Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disorder. Hydrogen sulfide (H2S), the third physiological gasotransmitter, is well recognized as an anti-inflammatory mediator in various inflammatory conditions. Herein, we explored the protective effects of S-propargyl-cysteine (SPRC, also known as ZYZ-802), an endogenous H2S modulator, on RA and determined the underlying mechanisms. In the present study, SPRC concentration-dependently attenuated inflammatory mediator expression, reactive oxidase species generation, and the expression and activity of matrix metalloproteinases (MMP)-9 in interleukin (IL)-1β-induced human rheumatoid fibroblast-like synoviocytes MH7A. In addition, SPRC blocked IL-1β-mediated migration and invasion of MH7A cells. As expected, the protective effects of SPRC were partially abrogated by DL-propargylglycine (PAG, a H2S biosynthesis inhibitor). In vivo study also demonstrated that SPRC treatment markedly ameliorated the severity of RA in adjuvant-induced arthritis rats, and this effect was associated with the inhibition of inflammatory response. We further identified that SPRC remarkably induced heme oxygenase-1 expression associated with the degradation of Kelch-like ECH-associated protein 1 (Keap1) and nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2); this effect was attributed to the sulfhydrylation of the cysteine residue of Keap1. Our data demonstrated for the first time that SPRC, an endogenous H2S modulator, exerted anti-inflammatory properties in RA by upregulating the Nrf2-antioxidant response element (ARE) signaling pathway.
Abbreviations: SPRC, S-propargyl-cysteine; CSE, cystathionine γ-synthase; FLS, fibroblast-like synoviocytes; AIA, adjuvant-induced arthritis; Nrf2, nuclear factor erythroid-2-related factor 2; ARE, antioxidant response element; Keap1, kelch-like ECH-associated protein 1; COX-2, cyclooxygenase 2; ICAM-1, intercellular adhesion molecule 1; iNOS, nitric oxide synthase; MMP9, matrix metalloproteinase 9; ROS, reactive oxygen species; GSH, glutathione; GSSH, oxidized glutathione; LAT1, L-type amino acid transporter-1; BCH, 2-aminobicyclo-2(2,2,1)-heptane-2-caboxylic acid; SOD1, superoxide dismutase 1; HO-1, heme oxygenase-1; PAG, DL-propargylglycine
Keywords: S-propargyl-cysteine, Arthritis, Cystathionine γ-synthase, Nuclear factor erythroid-2-related factor 2
Graphical abstract
Highlights
-
•
SPRC blocks IL-1β-mediated migration and invasion of MH7A cells.
-
•
SPRC reduces inflammatory response in rats with adjuvant induced arthritis.
-
•
SPRC inhibits inflammatory mediators by activation CSE-H2S.
-
•
SPRC activates the Nrf2/ARE antioxidant pathway in RA.
-
•
SPRC enters cells through LAT1 transporters.
1. Introduction
Rheumatoid arthritis (RA) is a global intractable autoimmune disease that affects about 1% of the whole world population [1]. Arbitrarily active RA causes joint damage, disability, and reduced life quality, and patients require a long-term therapy. A common effect of long-term therapy is the development of resistance to treatment and an increased occurrence of adverse effects. Hence, a continuous need for new agents in the therapy of RA is envisaged. Primary and dominant processes in the etiopathogenesis of RA are autoimmunological mechanisms, and pathogenesis of such a disease is also associated with the formation of free radicals produced by activated macrophages and neutrophils at the site of inflammation [2], [3].
RA is a chronic inflammatory disease, characterized by synovial proliferation and destruction of articular cartilage at multiple joints [4], [5]. It is believed that fibroblast-like synoviocytes (FLSs), which secrete synovial fluid and produce cytokines, are thought to play a key role involving the joint destruction [6]. The overexpression of the proinflammatory molecules, including cyclooxygenase 2 (COX-2), intercellular adhesion molecule-1 (ICAM-1), and inducible nitric oxide synthase (iNOS), are frequently detected in the synovial fluid and plasma of patients with RA. Matrix metalloproteinases (MMPs) also contribute to tissue remodeling during inflammation, and the elevated levels of MMPs in synovial fluid mainly secreted by FLSs may be the main cause of synovium and cartilage erosion [7], [8], [9].
Hydrogen sulfide (H2S), the third endogenous gasotransmitter following nitric oxide and carbon monoxide [10], has multiple effects on disparate physiological and pathophysiological processes. Endogenous H2S is generated from cysteine in a reaction catalyzed by three main enzymes: cystathionine β-synthase (CBS), cystathionineγ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase [11]. CSE has been identified to be the primary enzyme responsible for the generation of H2S in peripheral organs [12]. The numerous conflicting data concerning about the role of H2S in inflammation attract increasing attention [13]. Recent evidence demonstrated that H2S could activate the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) signaling pathway and upregulate the expression of the antioxidant protein heme oxygenase-1 (HO-1) [14]. Conversely, in several inflammation animal models, higher concentration of H2S poses a threat to the cells, involving the generation of free radical, calcium mobilization, glutathione depletion, and so on [15]. Exogenous sources of H2S, NaHS, and GYY4137 (a slow H2S-releasing agent) have shown significant anti-inflammatory property in the model of osteoarthritis [16], [17], [18], [19].
Our previous studies revealed that S-propargyl-cysteine (SPRC, also known as ZYZ-802), an endogenous H2S modulator, exerted protective effects against various inflammatory conditions by elevating the level and the activity of CSE [20], [21]. Nevertheless, the role of SPRC in other inflammatory diseases such as RA is yet to be defined. Considering that SPRC has antioxidant and anti-inflammatory properties, in the present study, we attempted to clarify whether SPRC could prevent the development of autoimmune arthritis and to determine the underlying mechanisms.
2. Materials and methods
2.1. Materials
Recombinant human IL-1β was purchased from Peprotech (Rocky Hill, NJ, USA). Freund's adjuvant (FA, heat-inactivated Mycobacterium tuberculosis) was obtained from Chondrex. 3-(4,5-Dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide (MTT), DL-propargylglycine (PAG), 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA), 2-aminobicyclo-2(2,2,1)-heptane-2-caboxylic acid (BCH), and N,N-dimethyl-p-phenylenediamine sulfate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies were obtained from the following commercial sources: MMP-9, IL-6, and ICAM-1 were purchased from Cell Signaling Biotechnology (Danvers, MA, USA); Keap-1, HO-1, CSE, CBS, Nrf-2, SOD1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); and antibody against Lamin A/C was obtained from Epitomics (Burlingame, CA, USA). SPRC was synthesized in our laboratory and purified by recrystallization from ethanol–water mixture (99%).
2.2. Establishment of arthritis and drug treatment
All animals used in this study received humane care in compliance with the principles of laboratory animal care formulated by Fudan University for medical research and the Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley (SD) rats weighing 200–230 g were randomly divided into five groups of eight animals each. Group 1 served as vehicle control and received an injection of 0.1 ml of paraffin oil into the left hind paw. All the other groups received a single subcutaneous injection of 0.1 ml of FA in the palmar surface of the left hind paw to induce experimental adjuvant-induced arthritis (AIA). Seven days after injection, the rats were started on various drug regimens that continued up to 4 weeks. Group 2: assigned as AIA. Group 3–5: received SPRC (25, 50, or 100 mg/kg, p.o., respectively). All animals were sacrificed on the 29th day, and ankle joints samples and serum were collected from all groups. The samples were stored at −70 °C until use.
2.3. Hind paw volume measurement
The hind paw volume (HPV) of all animal groups was measured by a plethysmometer at 0, 5, 10, and 20th day after the injection of FA emulsion. AIA legs of experimental rats were examined, and the percentage of inhibition was calculated according to the method of Coelho [14].
2.4. Cell culture
FLS MH7A cell line was gifted by Prof. Zhang Peng (Chinese Academy of Science, Shenzhen), the cells were purchased from the Riken cell bank (Tsukuba, Japan). Human acute monocytic leukemia cell line (THP-1) was obtained from the American Type Culture Collection (Manassas, VA, USA). THP-1 and MH7A cells were cultured in Roswell Park Memorial Institute 1640 medium (Hyclone), supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin/streptomycin in a 5% CO2 humidified atmosphere at 37 °C.
2.5. Cell viability assay
Cell viability was evaluated by the MTT assay as described previously [22]. In brief, MH7A cells were subcultured in a 96-well plate at the density of 2×104 cells/ml and then incubated for 12 h. After incubation with indicated concentration of SPRC for 24 h, final concentration of MTT (0.5 mg/ml) was added to the cell culture and incubated for another 4 h. Dimethylsulfoxide was then added to each well, and the absorbance at 570 nm was measured using a microplate reader (M1000, TECAN, Austria GmbH, Austria). Fold changes were finally used to indicate the data normalized.
2.6. H2S concentration measurement
H2S concentration was measured as described previously [23]. Briefly, 500 μl of culture medium from different treated cells was mixed with 250 μl of 1% zinc acetate in a test tube. Subsequently, N-dimethyl-p-phenylenediamine sulfate (20 mM, 133 μl) in 7.2 mM HCl and FeCl3 (133 μl) in 1.2 mM HCl were added to this test tube and incubated for 10 min at room temperature. To remove the protein in the culture, trichloroacetic acid (10% w/v, 250 μl) was added to the reaction, and the protein was pelleted by centrifugation at 12,000 rpm for 5 min. The absorbance (670 nm) intensity was measured by a spectrophotometer (M1000, TECAN, Austria GmbH, Austria). The H2S concentrations of each sample were calculated against a calibration curve of NaHS (3.125–250 μM).
2.7. Measurement of serum TNF-α level by enzyme-linked immunosorbent assay
Plasma obtained from the rats after sacrifice was analyzed for the concentrations of TNF-α with a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Boatman Biotechnology, Shanghai, China) according to the manufacturer's instructions. Optical densities were read on a microplate reader (M1000, TECAN, Austria GmbH, Austria) at 450 nm. Results are presented as pg/ml.
2.8. Cell adhesion assay
THP-1 cells were labeled by BCECF-AM (10 μM, Meilune Biotechnology, Dalian, China) at 37 °C for 1 h in RPMI-1640. The cells were then washed with culture medium and centrifuged. MH7A cells grown on glass coverslip were incubated with SPRC (10 μM) with or without PAG (2 mM) for 1 h and then stimulated with IL-1β (5 ng/ml) for 12 h. After washing twice with phosphate-buffered saline (PBS), the labeled THP-1 cells (2×106 cells/ml) were seeded onto MH7A cell monolayers and incubated for 1 h in a CO2 incubator. The nonadherent THP-1 cells were removed by washing with PBS. The cell adhesion was detected using a fluorescence microscope.
2.9. Cell invasion assay
The invasion assay of MH7A cells was determined as previously described with modification [24]. MH7A cells were pretreated with SPRC (10 μM) or together with PAG for 1 h; the cells were then re-suspended and a total of 2×105 cells in 300 μl of medium with 1% fetal calf serum were seeded in the upper chamber with 8-μm pore size membrane. The lower wells of the chamber were filled with medium, and the chamber was then incubated at 37 °C for 12 h to initiate migration. Nonmigrated cells were wiped off with a cotton swab, and the filter was fixed and stained with viola crystalline and counted. The number of migrated cells in five random microscopy fields per well were counted at 400× magnification. Experiments were repeated at least three times, each time in triplicate.
2.10. Intracellular ROS production, GSH levels, and catalase and glutathione peroxidase activity
Intracellular reactive oxygen species (ROS) generation was detected using DCFH-DA as described previously with modification [25]. Briefly, MH7A cells were washed with serum-free DMEM and incubated with DCFH-DA (10 μM) for 30 min at 37 °C. The cells were then washed with PBS, and the fluorescence intensity of ROS production was first quantified using a fluorescence spectrophotometer (M1000, TECAN, Austria GmbH, Austria) at excitation and emission wavelengths of 485 and 530 nm, respectively. Intracellular ROS production was also visualized by a fluorescence microscope (Carl Zeiss Inc.). Relative fluorescence units were normalized to the control cells.
Intracellular activities of reduced glutathione (GSH), catalase, and glutathione peroxidase (GPx) were determined using commercial kits (Beyotime Biotechnology, Jiangsu, China). All procedures were performed according to the manufacturer's instructions.
2.11. Gelatin zymography
MMP-9 was assayed for gelatinolytic activity by gelatin zymography as previously described [26]. Briefly, 8% polyacrylamide gel of 1 mm thickness containing 1 mg/ml gelatin was used. A total of 20 μl of the supernatant of MH7A cells was loaded and separated on 10% SDS-polyacrylamide gels (containing 1 mg/ml gelatin) under nonreducing conditions. After electrophoresis, the gels were soaked in 2.5% Triton X-100 for 30 min to remove SDS and incubated in Tris–HCl (50 mM, pH 7.5) containing CaCl2 (5 mM) and ZnCl2 (1 mM) overnight at 37 °C. After Coomassie blue staining, white bands of lysis indicated digestion of the gelatin by MMP-9. The gels were scanned, and the optical density of the band was analyzed by Image J software.
2.12. Preparation of whole cell extracts and nuclear fraction
For whole cell extraction, cells were washed twice with ice-cold PBS and lyzed in RIPA buffer with protease and phosphatase inhibitor. After centrifugation (4 °C, 10 min, 10,000g), samples were prepared for western blot analysis.
For preparation of nuclear fraction, nuclear proteins of MH7A cells were extracted using the NE-PER@ Nuclear and Cytoplasmic Extraction Reagents (ThermoFisher Scientific, Shanghai, China) according to the manufacturer's instructions.
2.13. Western blot analysis
Western blot analysis was performed as previously described [27]. Equal amounts (50 μg) of proteins were separated and transferred to a polyvinyl difluoride membrane. After blocking with 5% nonfat dried milk, the membranes were probed with antibodies MMP9, IL-6, ICAM-1, GAPDH, Keap1, Nrf2, SOD1, HO-1, and Lamin A/C and incubated with either horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antibody (1:5000, ThermoFisher Scientific, Shanghai, China). Immunoreactive proteins were visualized by enhanced chemiluminescence and signal intensity was detected and quantified by Alpha Imager (Alpha Innotech Corp, San Leandro, CA, USA).
2.14. Quantitative real-time reverse transcription polymerase chain reaction analysis
Total RNA from different treatment MH7A cells was extracted using Trizol reagent (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions. RNA (1 μg) of each sample was reverse transcribed using the reverse transcription system of Takara. An equal volume of cDNA was used as a polymerase chain reaction (PCR) template for determining the mRNA expression level using SYBR-Green Quantitative PCR kit (Takara Biotechnology, Dalian, China) by iCycler iQ system (Bio-Rad, Hercules, CA, USA). Human-specific primers were as follows: GAPDH (forward: 5′-TGTTGCCATCAATGACCCCTT-3′; reverse: 5′-CTCCACGACGTACTCAGC-3′); ICAM-1 (forward: 5′- ATGCCCAGACATCTGTGTCC-3′; reverse: 5′-GGGGTCTCTATGCCCAACAA-3′); IL-6 (forward: 5′- CCTGAACCTTCCAAAGATGGC-3′; reverse: 5′-TTCACCAGGCAAGTCTCCTC A-3′). Relative gene expression was calculated by the △△Ct method.
2.15. CSE-shRNA lentivirus generation and infection
The specific Short Hairpin RNA (shRNA) CSE and shRNA-control (CTR) plasmids were gifted by Prof. Zha (Department of Biochemistry and Molecular Biology, Fudan University, Shanghai, China). To obtain the lentivirus, the recombinant plasmid and packaging vectors pΔ8.2 and pVSVG were co-transfected into 293T cells using the transfection reagent lipofectamine 2000 (Invitrogen, USA). After 48 h, the lentivirus in the culture medium was collected by filtration with 0.45-μm filters. The lentivirus was added to the culture medium with 8 μg/ml polybrene (Sigma, America). The infection efficiency of lentivirus was verified by fluorescence microscopy. In experiments with MH7A cells, 1×106 cells were spun and then mixed with 50 μl of concentrated virus. The cells were incubated for 4 h at 37 °C. Subsequently, MH7A culture medium (500 μl) was added to prepare a suspension of the infected cells, and the cells were incubated for additional 72 h. To generate stable transfected cells, puromycin (3 μg/ml) was added after 72 h of incubation.
2.16. Small interfering RNA transfection
To introduce Nrf2 small interfering RNA (siRNA) into MH7A cells, the cells were plated on 6-well plates at 30–50% confluence before transfection. Individual siRNA (30 nM), lipofectamine RNAiMAX, and Opti-MEM were mixed and incubated at room temperature for 5 min siRNA–lipofectamine RNAiMAX complexes were added to cells for 24 h, and the medium was replaced by fresh serum DMEM medium after transfection. Nrf2 siRNA and scrambled siRNA were purchased from GenePharma Co. Ltd (Shanghai, China). The following is the sequence of human Nrf2 siRNA used in this study: sense: 5′-CGCUCAGUUACAACUAGAUTT-3′; antisense: 5′-AUCUAGUUGUAACUGAGCGTT-3′. Experiments were performed 72 h after transfection. Knockdown of Nrf2 was assessed by western blot assay.
2.17. Statistical analysis
Results of the experimental studies are expressed as mean ± standard error of the mean (SEM). All data analysis was performed using GraphPad Prism 5 software (GraphPad, La Jolla, CA, USA). Statistical analyses were performed by one-way analysis of variance with Tukey's test for post hoc comparisons and Student's t-test when comparing between two groups. Statistical significance was set at p<0.05.
3. Results
3.1. SPRC inhibited IL-1β-mediated inflammatory mediators in MH7A cells
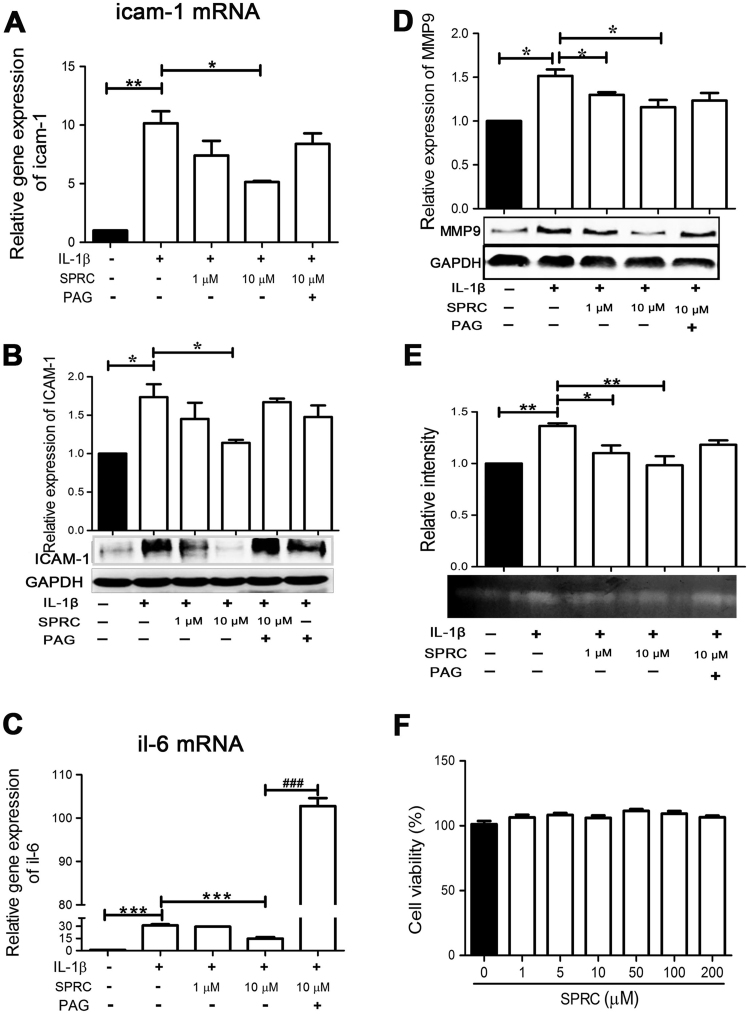
To demonstrate the anti-inflammatory activity of SPRC, the effects of SPRC on inflammatory mediators (IL-6 and ICAM-1) and MMP-9 and its activity were measured in IL-1β-stimulated MH7A cells. As shown in Fig. 1A and B, IL-1β stimulation resulted in a significant increase in ICAM-1 expression at mRNA and protein levels, which was remarkably inhibited by pretreatment with SPRC in a concentration-dependent manner. Similarly, SPRC also concentration-dependently suppressed IL-1β-induced IL-6 expression at the mRNA level (Fig. 1C). SPRC also concentration-dependently reduced IL-1β-mediated MMP-9 expression and its activity (Fig. 1D and E). However, the effects of SPRC on IL-1β-stimulated MH7A cells were partly abolished by pretreatment with PAG (a specific CSE inhibitor, Fig. 1A–E). To assess the potential cytotoxicity of SPRC, cell viability was evaluated by the MTT assay. As shown in Fig. 1F, SPRC alone at concentration in the range of 1–200 µM did not affect cell viability after 24 h incubation. Taken together, our results suggested SPRC exhibited prominent anti-inflammatory activities in IL-1β-stimulated MH7A cells, but not due to cytotoxicity.
Fig. 1.
SPRC-attenuated IL-1β-induced inflammatory mediators in MH7A cells. MH7A cells were incubated with SPRC or together with PAG (2 mM) for 1 h and then stimulated with IL-1β (5 ng/ml) for indicated periods, and the mRNA and protein levels of inflammatory mediators were analyzed as described in Section 2. Bar graphs showed quantitative analysis of the mRNA level (A) and protein level (B) of ICAM-1, the mRNA level of IL-6 (C), the protein level of MMP-9 (D), and the activity of MMP-9 activity (E). (F) MH7A cells were incubated with SPRC (1–200 μM) for 24 h, and the cell viability was determined by the MTT assay. Data are expressed as mean±SEM from triplicate experiments. *p<0.05, **p<0.01, ***p<0.001 vs. IL-1β-stimulated cells. ###p<0.001 vs. SPRC-treated cells.
3.2. SPRC suppressed monocyte adhesion and MH7A cells migration
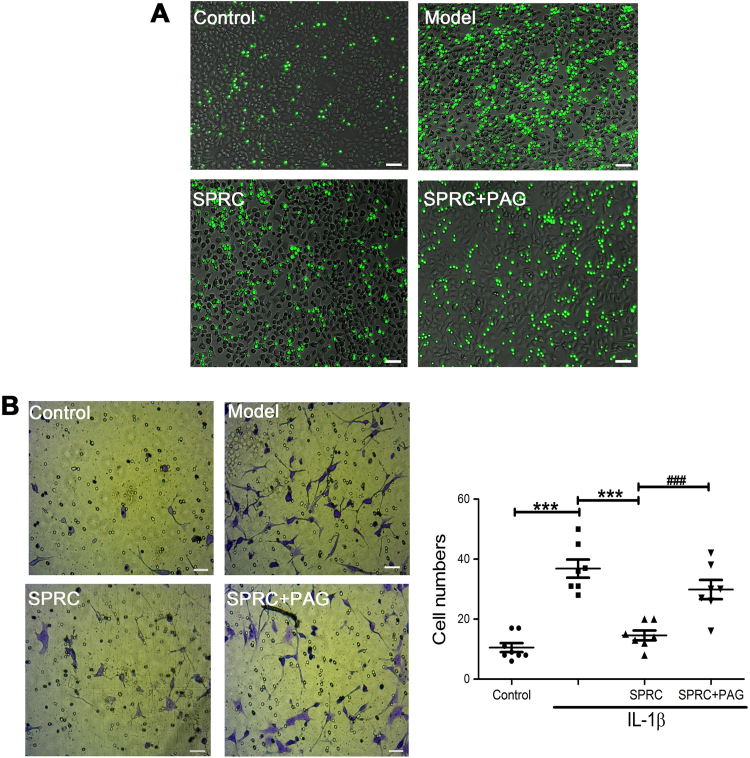
We first investigated the effect of SPRC on the adhesion of THP-1 cells to IL-1β-activated MH7A cells, a critical inflammatory process in arthritis. As shown in Fig. 2A, the adhesion of THP-1 cells was remarkably increased when MH7A cells were stimulated with IL-1β for 12 h, which was significantly attenuated by SPRC (10 µM) treatment. Next, we examined the migratory potential of MH7A cells treated without or with SPRC (10 µM) prior to IL-1β exposure. As shown in Fig. 2B, IL-1β markedly induced the migration of MH7A cells. SPRC (10 µM) also suppressed IL-1β-induced MH7A cell migration. Intriguingly, the effects of SPRC on the adhesion of THP-1 cells to IL-1β-activated MH7A cells and the migration of MH7A cells were reversed by PAG pretreatment (Fig. 2A and B). Taken together, our results indicated that SPRC effectively inhibited the adhesion of THP-1 cells to MH7A cells and the migration of MH7A cells, at least in part, through modulation of the endogenous CSE/H2S pathway.
Fig. 2.
SPRC-inhibited IL-1β-induced adhesion of THP-1 cells and migration of MH7A cells. MH7A cells were pre-incubated with SPRC (10 μM) or together with PAG (2 mM) for 1 h and stimulated with IL-1β for another 12 h, and the adhesion of THP-1 on MH7A cells and migration of MH7A cells were analyzed as described in Section 2. (A) Representative images show that cell adhesion detected by a fluorescence microscope (magnification, 100×). (B) Representative images and quantitative analysis of migration of MH7A cells (magnification, 200×). Data are expressed as mean±SEM from triplicate experiments. ***p<0.001 vs. IL-1β-stimulated cells. ###p<0.001 vs. SPRC-treated cells.
3.3. SPRC-modulated intracellular redox balance in IL-1β-stimulated MH7A cells
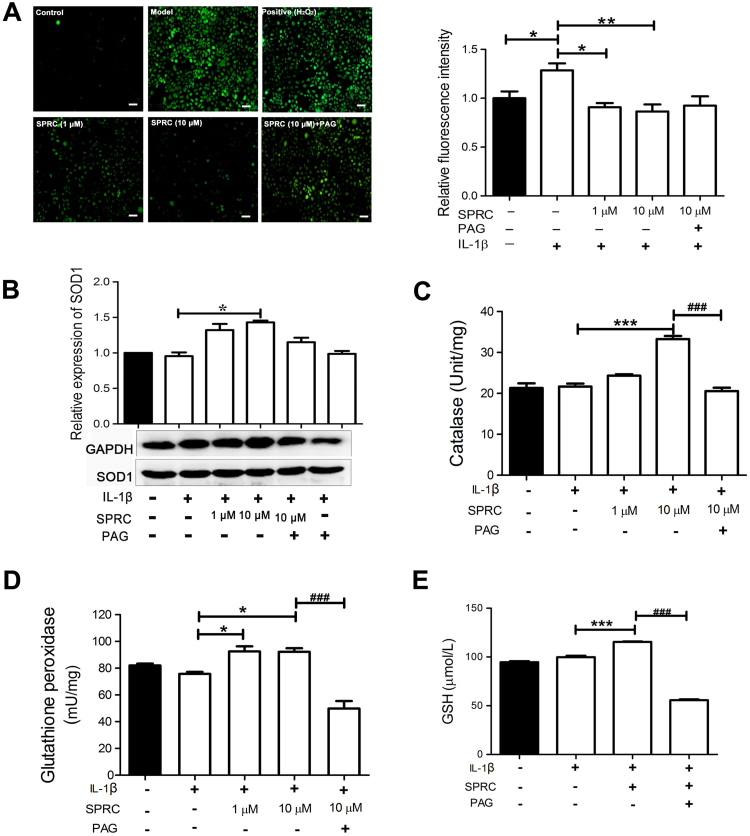
To elucidate the protective effects of SPRC on IL-1β-induced cellular injury, intracellular ROS production, SOD1 expression, and the activities of GSH, catalase, and GPx were measured. As shown in Fig. 3A, IL-1β stimulation significantly increased intracellular ROS production, which was evidently ameliorated by SPRC pretreatment in a concentration-dependent manner. In addition, SPRC treatment significantly increased intercellular antioxidative capacity, as evidenced by upregulation of SOD1 expression (Fig. 3B) and activities of catalase (Fig. 3C), GPx (Fig. 3D), and GSH (Fig. 3E) in IL-1β-stimulated MH7A cells. SPRC-mediated expression of SOD1 and activities of catalase, GPx, and GSH in IL-1β-stimulated MH7A cells were also abrogated by PAG (Fig. 3C). These results indicated that the CSE/H2S pathway was involved in SPRC-mediated intracellular redox balance in MH7A cells.
Fig. 3.
SPRC-modulated intracellular redox balance in IL-1β-stimulated MH7A cells. (A) MH7A cells were pretreated with SPRC (10 μM) or together with PAG (2 mM) for 1 h and then stimulated with IL-1β (5 ng/ml) for 24 h, and intracellular ROS production was analyzed as described in Section 2. H2O2 stimulation served as positive control. Representative images and quantitative analysis of intracellular ROS production (control set as 1) are shown. MH7A Cells were pretreated with indicated concentration of SPRC or together with PAG for 1 h, and stimulated with IL-1β (5 ng/ml) for 24 h, and the activities and expression of intracellular antioxidative enzymes were analyzed as described in Section 2. Bar graphs showed quantitative analysis of the expression of SOD1 (B) and activities of catalase (C), GPx (D), and GSH (E), GAPDH was used as a loading control for western blot analysis. Data are expressed as mean±SEM from triplicate experiments. *p<0.05, **p<0.01, ***p<0.001 vs. IL-1β-stimulated cells; ###p<0.001 vs. SPRC-treated cells.
3.4. SPRC-modulated CSE expression and endogenous H2S production
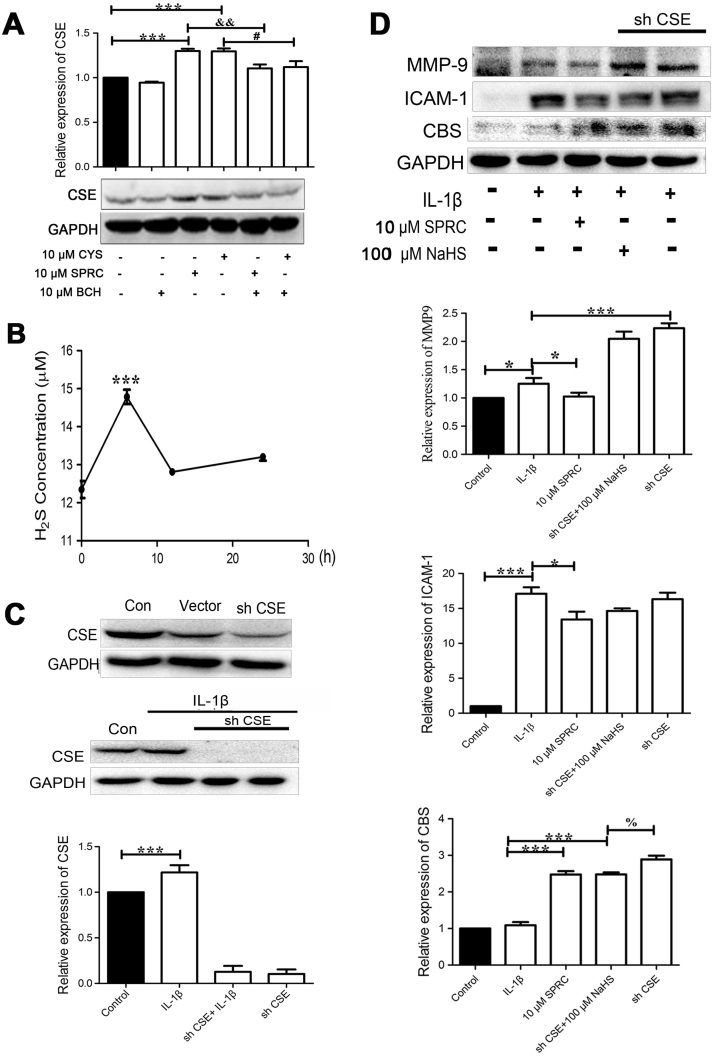
To confirm the involvement of the CSE/H2S pathway in the anti-inflammation activity of SPRC, the CSE expression and H2S production in MH7A cells were analyzed. Our results showed that SPRC (10 µM) or cysteine (CYS, 10 µM) treatment markedly induced CSE expression, which was abolished by BCH (a competitive L-type amino acid transporter 1 inhibitor, LAT1) (Fig. 4A). SPRC (10 µM) treatment for 6 h markedly increased H2S level in MH7A cells (Fig. 4B). Furthermore, CSE expression was silenced by lentiviral CSE shRNA knockdown strategy. As shown in Fig. 4C, transfection with lentiviral CSE shRNA, but not scramble shRNA, dramatically reduced CSE in both MH7A cells and IL-1β-stimulated MH7A cells. As shown in Fig. 4D–F, SPRC (10 µM) or NaHS (an exogenous H2S donor, 100 µM) treatment significantly attenuated IL-1β-induced ICAM-1 and MMP-9 expression. However, transfection with lentiviral CSE shRNA upregulated IL-1β-induced MMP-9 expression. Knockdown CSE feedback increased CBS expression in IL-1β-stimulated MH7A cells. Taken together, our results clearly demonstrated that SPRC exerted anti-inflammatory activity in MH7A cells by modulation of the endogenous CSE/H2S pathway.
Fig. 4.
Modulation of endogenous H2S by SPRC contributed to anti-inflammation activity in IL-1β-stimulated MH7A cells. (A) After pre-incubation with BCH for 1 h, MH7A cells were treated for 24 h with or without SPRC or CYS. Bar graph showed quantitative analysis of CSE expression; data are expressed as mean±SEM from triplicate experiments. ***p<0.001 vs. untreated cells; &&p<0.01 vs. SPRC-treated cells; #p<0.05 vs. CYS-treated cells. (B) MH7A cells were incubated by SPRC (10 μM) for indicated time, curve chart showed quantitative analysis of H2S level in the culture medium. Data are expressed as mean±SEM from triplicate experiments. ***p<0.001 vs. untreated cells; (C) MH7A cells were transfected with lentiviral scrambled shRNA or CSE shRNA; the representative image shows silencing efficiency of CSE. GAPDH was used as a loading control. (D) MH7A cells were transfected with lentiviral CSE shRNA or scrambled shRNA and then stimulated with IL-1β for 24 h with or without SPRC (10 μM) or NaHS (100 μM). Western blot result and quantitative analysis of MMP-9, ICAM-1, and COX-2 expression are shown; data are expressed as mean±SEM from triplicate experiments. *p<0.05, **p<0.01, ***p<0.001 vs. IL-1β-stimulated cells; %p<0.05 vs. NaHS-treated cells.
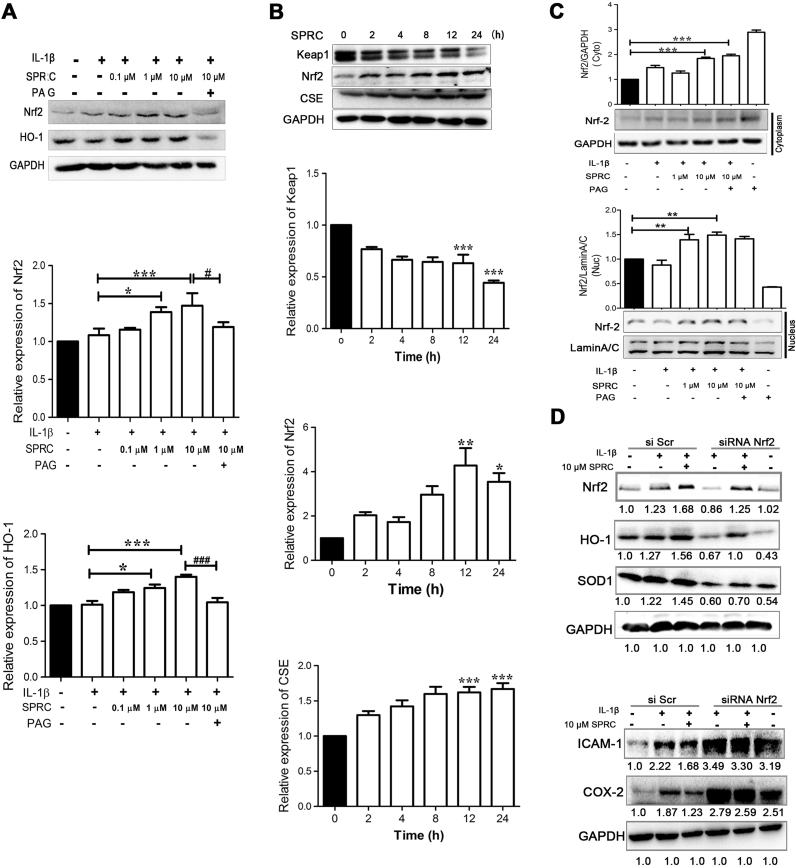
3.5. SPRC-induced HO-1 expression by activation of Nrf2 signaling in MH7A cells
Maintaining high levels of HO-1 is a promising strategy to protect from inflammation and arthritis [28]. As shown in Fig. 5A and B, SPRC incubation for 12 h concentration-dependently induced the expression of HO-1 and Nrf2 protein in IL-1β-stimulated MH7A cells, and IL-1β alone did not significantly affect HO-1 and Nrf2 expression. However, SPRC-mediated HO-1 and Nrf2 expression was abolished by PAG pretreatment. SPRC treatment time-dependently induced CSE expression. Consistent with the increase in CSE expression, SPRC treatment also time-dependently induced Nrf2 expression and decreased Keap1 expression (Fig. 5C). In addition, SPRC-mediated increase in Nrf2 protein expression levels resulted in increased nuclear translocation of Nrf2 (Fig. 5D). However, Nrf2 silencing by siRNA abolished SPRC-mediated Nrf2 expression, accompanied by decrease in HO-1 and SOD1 expression and increase in ICAM-1 and COX-2 expression (Fig. 5E).
Fig. 5.
SPRC-induced HO-1 expression by activation of Nrf2 signaling in MH7A cells. (A) MH7A cells were pretreated with SPRC (0.1–10 μM) or together with PAG for 1 h and then stimulated with IL-1β (5 ng/ml) for 24 h; western blot result and quantitative analysis of Nrf2 and HO-1 expression are shown. Data are expressed as mean±SEM from triplicate experiments. *p<0.05, **p<0.01, ***p<0.001 vs. IL-1β-stimulated cells; ###p<0.001 vs. SPRC (10 μM)-treated cells. (B) MH7A cells were incubated with 10 μM SPRC for indicated time; western blot result and quantitative analysis of Nrf2, CSE, and Keap1 expression are shown. Data are expressed as mean±SEM from triplicate experiments. *p<0.05, **p<0.01, ***p<0.001 vs. unstimulated cells; (D) MH7A cells pretreated with SPRC (1 or 10 μM) with or without PAG (2 mM) for 1 h, and then stimulated with IL-1β (5 ng/ml) for another 4 h; western blot result and quantitative analysis of Nrf2 in cytosolic and nuclear fractions, respectively, are shown. GAPDH and Lamin A/C were used as loading controls for cytosolic and nuclear fractions, respectively, and data are expressed as mean±SEM from triplicate experiments. *p<0.05, **p<0.01, ***p<0.001 vs. unstimulated cells. (D) MH7A cells were transfected with lentiviral scrambled siRNA or Nrf siRNA and then stimulated with IL-1β (5 ng/ml) for 24 h after incubation with SPRC (10 μM); the representative image shows silencing efficiency of Nrf2 and the expression of HO-1, SOD1, ICAM-1, and COX-2 expression. GAPDH was used as a loading control, and data are from triplicate experiments.
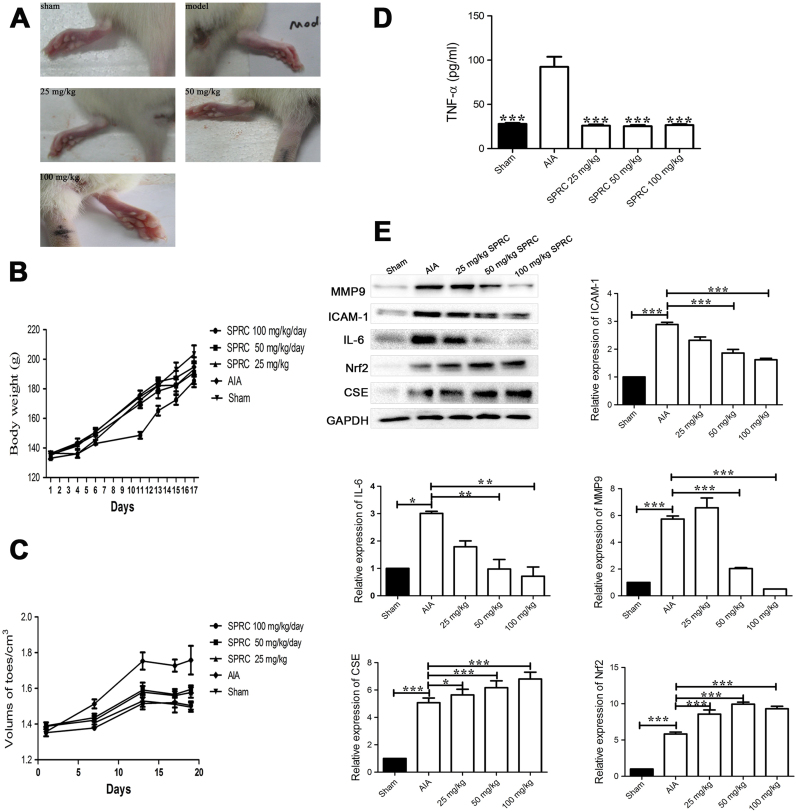
3.6. SPRC-inhibited inflammatory response and ameliorated symptoms of arthritis in AIA rats
The effect of SPRC was evaluated in AIA rats, a well-established in vivo model of inflammatory joint diseases. Supplementation of SPRC (i.g.) once a day started from day 1 to day 28 after initial immunization. As shown in Fig. 6A, SPRC treatment dose-dependently attenuated the severity of AIA. The body weight of each rat was recorded following arthritis induction. In the present study, the mean body weights of rats in all groups increased during the experiment and from the beginning to the end of investigation. However, the model group rats showed slow weight gain after 1 week immunization. In contrast, the mean body weights of rats that received SPRC treatment did not show abnormality compared with the sham group (Fig. 6B). In addition, SPRC (25 mg/kg) supplementation yielded a slight decrease in paw volume, and excellent anti-rheumatic activities were observed in the groups given 50 or 100 mg/kg of SPRC (Fig. 6C).
Fig. 6.
SPRC inhibited inflammatory response and ameliorated symptoms of arthritis in AIA rats. Beginning on day 8 and continuing until day 28, rats were treated daily with vehicle or SPRC (25, 50, and 100 mg/kg, i.g.). (A) Images of representative paws from different treated AIA rats. (B) The body weights of rats were measured. (C) The swelling of ankle joints was quantified by measuring using a plethysmometer. (D) The levels of serum TNF-α of AIA rats measured by ELISA. (E) Western blot result and quantitative analysis of MMP-9, ICAM-1, IL-6, Nrf2, and CSE expression in synovial tissues from AIA rats are shown; GAPDH was used as a loading control. The values are expressed as mean±SEM; n=6–8 rats per group. *p<0.05, **p<0.01, ***p<0.001 vs. AIA rats.
Because inflammatory response is thought to be dominant in the induction of RA, we monitored the production of TNF-α in the sera and inflammatory mediators (MMP-9, ICAM-1, and IL-6) in inflamed joints of AIA rats. As shown in Fig. 6D, the level of TNF-α was dramatically increased in the sera of AIA rats, which was reduced by SPRC (25–100 mg/kg) supplementation in a dose-dependent manner. The expression of MMP-9, ICAM-1, and IL-6 in inflamed joints was also analyzed. As shown in Fig. 6E, compared with vehicle-treated AIA rats, SPRC therapy dose-dependently reduced the expression of MMP-9, ICAM-1, and IL-6 in inflamed joints. Intriguingly, SPRC treatment markedly increased the expression of CSE and Nrf2 in inflamed joints (Fig. 6E).
4. Discussion
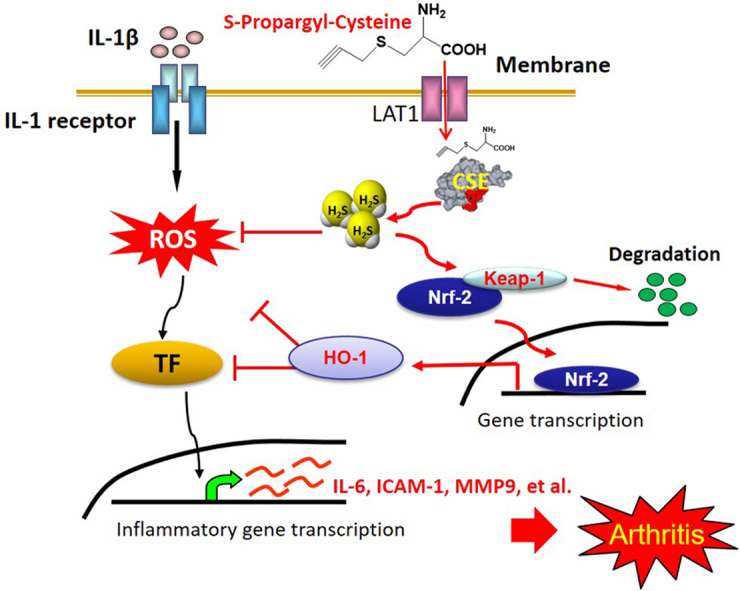
H2S, the third gasotransmitter, is considered as the crucial mediator of the inflammation process [13], [29]. However, the role of H2S in inflammation remains controversial because it is reported to have both inflammatory and anti-inflammatory activities [18], [30], [31]. Our recent findings showed that SPRC, an endogenous H2S modulator, exerted salubrious effects because of its various biological activities, including antioxidant and anti-inflammatory activities [32], [33]. Therefore, we hypothesized that SPRC played a positive role in RA. In this study, we found that SPRC remarkably ameliorated inflammatory response in IL-1β-stimulated FLS and AIA rats associated with the modulation of the CSE/H2S pathway. We further elucidated that SPRC exerted beneficial effects in RA in vitro and in vivo and was associated with the activation of Nrf2 signaling axis and an increase in HO-1 expression, and that this process requires sulfhydrylation of the cysteine residue of Keap1 by endogenous H2S.
FLSs, a specialized cell type located in the inner layer of the synovium, are the main source of synovial fluid and are important in maintaining the homeostasis of the internal joints. FLSs also play crucial roles in the damage, destruction, and deformation of cartilage and joints in the pathogenesis of RA [34]. Activated FLSs exhibit an aggressive/transformed phenotype and induced and/or enhanced the production of MMPs and inflammatory mediators in the synovial tissue and eventually leading to ongoing inflammation and destruction of cartilage and bone [8], [35], [36]. Therefore, the activation of FLSs is generally regarded as a key process in the development of RA. In this study, we clearly demonstrated that SPRC, an endogenous H2S modulator, significantly inhibited the IL-1β-induced migration and adhesion of cells. SPRC also attenuated IL-1β-mediated ICAM-1, IL-6, and MMP-9 expression and MMP-9 activity. Consistent with the results in vitro, we further confirmed that SPRC downregulated the production of pro-inflammatory cytokines and ameliorated the symptoms of arthritis in AIA rats. CSE represents the prominent enzyme for the generation of H2S in peripheral organs [37]. Consistent with our previous studies [38], [39], in the study, SPRC treatment markedly enhanced the expression of CSE and level of H2S in vitro and in vivo. This was associated with decreased inflammatory response and ameliorated knee joint swelling in AIA rats. However, pharmacological inhibition or knockdown strategy of CSE reversed the anti-inflammatory activities of SPRC. Taken together, our findings established that SPRC attenuated inflammatory response in RA associated with the modulation of the CSE/H2S pathway.
The role of oxidative stress is represented by a significant increase in the concentration of ROS in RA patients [40], [41]. Oxidative stress plays a crucial role in the development of arthritis; ROS distinctly contribute to the destructive, proliferative synovitis of RA and play a prominent role in cell signaling events [41]. In addition, several previous studies have showed higher total oxidative stress in RA patients. Intriguingly, supplementation of antioxidants or modulation of intracellular antioxidative capacities has been shown to ameliorate arthritis in animal models [40], [41]. In the present study, IL-1β stimulation significantly attenuated intracellular antioxidative process. However, SPRC significantly enhanced intracellular antioxidative capacities in IL-1β-stimulated FLS, as evidenced by the increase in CAT, GPx, and GSH activities as well as SOD1 expression and decrease in intracellular ROS production. Importantly, the beneficial effects of SPRC on intracellular antioxidative ability were blocked by PAG treatment, indicating SPRC-modulated intracellular redox balance through the CSE/H2S signaling pathway. HO-1, a member of the family of heat shock protein, is an important anti-inflammatory, antioxidative, and cytoprotective enzyme that is regulated by the activation of the major transcription factor Nrf2 [42], [43]. Another major finding of the present study relates to SPRC-mediated upregulation of HO-1 expression in association with reduced inflammatory response in vivo and in vitro. However, SPRC-mediated HO-1 expression was abolished by Nrf2 silencing in IL-1β-stimulated FLS. The results were consistent with the report that of HO-1 is upregulated by transcriptional factor Nrf2 [44]. Interestingly, our data showed that SPRC significantly increased the expression and nuclear translocation of Nrf2 and promoted degradation of Keap1, which were reversed by pharmacological inhibition or knockdown strategy of CSE. Considering that HO-1 acts as a regulator of inflammatory response, it is conceivable that the upregulation of HO-1 by SPRC, an endogenous H2S modulator, was involved in the regulation of inflammatory response in RA by activation of the Nrf2/ARE pathway.
5. Conclusions
In conclusion, this study highlighted the role of SPRC supplementation in inflammatory response in IL-1β-stimulated FLS and AIA rats, and showed that SPRC treatment suppressed inflammatory mediators in IL-1β-stimulated FLS and AIA rats, at least in part, through modulation of Nrf2/HO-1 and CSE/H2S signaling. Therefore, the results of this study suggested that SPRC has the potential for beneficial therapeutic interventions for RA.
Author disclosure statement
The authors declare no conflict of interest.
Acknowledgments
We thank Prof. Peng Zhang for gifting the MH7A cells and Prof. Xiliang Zha for giving the shRNA plasmid targeting CSE. This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81330080, 81173054, 81573420, and 81470164); a Key Laboratory Program of the Education Commission of Shanghai Municipality (No. ZDSYS14005); the Shanghai Committee of Science and Technology (No. 14JC1401100).
Contributor Information
Xin-Hua Liu, Email: liuxinhua@fudan.edu.cn.
Yi Zhun Zhu, Email: yzzhu@must.edu.mo, zhuyz@fudan.edu.cn.
References
- 1.Scott D.L., Wolfe F., Huizinga T.W. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Filippin L.I., Vercelino R., Marroni N.P., Xavier R.M. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin. Exp. Immunol. 2008;152:415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firestein G.S. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 4.Goronzy J.J., Weyand C.M. T-cell regulation in rheumatoid arthritis. Curr. Opin. Rheumatol. 2004;16:212–217. doi: 10.1097/00002281-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Gao W., McGarry T., Orr C., McCormick J., Veale D.J., Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann. Rheum. Dis. 2016;75:311–315. doi: 10.1136/annrheumdis-2014-207201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller-Ladner U., Gay R.E., Gay S. Activation of synoviocytes. Curr. Opin. Rheumatol. 2000;12:186–194. doi: 10.1097/00002281-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Ban J.O., Kim D.H., Lee H.P., Hwang C.J., Shim J.H., Kim D.J., Kim T.M., Jeong H.S., Nah S.S., Chen H., Dong Z., Ham Y.W., Kim Y., Han S.B., Hong J.T. Anti-arthritis effects of (E)-2,4-bis(p-hydroxyphenyl)-2-butenal are mediated by inhibition of the STAT3 pathway. Br. J. Pharmacol. 2014;171:2900–2912. doi: 10.1111/bph.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Rooy D.P., Zhernakova A., Tsonaka R., Willemze A., Kurreeman B.A., Trynka G., van Toorn L., Toes R.E., Huizinga T.W., Houwing-Duistermaat J.J., Gregersen P.K., van der Helm-van Mil A.H. A genetic variant in the region of MMP-9 is associated with serum levels and progression of joint damage in rheumatoid arthritis. Ann. Rheum. Dis. 2014;73:1163–1169. doi: 10.1136/annrheumdis-2013-203375. [DOI] [PubMed] [Google Scholar]
- 9.Hellio le Graverand M.P., Clemmer R.S., Redifer P., Brunell R.M., Hayes C.W., Brandt K.D., Abramson S.B., Manning P.T., Miller C.G., Vignon E. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann. Rheum. Dis. 2013;72:187–195. doi: 10.1136/annrheumdis-2012-202239. [DOI] [PubMed] [Google Scholar]
- 10.Hourihan J.M., Kenna J.G., Hayes J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid. Redox Signal. 2013;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 11.Kimura H. Production and physiological effects of hydrogen sulfide. Antioxid. Redox Signal. 2014;20:783–793. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 13.Tokuda K., Kida K., Marutani E., Crimi E., Bougaki M., Khatri A., Kimura H., Ichinose F. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by altering sulfide metabolism in mice. Antioxid. Redox Signal. 2012;17:11–21. doi: 10.1089/ars.2011.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., Khaper N., Wu L., Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 15.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2012;17:68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L., Fox B., Keeble J., Salto-Tellez M., Winyard P.G., Wood M.E., Moore P.K., Whiteman M. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. J. Cell. Mol. Med. 2013;17:365–376. doi: 10.1111/jcmm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandiver M., Snyder S.H. Hydrogen sulfide: a gasotransmitter of clinical relevance. J. Mol. Med. 2012;90:255–263. doi: 10.1007/s00109-012-0873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burguera E.F., Vela-Anero A., Magalhaes J., Meijide-Failde R., Blanco F.J. Effect of hydrogen sulfide sources on inflammation and catabolic markers on interleukin 1beta-stimulated human articular chondrocytes. Osteoarthr. Cartil. 2014;22:1026–1035. doi: 10.1016/j.joca.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Fox B., Keeble J., Salto-Tellez M., Winyard P.G., Wood M.E., Moore P.K., Whiteman M. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. J. Cell. Mol. Med. 2013;17:365–376. doi: 10.1111/jcmm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang Y.H., Shen Y.Q., Guo W., Zhu Y.Z. SPRC protects hypoxia and re-oxygenation injury by improving rat cardiac contractile function and intracellular calcium handling. Nitric Oxide. 2014;41:113–119. doi: 10.1016/j.niox.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Huang C., Kan J., Liu X., Ma F., Tran B.H., Zou Y., Wang S., Zhu Y.Z. Cardioprotective effects of a novel hydrogen sulfide agent-controlled release formulation of S-propargyl-cysteine on heart failure rats and molecular mechanisms. Plos One. 2013;8:e69205. doi: 10.1371/journal.pone.0069205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan L.L., Liu X.H., Jia Y.L., Wu D., Xiong Q.H., Gong Q.H., Wang Y., Zhu Y.Z. A novel compound derived from danshensu inhibits apoptosis via upregulation of heme oxygenase-1 expression in SH-SY5Y cells. Biochim. Biophys. Acta. 1830;2013:2861–2871. doi: 10.1016/j.bbagen.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y.Z., Wang Z.J., Ho P., Loke Y.Y., Zhu Y.C., Huang S.H., Tan C.S., Whiteman M., Lu J., Moore P.K. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J. Appl. Physiol. 1985;102(2007):261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 24.Kong N., Lan Q., Chen M., Zheng T., Su W., Wang J., Yang Z., Park R., Dagliyan G., Conti P.S., Brand D., Liu Z., Zou H., Stohl W., Zheng S.G. Induced T regulatory cells suppress osteoclastogenesis and bone erosion in collagen-induced arthritis better than natural T regulatory cells. Ann. Rheum. Dis. 2012;71:1567–1572. doi: 10.1136/annrheumdis-2011-201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yae T., Tsuchihashi K., Ishimoto T., Motohara T., Yoshikawa M., Yoshida G.J., Wada T., Masuko T., Mogushi K., Tanaka H., Osawa T., Kanki Y., Minami T., Aburatani H., Ohmura M., Kubo A., Suematsu M., Takahashi K., Saya H., Nagano O. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat. Commun. 2012;3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- 26.McGarry T., Veale D.J., Gao W., Orr C., Fearon U., Connolly M. Toll-like receptor 2 (TLR2) induces migration and invasive mechanisms in rheumatoid arthritis. Arthritis Res. Ther. 2015;17:153. doi: 10.1186/s13075-015-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X.H., Zhang Q.Y., Pan L.L., Liu S.Y., Xu P., Luo X.L., Zou S.L., Xin H., Qu L.F., Zhu Y.Z. NADPH oxidase 4 contributes to connective tissue growth factor expression through Smad3-dependent signaling pathway. Free Radic. Biol. Med. 2016;94:174–184. doi: 10.1016/j.freeradbiomed.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Poulet B., Beier F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Res. Ther. 2016;18:32. doi: 10.1186/s13075-015-0908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace J.L., Blackler R.W., Chan M.V., Da Silva G.J., Elsheikh W., Flannigan K.L., Gamaniek I., Manko A., Wang L., Motta J.P., Buret A.G. Anti-inflammatory and cytoprotective actions of hydrogen sulfide: translation to therapeutics. Antioxid. Redox Signal. 2015;22:398–410. doi: 10.1089/ars.2014.5901. [DOI] [PubMed] [Google Scholar]
- 30.Zhi L., Ang A.D., Zhang H., Moore P.K., Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J. Leukoc. Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 31.Muniraj N., Stamp L.K., Badiei A., Hegde A., Cameron V., Bhatia M. Hydrogen sulfide acts as a pro-inflammatory mediator in rheumatic disease. Int. J. Rheum. Dis. 2014 doi: 10.1111/1756-185X.12472. [DOI] [PubMed] [Google Scholar]
- 32.Tran B.H., Huang C., Zhang Q., Liu X., Lin S., Liu H., Wang S., Zhu Y.Z. Cardioprotective effects and pharmacokinetic properties of a controlled release formulation of a novel hydrogen sulfide donor in rats with acute myocardial infarction. Biosci. Rep. 2015;35 doi: 10.1042/BSR20140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan L.L., Liu X.H., Gong Q.H., Zhu Y.Z. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids. 2011;41:205–215. doi: 10.1007/s00726-011-0834-1. [DOI] [PubMed] [Google Scholar]
- 34.Bartok B., Firestein G.S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Carbonell R., Divakaruni A.S., Lodi A., Vicente-Suarez I., Saha A., Cheroutre H., Boss G.R., Tiziani S., Murphy A.N., Guma M. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheuma. 2016;68:1614–1626. doi: 10.1002/art.39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takemura S., Braun A., Crowson C., Kurtin P.J., Cofield R.H., O’Fallon W.M., Goronzy J.J., Weyand C.M. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 2001;167:1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 37.Pan L.L., Liu X.H., Gong Q.H., Yang H.B., Zhu Y.Z. Role of cystathionine gamma-lyase/hydrogen sulfide pathway in cardiovascular disease: a novel therapeutic strategy? Antioxid. Redox Signal. 2012;17:106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma K., Liu Y., Zhu Q., Liu C.H., Duan J.L., Tan B.K., Zhu Y.Z. H2S do, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a el anti-cancer effect of endoges H2S? PLoS One. 2011;6:e20525. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kan J., Guo W., Huang C., Bao G., Zhu Y., Zhu Y.Z. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid. Redox Signal. 2014;20:2303–2316. doi: 10.1089/ars.2013.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riveiro-Naveira R.R., Valcarcel-Ares M.N., Almonte-Becerril M., Vaamonde-Garcia C., Loureiro J., Hermida-Carballo L., Lopez-Pelaez E., Blanco F.J., Lopez-Armada M.J. Resveratrol lowers synovial hyperplasia, inflammatory markers and oxidative damage in an acute antigen-induced arthritis model. Rheumatology. 2016 doi: 10.1093/rheumatology/kew255. [DOI] [PubMed] [Google Scholar]
- 41.Khojah H.M., Ahmed S., Abdel-Rahman M.S., Hamza A.B. Reactive oxygen and nitrogen species in patients with rheumatoid arthritis as potential biomarkers for disease activity and the role of antioxidants. Free Radic. Biol. Med. 2016;97:285–291. doi: 10.1016/j.freeradbiomed.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Rosillo M.A., Sanchez-Hidalgo M., Gonzalez-Benjumea A., Fernandez-Bolanos J.G., Lubberts E., Alarcon-de-la-Lastra C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015;59:2537–2546. doi: 10.1002/mnfr.201500304. [DOI] [PubMed] [Google Scholar]
- 43.Joo Choi R., Cheng M.S., Kim Y. Shik. Desoxyrhapontigenin up-regulates Nrf2-mediated heme oxygenase-1 expression in macrophages and inflammatory lung injury. Redox Biol. 2014;2:504–512. doi: 10.1016/j.redox.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Na H.K., Surh Y.J. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic. Biol. Med. 2014;67:353–365. doi: 10.1016/j.freeradbiomed.2013.10.819. [DOI] [PubMed] [Google Scholar]