. Tubular structures were assessed by measuring the total tubule length in each field of view using Image-Pro Plus software.
Animals
Wild-type C57BL/6 mice (8 to 12 weeks old) were maintained on an autoclaved chow diet in filter-topped cages in a specific pathogen-free (SPF) animal room (controlled temperature (24 ± 2 °C), with approximately 40% humidity and a 12/12 h light/dark cycle. The presence of a vaginal plug was designated as day 0.5 of pregnancy. To deliver cholesterol-conjugated miRNAs (Austin, TX, USA), 10 nmol miRNAs in 50 μl saline buffer was locally injected into the placenta using apercutaneous ultrasound-guided approach on day 7.5 of pregnancy. All placentas in a single litter received the same miRNA (control or miR-16 mimic/inhibitor) injection. Pregnant females were killed on day 14.5 of pregnancy, and placenta samples were collected. All animal experiments were approved by the Ethics Review Board of the Ningxia Medical University and conducted following institutional guidelines.
Immunohistochemistry assay
Paraffin-embedded tissue sections (4-μm thick) were deparaffinized in xylene and rehydrated through a graded series of methanol. Endogenous peroxidase activity was quenched with 3% H2O2 for 10 min. Subsequently, the slides were subjected to antigen retrieval in a microwave oven in Tris-buffered citric acid (pH = 6) for 10 min. The sections were incubated in 10% goat serum albumin in phosphate buffered saline (PBS) for 30 min, followed by incubation with a mouse monoclonal anti-VEGF antibody (C-1, 1/50 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-PCNA antibody (1/500 dilution; Abcam, Cambridge, UK) or rabbit monoclonal anti-CD34 antibody (1/50 dilution, Boshide Biotechnology Company, Wuhan, China) at 4 °C overnight. The sections were then incubated with the appropriate secondary antibody for 1 h and washed with PBS. Lastly, the visualization signal was developed by incubating the sections in 3, 3′-diaminobenzidine in buffered substrate for 5 min.
The immunostaining intensity was evaluated according to the immunoreactive score (IRS). IRS = staining intensity × percentage of positive cells. Staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The percentages of positive cells were scored as 0 (0% positive cells), 1 (<10% positive cells), 2 (10–50% positive cells), 3 (51–80% positive cells), or 4 (>80% positive cells). The microvascular density (MVD) was determined using the Weidner method on the basis of CD34 staining31.
Western blot assays
Protein extraction and western blotting were performed as previously described in more detail32. Briefly, proteins (50 μg) were separated by 10% sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) and transferred to a nitrocellulose membrane (Amersham Bioscience, Buckinghamshire, U.K.). Membranes were blotted with an anti-VEGF antibody (sc-152, 1/1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C and then incubated with horseradish peroxidase-conjugated secondary antibody (Transduction Laboratories, Lexington, UK). GAPDH antibody (1/1000 dilution, Cell Signaling Technology, Boston, MA, USA) was used as a control. The bands were detected with Enhanced Chemiluminescence (Amersham Biosciences, Piscataway, NJ) and visualized with the ChemiDoc XRS System (Bio-Rad, Hercules, CA, USA).
Plasmid construction
To generate a luciferase reporter vector, a 376-bp fragment of the VEGF 3′UTR sequence, as well as the mutant VEGF sequence, were synthesized by PCR. The primers contained the following restriction sites: VEGF 3′UTR forward, 5′-GGAATTCCCACACCATCACCATCGACAGA-3′, reverse, 5′-CAAGCTTGACACCAATAACATTAGCACTG-3′; and Mut VEGF 3′UTR forward, 5′-TTATTTTTCTTAATCCTAAATCACCGA-3′, reverse, 5′- TCGGTGATTTAGGATTAAGAAAAATAA-3′. The PCR product was cloned into the EcoRΙ and HindΙΙΙ restriction sites downstream of the luciferase open reading frame in the pGL3-luciferase reporter plasmid (Promega, Madison, WI, USA). The correct clones were confirmed by sequencing.
Luciferase reporter assays
The cells (HTR-8/SVNEO and JEG-3) were grown to 70–80% confluence in 24-well plates and co-transfected with the luciferase reporter vector described above (200 ng) and the appropriate miRNA (50 nM) using LipofectamineTM 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After 48 h, cells were washed and lysed with passive lysis buffer (Promega, Madison, WI, USA), and luciferase activity was analyzed using dual luciferase assays (Promega, Madison, WI, USA). The relative reporter activity was obtained by normalization to the Renilla luciferase activity.
Statistical analysis
SPSS13.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Values are expressed as the mean ± standard deviation (SD). Significant differences between groups were analyzed using Student’s t-test. Differences were considered statistically significant at P < 0.05.
Additional Information
How to cite this article: Zhu, Y. et al. MicroRNA-16 inhibits foeto-maternal angiogenesis and causes recurrent spontaneous abortion by targeting vascular endothelial growth factor. Sci. Rep. 6, 35536; doi: 10.1038/srep35536 (2016).
Supplementary Material
Acknowledgments
This research was supported by the National Science Foundation of China (NSFC31360257 and NSFC 31460272) and the Ningxia Science Foundation (NZ14073).
Footnotes
Author Contributions Z.H. conceived the study and drafted the manuscript. Y.Z and H.L. performed the experiments and collected data in the field. Z.M. and J.D. performed the data analysis. W.D. and L.P. participated in the proposal preparation and interpretation of data. J.C. and H.Z. participated in the interpretation of data and critically reviewed the manuscript. All authors read and approved the final manuscript.
References
- Krieg S. A. et al. Global alteration in gene expression profiles of deciduas from women with idiopathic recurrent pregnancy loss. Molecular human reproduction 18, 442–450, doi: 10.1093/molehr/gas017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R. & Regan L. Recurrent miscarriage. Lancet 368, 601–611, doi: 10.1016/S0140-6736(06)69204-0 (2006). [DOI] [PubMed] [Google Scholar]
- Pang L. et al. An increase in vascular endothelial growth factor (VEGF) and VEGF soluble receptor-1 (sFlt-1) are associated with early recurrent spontaneous abortion. PloS one 8, e75759, doi: 10.1371/journal.pone.0075759 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo Andersen A. M., Wohlfahrt J., Christens P., Olsen J. & Melbye M. Maternal age and fetal loss: population based register linkage study. Bmj 320, 1708–1712 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont K., Scott N. W., Jones G. T. & Bhattacharya S. Risk of recurrent stillbirth: systematic review and meta-analysis. Bmj 350, h3080, doi: 10.1136/bmj.h3080 (2015). [DOI] [PubMed] [Google Scholar]
- Garrido-Gimenez C. & Alijotas-Reig J. Recurrent miscarriage: causes, evaluation and management. Postgraduate medical journal 91, 151–162, doi: 10.1136/postgradmedj-2014-132672 (2015). [DOI] [PubMed] [Google Scholar]
- Dong F. et al. Genome-wide miRNA profiling of villus and decidua of recurrent spontaneous abortion patients. Reproduction 148, 33–41, doi: 10.1530/REP-14-0095 (2014). [DOI] [PubMed] [Google Scholar]
- Avagliano L., Garo C. & Marconi A. M. Placental amino acids transport in intrauterine growth restriction. Journal of pregnancy 2012, 972562, doi: 10.1155/2012/972562 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. J., Bulmer J. N. & Innes B. A. & Broughton Pipkin, F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biology of reproduction 84, 1148–1153, doi: 10.1095/biolreprod.110.088351 (2011). [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature 438, 932–936, doi: 10.1038/nature04478 (2005). [DOI] [PubMed] [Google Scholar]
- Gourvas V. et al. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review). Molecular medicine reports 6, 23–27, doi: 10.3892/mmr.2012.898 (2012). [DOI] [PubMed] [Google Scholar]
- Lewis B. P., Burge C. B. & Bartel D. P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20, doi: 10.1016/j.cell.2004.12.035 (2005). [DOI] [PubMed] [Google Scholar]
- Giraldez A. J. et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312, 75–79, doi: 10.1126/science.1122689 (2006). [DOI] [PubMed] [Google Scholar]
- Gangaraju V. K. & Lin H. MicroRNAs: key regulators of stem cells. Nature reviews. Molecular cell biology 10, 116–125, doi: 10.1038/nrm2621 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reproductive biomedicine online 25, 415–424, doi: 10.1016/j.rbmo.2012.06.022 (2012). [DOI] [PubMed] [Google Scholar]
- Di Simone N. et al. Potential new mechanisms of placental damage in celiac disease: anti-transglutaminase antibodies impair human endometrial angiogenesis. Biology of reproduction 89, 88, doi: 10.1095/biolreprod.113.109637 (2013). [DOI] [PubMed] [Google Scholar]
- Ishii T. et al. Genetically induced oxidative stress in mice causes thrombocytosis, splenomegaly and placental angiodysplasia that leads to recurrent abortion. Redox biology 2, 679–685, doi: 10.1016/j.redox.2014.05.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P. et al. Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. Plos one 8, e80940, doi: 10.1371/journal.pone.0080940 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E., Poston L. & Burton G. J. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Human reproduction update 12, 747–755, doi: 10.1093/humupd/dml016 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria X. & Taylor H. MicroRNA and gynecological reproductive diseases. Fertility and sterility 101, 1545–1551, doi: 10.1016/j.fertnstert.2014.04.044 (2014). [DOI] [PubMed] [Google Scholar]
- Hu Y. et al. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clinical chemistry and laboratory medicine: CCLM/FESCC 47, 923–929, doi: 10.1515/CCLM.2009.228 (2009). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. miR-16 inhibits the proliferation and angiogenesis-regulating potential of mesenchymal stem cells in severe pre-eclampsia. The FEBS journal 279, 4510–4524, doi: 10.1111/febs.12037 (2012). [DOI] [PubMed] [Google Scholar]
- Fang Y. et al. Association of Dll4/notch and HIF-1a -VEGF signaling in the angiogenesis of missed abortion. PloS one 8, e70667, doi: 10.1371/journal.pone.0070667 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher A., Sundrani D. & Joshi S. Maternal nutrition influences angiogenesis in the placenta through peroxisome proliferator activated receptors: A novel hypothesis. Molecular reproduction and development, doi: 10.1002/mrd.22518 (2015). [DOI] [PubMed] [Google Scholar]
- McKeeman G. C., Ardill J. E., Caldwell C. M., Hunter A. J. & McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. American journal of obstetrics and gynecology 191, 1240–1246, doi: 10.1016/j.ajog.2004.03.004 (2004). [DOI] [PubMed] [Google Scholar]
- Bardin N., Murthi P. & Alfaidy N. Normal and pathological placental angiogenesis. BioMed research international 2015, 354359, doi: 10.1155/2015/354359 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirchaghmaghi E. et al. Gene expression analysis of VEGF and its receptors and assessment of its serum level in unexplained recurrent spontaneous abortion. Cell journal 16, 538–545 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutluer G. et al. Low VEGF expression in conceptus material and maternal serum AFP and beta-hCG levels as indicators of defective angiogenesis in first-trimester miscarriages. Journal of the Turkish German Gynecological Association 13, 111–117, doi: 10.5152/jtgga.2012.06 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y., Qian Z. & Huang L. Elevated expression levels of matrix metalloproteinase-9 in placental villi and tissue inhibitor of metalloproteinase-2 in decidua are associated with prolonged bleeding after mifepristone-misoprostol medical abortion. Fertil Steril 101, 166–171 e162, doi: 10.1016/j.fertnstert.2013.09.027 (2014). [DOI] [PubMed] [Google Scholar]
- Lv Z. et al. Downregulation of HDAC6 promotes angiogenesis in hepatocellular carcinoma cells and predicts poor prognosis in liver transplantation patients. Molecular carcinogenesis, doi: 10.1002/mc.22345 (2015). [DOI] [PubMed] [Google Scholar]
- Ye B. et al. Anti-tumor activity of CrTX in human lung adenocarcinoma cell line A549. Acta Pharmacol Sin 32, 1397–1401, doi: 10.1038/aps.2011.116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M. et al. MicroRNA-450a-3p represses cell proliferation and regulates embryo development by regulating Bub1 expression in mouse. PloS one 7, e47914, doi: 10.1371/journal.pone.0047914 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.