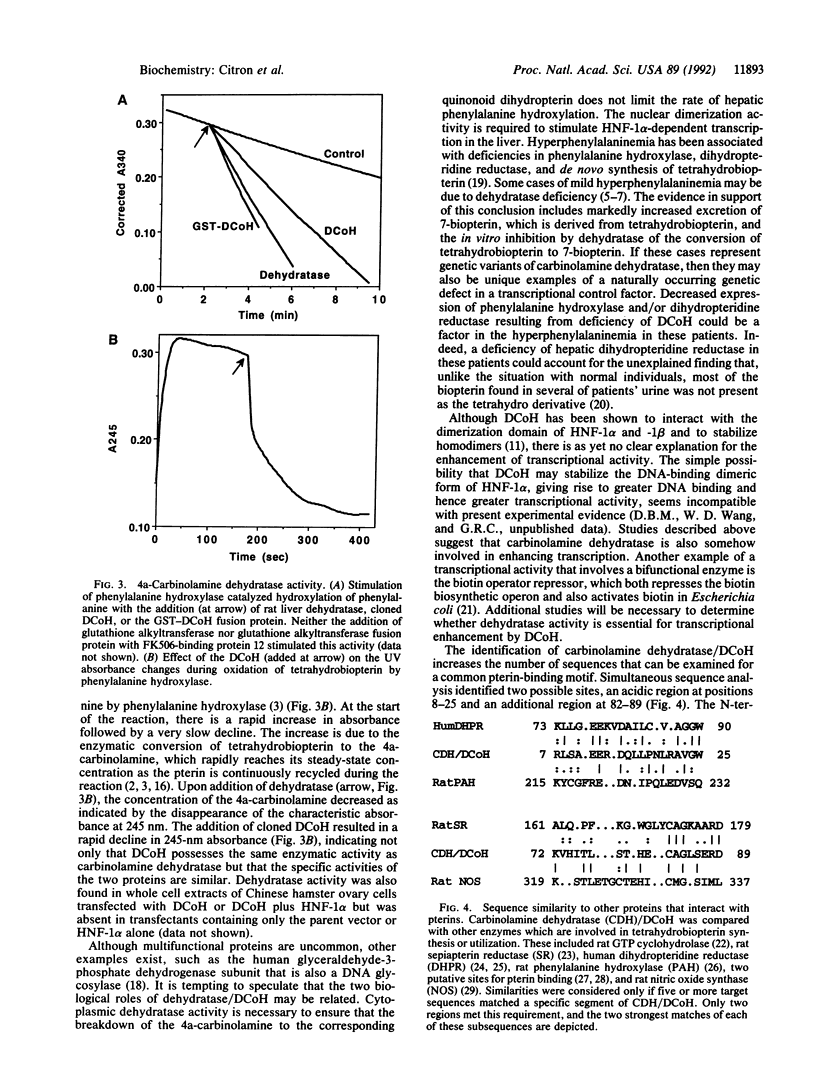
Abstract
The principal pathway for the metabolism of phenylalanine in mammals is via conversion to tyrosine in a tetrahydrobiopterin-dependent hydroxylation reaction occurring predominantly in the liver. Recently, the proposal that certain hyperphenylalaninemic children may have a deficiency of carbinolamine dehydratase, a component of the phenylalanine hydroxylation system, has widened the interest in this area of metabolism. Upon cloning and sequencing the dehydratase, we discovered that this protein is identical to DCoH, the cofactor which regulates the dimerization of hepatic nuclear factor 1 alpha, a homeodomain transcription factor. The identity of the nuclear and cytoplasmic proteins is demonstrated by size, immunoblotting, stimulation of phenylalanine hydroxylase, and dehydratase activity. The evolution of the dual functions of regulation of phenylalanine hydroxylation activity and transcription activation in a single polypeptide is unprecedented.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D. F., Campbell A. M. Genetic and biochemical characterization of the birA gene and its product: evidence for a direct role of biotin holoenzyme synthetase in repression of the biotin operon in Escherichia coli. J Mol Biol. 1981 Mar 15;146(4):469–492. doi: 10.1016/0022-2836(81)90043-7. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Citron B. A., Milstien S., Gutierrez J. C., Levine R. A., Yanak B. L., Kaufman S. Isolation and expression of rat liver sepiapterin reductase cDNA. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6436–6440. doi: 10.1073/pnas.87.16.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- Curtius H. C., Adler C., Rebrin I., Heizmann C., Ghisla S. 7-Substituted pterins: formation during phenylalanine hydroxylation in the absence of dehydratase. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1060–1066. doi: 10.1016/0006-291x(90)91554-6. [DOI] [PubMed] [Google Scholar]
- Curtius H. C., Kuster T., Matasovic A., Blau N., Dhondt J. L. Primapterin, anapterin, and 6-oxo-primapterin, three new 7-substituted pterins identified in a patient with hyperphenylalaninemia. Biochem Biophys Res Commun. 1988 Jun 16;153(2):715–721. doi: 10.1016/s0006-291x(88)81153-7. [DOI] [PubMed] [Google Scholar]
- Curtius H. C., Matasovic A., Schoedon G., Kuster T., Guibaud P., Giudici T., Blau N. 7-Substituted pterins. A new class of mammalian pteridines. J Biol Chem. 1990 Mar 5;265(7):3923–3930. [PubMed] [Google Scholar]
- Dahl H. H., Hutchison W., McAdam W., Wake S., Morgan F. J., Cotton R. G. Human dihydropteridine reductase: characterisation of a cDNA clone and its use in analysis of patients with dihydropteridine reductase deficiency. Nucleic Acids Res. 1987 Mar 11;15(5):1921–1932. doi: 10.1093/nar/15.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Mercer J. F. Isolation and sequence of a cDNA clone which contains the complete coding region of rat phenylalanine hydroxylase. Structural homology with tyrosine hydroxylase, glucocorticoid regulation, and use of alternate polyadenylation sites. J Biol Chem. 1986 Mar 25;261(9):4148–4153. [PubMed] [Google Scholar]
- Davis M. D., Kaufman S., Milstien S. Conversion of 6-substituted tetrahydropterins to 7-isomers via phenylalanine hydroxylase-generated intermediates. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):385–389. doi: 10.1073/pnas.88.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. D., Kaufman S., Milstien S. Distribution of 4a-hydroxytetrahydropterin dehydratase in rat tissues. Comparison with the aromatic amino acid hydroxylases. FEBS Lett. 1992 May 4;302(1):73–76. doi: 10.1016/0014-5793(92)80288-r. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs B. S., Benkovic S. J. Affinity labeling of the active site and the reactive sulfhydryl associated with activation of rat liver phenylalanine hydroxylase. Biochemistry. 1991 Jul 9;30(27):6795–6802. doi: 10.1021/bi00241a024. [DOI] [PubMed] [Google Scholar]
- Hatakeyama K., Inoue Y., Harada T., Kagamiyama H. Cloning and sequencing of cDNA encoding rat GTP cyclohydrolase I. The first enzyme of the tetrahydrobiopterin biosynthetic pathway. J Biol Chem. 1991 Jan 15;266(2):765–769. [PubMed] [Google Scholar]
- Huang C. Y., Kaufman S. Studies on the mechanisms of action of phenylalanine hydroxylase and its protein stimulator. I. Enzyme concentration dependence of the specific activity of phenylalanine hydroxylase due to a nonenzymatic step. J Biol Chem. 1973 Jun 25;248(12):4242–4251. [PubMed] [Google Scholar]
- Huang C. Y., Max E. E., Kaufman S. Purification and characterization of phenylalanine hydroxylase-stimulating protein from rat liver. J Biol Chem. 1973 Jun 25;248(12):4235–4241. [PubMed] [Google Scholar]
- Jennings I., Cotton R. Structural similarities among enzyme pterin binding sites as demonstrated by a monoclonal anti-idiotypic antibody. J Biol Chem. 1990 Feb 5;265(4):1885–1889. [PubMed] [Google Scholar]
- Kaufman S. A protein that stimulates rat liver phenylalanine hydroxylase. J Biol Chem. 1970 Sep 25;245(18):4751–4759. [PubMed] [Google Scholar]
- Lockyer J., Cook R. G., Milstien S., Kaufman S., Woo S. L., Ledley F. D. Structure and expression of human dihydropteridine reductase. Proc Natl Acad Sci U S A. 1987 May;84(10):3329–3333. doi: 10.1073/pnas.84.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel D. B., Crabtree G. R. HNF-1, a member of a novel class of dimerizing homeodomain proteins. J Biol Chem. 1991 Jan 15;266(2):677–680. [PubMed] [Google Scholar]
- Mendel D. B., Khavari P. A., Conley P. B., Graves M. K., Hansen L. P., Admon A., Crabtree G. R. Characterization of a cofactor that regulates dimerization of a mammalian homeodomain protein. Science. 1991 Dec 20;254(5039):1762–1767. doi: 10.1126/science.1763325. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler K., Mauro D. J., Seal G., Wurzer J., deRiel J. K., Sirover M. A. A human nuclear uracil DNA glycosylase is the 37-kDa subunit of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8460–8464. doi: 10.1073/pnas.88.19.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Monaci P., Tomei L., De Francesco R., Nuzzo M., Stunnenberg H., Cortese R. A myosin-like dimerization helix and an extra-large homeodomain are essential elements of the tripartite DNA binding structure of LFB1. Cell. 1990 Jun 29;61(7):1225–1236. doi: 10.1016/0092-8674(90)90687-a. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]