Summary
Background
Cabozantinib is approved in the treatment of progessive, metastatic medullary thyroid cancer (MTC). It is a small molecule inhibitor, which targets multiple receptors including vascular endothelial growth factor receptor, tyrosine kinase with Ig and epidermal growth factor homology domains-2 and the proto-oncogenes MET (mesenchymal-epithelial transition factor) and RET (rearranged during transfection). The drug is currently in phase I/II/III clinical trials for a number of other solid tumours and haematological malignancies. The adverse event (AE) profile is similar to that of other newer angiogenesis inhibitors. Hand–foot skin reaction (HFSR) is an important dose-limiting dermatological adverse event of this class of drugs.
Aim
To ascertain the incidence and risk of HFSR in patients with cancer during treatment with cabozantinib.
Methods
Electronic databases (PubMed, Web of Science) and the American Society for Clinical Oncology website were queried from inception to July 2014. Only phase II/III studies investigating cabozantinib for the treatment of cancer were shortlisted. The incidence, relative risk (RR) and 95% CI were calculated using random- or fixed-effects models, depending on the heterogeneity of included studies.
Results
We included 831 patients treated with cabozantinib for various solid malignancies in the analysis. The overall incidence was 35.3% (95% CI 27.9–43.6%) for all-grade and 9.5% (95% CI 7.6–11.7%) for high-grade HFSR. The RR of all-grade and high-grade HFSR with cabozantinib, compared with controls, was increased for both all-grade (27.3; 95% CI 6.9–108.3; P < 0.001) and high-grade, 28.1; 95% CI 1.7–457; P < 0.02) HFSR, respectively.
Conclusions
The incidence and risk of developing HFSR with cabozantinib are high. Timely recognition of this dose-limiting AE is critical to direct supportive care efforts including patient counselling, and to institute preventative and/or treatment interventions.
Introduction
Cabozantinib (XL184 or Cometriq; Exelixis Inc., South San Francisco, CA, USA) is a is a novel orally bioavailable multikinase inhibitor (MKI) with potent target activity against the vascular endothelial growth factor receptor (VEGFR)-2 and the proto-oncogenesMET (mesenchymal-epithelial transition factor), KIT and RET (rearranged during transfection).1 The drug was approved after it met its primary endpoint of progression-free survival for the treatment of metastatic medullary thyroid cancer (MTC).2 Currently, it is under investigation for solid tumours3 and haematological malignancies in over 50 clinical trials being conducted across North America, Europe, China, Australia and the Middle East.
The associated adverse events (AEs) include fatigue, decreased appetite, taste alterations, nausea, diarrhoea, dehydration, weight loss, hypertension, pulmonary embolism and dermatological manifestations such as hand–foot skin reaction (HFSR), rashes, dry skin, hair colour changes and alopecia.2,4 These AEs affect patients’ health-related quality of life (HRQoL), add financial burden and may lead to anti-cancer dosing inconsistencies.5
Therefore, we conducted a systematic review of the literature and performed a meta-analysis to determine the overall incidence and risk of developing HFSR with cabozantinib.
Methods
Data sources and search strategy
We queried the electronic databases of PubMed, Web of Science and the American Society of Clinical Oncology abstracts from inception until July 2014, using ‘cabozantinib’ or ‘Cometriq’ or ‘XL-184’ as the search terms.
Selection of studies
We included only phase II and phase III clinical trials in patients with any tumour type treated with cabozantinib, which reported safety data on HFSR. Phase I trials were excluded because of their dose-escalation model and smaller enrolment numbers. For the incidence analysis, all trials were included, and for the relative risk (RR) analysis, only randomized controlled trials (RCTs) were considered.
Data extraction and clinical endpoints
The following data were independently extracted by two of the researchers (VRB and CS) and compared: first author’s name, year of publication, trial design, underlying cancer, accrual/treatment numbers, treatment arms, number of patients with all-grade and high-grade HFSR in each treatment group, and the Common Terminology Criteria for Adverse Events (CTCAE) grading. The number of patients evaluable for AEs was used as the analysable number for each trial. Only data from the latest version among duplicates was considered.
The safety data were used for extraction of clinical endpoints. HFSR had been recorded according to CTCAE version 3.0, which defines the severity of HFSR as: grade 1, minimal skin changes or dermatitis (e.g. erythema) without pain; grade 2, skin changes (e.g. peeling, blisters, bleeding, oedema) or pain, not interfering with function; and grade 3, ulcerative dermatitis or skin changes with pain, interfering with function. All-grade HFSR includes grades 1, 2 and 3, while high-grade HFSR covers grade 3 only.
Statistical analysis
All statistical analyses were performed using comprehensive MetaAnalysis program (v2; Biostat, Englewood, NJ USA). For each clinical trial, the incidence of HFSR was calculated, and the 95% CI was derived. The RR of HFSR among patients assigned to cabozantinib was calculated and compared with the RR of those assigned to control treatment in the same trial. Forest plots were constructed. For the meta-analysis, both the fixed-effects model (weighted with inverse variance) and the random-effects model were considered. For each meta-analysis, Cochran’s Q statistic was first calculated to assess the heterogeneity of the included trials. For P < 0.1, the assumption of homogeneity was deemed invalid, and the random-effects model was used. Otherwise, results from both the fixed-effects model and the random-effects model were evaluated. If the results of the fixed-effects and the random-effects model were similar, only fixed-effects model results were reported. A two-tailed P < 0.05 (two-tailed) was considered as statistically significant.
Results
Search results
Our database search yielded 157 potentially relevant records. After initial exclusion (Fig. 1), we reviewed the safety data from trials of cabozantinib in MTC (phase III, RCT),2 glioblastoma,6 and six (of nine) other cancer types, namely, breast, ovarian and prostate cancer, non-small cell lung cancer (NSCLC), hepatocellular carcinoma (HCC) and melanoma [subsets of a large phase II, randomized discontinuation trial (RDT)].3
Figure 1.
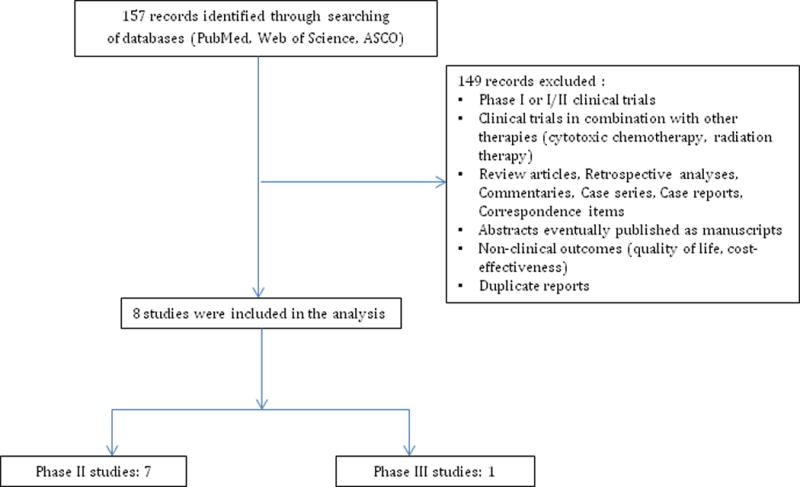
Selection process for studies included in the meta-analysis.
Screening process
Overall, eight clinical trials were eligible for inclusion: seven phase II trials and one phase III trial. In total, 831 patients with underlying diagnoses of progressive unresectable MTC,2 castration-resistant prostate cancer,4 progressive glioblastoma,6 metastatic breast cancer,7 metastatic NSCLC,8 HCC,9 ovarian cancer10 and melanoma11 were analysed (Table 1).
Table 1.
Clinical trials of cabozantinib included in the meta-analysis.
| Study (author, year) | Trial design | Arm
|
Enrolled, n | Sample size, n* | Underlying Malignancy | CTCAE version | |
|---|---|---|---|---|---|---|---|
| Cabozantinib, mg/day | Control | ||||||
| Elisei et al., 20132 | Phase III RCT | 140 | Placebo | 330 | 214 | Metastatic medullary thyroid cancer | 3 |
| Wen et al., 20106 | Phase II (Arm A) Phase II (Arm B) |
175 125 |
NA | 195 | 46 107 |
Progressive glioblastoma | 3 |
| Winer et al., 20127 | Phase II RDT | 100 | NA | 45 | 45 | Metastatic breast cancer | 3 |
| Hellerstedt et al.,8 2012 | Phase II RDT | 100 | NA | 60 | 60 | Metastatic NSCLC | 3 |
| Verslype et al., 20129 | Phase II RDT | 100 | NA | 41 | 41 | HCC | 3 |
| Buckanovich et al., 201110 | Phase II RDT | 100 | NA | 70 | 70 | Ovarian cancer | 3 |
| Smith et al., 20134 | Phase II RDT | 100 | NA | 171 | 171 | Prostate cancer | 3 |
| Gordon et al., 201211 | Phase II RDT | 100 | NA | 77 | 77 | Metastatic melanoma | 3 |
CTCAE, Common Terminology Criteria for Adverse Events; HCC, Hepatocellular carcinoma; NA, not applicable; NSCLC, non-small cell lung cancer; RCT, randomized controlled trial; RDT, randomized discontinuation trial.
Sample size is the number of patients included in the meta-analysis.
Incidence of all-grade hand–foot skin reaction
For the incidence analysis, data was included from all 831 patients in eight trials. The calculated overall incidence was 35.3% (95% CI 27.9–43.6%), using the random effects model (ranging from 21.7% to 56.1% for the individual trials). The lowest incidence (21.7%) was noted in a phase II RDT of 60 patients with metastatic NSCLC treated with cabozantinib 100 mg/day,8 and the highest incidence (56.1%) was observed in a phase II RDT of 41 patients with HCC (100 mg/day).9
Incidence of high-grade hand–foot skin reaction
The calculated overall incidence of high-grade HFSR was 9.5% (95% CI 7.6–11.7%) using the fixed effects model (range 4.7%–14.6%). The lowest incidence (4.7%) was noted in Arm B (n = 107, 125 mg/day) of a two-arm phase II trial of patients with glioblastoma treated with cabozantinib.6 The highest incidence (14.6%) was observed in a phase II RDT of 41 patients with HCC (100 mg/day).9
Incidence of hand–foot skin reaction based on dose of cabozantinib
Trials investigating different doses of cabozantinib (100–175 mg) were analysed for any variation in the incidence. The incidences of all-grade HFSR were as follows: 34.0% (95% CI 25.9–43.1%) with 100 mg/day, 26.2% (95% CI 18.7–35.3%) with 125 mg/day, 50.0% (95% CI 43.3–56.7%) with 140 mg/day and 37.0% (95% CI 24.4–51.6%) with 175 mg/day, based on the random effects model (P < 0.001).
Incidence of hand–foot skin reaction based on tumour type
The calculated incidence of all-grade HFSR was 21.7% for NSCLC, 24.7% for melanoma, 30.4% for prostate cancer and glioblastoma, 42.2% for breast cancer, 50.0% for MTC and 56.1% for HCC according to the random effects model (P < 0.001).
Relative risk of hand–foot skin reaction
The risk of developing HFSR (versus placebo) was ascertained in a phase III RCT (n = 323).2 All-grade HFSR was noted in 107/214 of the patients treated with cabozantinib, giving an overall RR of 27.3 (95% CI 6.9–108.3%, P < 0.001). High-grade HFSR was observed in 27/214 of the patients treated with cabozantinib, representing a RR of 28.1 (95% CI 1.7–457%, P < 0.02).
Discussion
This is the first study to estimate the incidence and risk of HFSR in patients with cancer treated with cabozantinib. We found that the drug was associated with a high incidence and an increased RR of developing all-grade and high-grade HFSR. Our subgroup analysis showed that the incidence was highest in patients with MTC and HCC.
In recent years several angiogenesis inhibitors have been approved (e.g. bevacizumab, sorafenib, sunitinib, pazopanib, axitinib, regorafenib and cabozantinib), which exert their effects primarily by targeting the VEGF/VEGFR pathway and other molecules [e.g. fibroblast growth factor receptor, platelet derived growth factor receptor (PDGFR) and Ang-1/tyrosine kinase with Ig and epidermal growth factor homology domains (TIE)-2].12 Intriguingly, these agents also induce HFSR, which is distinct from the hand–foot syndrome (HFS) that develops with conventional cytotoxic agents such as fluorouracil (5-FU). However, the severity and degree of HFSR appears to vary significantly, despite the considerable overlap in the spectrum of target inhibition.
Based on our data and those of previous studies the incidence of HFSR with cabozantinib is among the highest in the class of MKIs (Table 2).13–17 It is possible that the receptor blocking profile of cabozantinib (VEGFR, MET, RET, KIT and TIE-2) may be responsible.17,18 In addition, the drug is a cytochrome P3A4 (CYP3A4) substrate and any impairment in the expression of CYP3A4 enzymes, possibly due to liver metastases and/or HCC, may result in increased plasma levels of the drug and hence greater toxicity.19 It is noteworthy that the incidence based on tumour type was highest (56.1%) with HCC, although the mechanisms underlying the variation with different cancers are not known. This is consistent with a risk tool20 that demonstrated that the number of liver metastases increased the risk of HFSR with sorafenib.
Table 2.
Incidence of all-grade and high-grade HFSR with cabozantinib and other multikinase inhibitors.
| Drug (brand name) | Approved indication(s) | Molecular target | Incidence, %
|
Use in thyroid cancer | |
|---|---|---|---|---|---|
| All-grade | High-grade | ||||
| Pazopanib13 (Votrient®) | Advanced soft tissue sarcoma; advanced RCC | VEGF-R1/-R2/-R3, PDGFR-α/β, KIT, RAF | 4.5 | 1.8 | Experimental |
| Sunitinib14 (Sutent®) | GIST; advanced RCC; pancreatic neuroendocrine tumour | VEGF-R1/-R2/-R3, PDGFR, KIT, RET, CSF-1R, Flt-3 | 18.9 | 5.5 | Experimental |
| Axitinib15 (Inlyta®) | Advanced RCC | VEGF-R1/-R2/-R3, PDGFR, c-KIT | 29.2 | 9.6 | Experimental |
| Sorafenib16 (Nexavar®) | Unresectable HCC; advanced RCC; locally recurrent or metastatic, progressive, differentiated thyroid cancer | VEGF-R1/-R2/-R3, PDGFR-β, KIT, RET, RAF (CRAF & BRAF) | 33.8 | 8.9 | Approved |
| Cabozantinib (Cometriq®) | Progressive, metastatic medullary thyroid cancer | VEGF-R1/-R2/-R3, Flt-3, MET, RET, KIT, AXL, TRKB, TEK, TIE-2 | 35.3 | 9.5 | Approved |
| Regorafenib17 (Stivarga®) | Advanced GIST; mCRC | VEGF-R1/-R2/-R3, PDGFR-α/β, TIE-2, FGFR-1, KIT, RET, RAF, p38 MAPK | 60.5 | 20.4 | Not studied* |
CSF-1R, macrophage colony-stimulating factor 1 receptor; Flt, FMS-like tyrosine kinase; FGFR, fibroblast growth factor receptor;GIST, gastrointestinal stromal tumour; HCC, hepatocellular carcinoma; MAPK, mitogen-activated protein kinase; mCRC, metastatic colorectal cancer; MET, mesenchymal-epithelial transition factor; MKI, multikinase inhibitor; PDGFR, platelet-derived growth factor receptor; RAF, rapidly accelerating fibrosarcoma proto-oncogene serine–threonine-protein kinase; RET, rearranged during transfection receptor tyrosine kinase; TEK, tunica interna endothelial cell kinase; TIE, tyrosine kinase with Ig and epidermal growth factor homology domains; TrkB, tropomyosin-related kinase B; RCC, renal cell carcinoma; VEGFR, vascular endothelial growth factor receptor. *Data are based on published clinical trials investigating the utility of this drug exclusively in thyroid cancer.
We also observed a higher incidence of HFSR in patients treated with cabozantinib 140 mg/day (50.1%) compared with 100 mg/day (34.0%), suggesting a dose-dependent relationship, as seen with other MKIs. However, the incidence did not increase with higher doses (175 mg/day), as calculated from the data reported in a separate study.6 Therefore, given this haphazard response and the relatively small number of studies conducted to date, firm conclusions cannot be drawn.
The causes of HFSR appear to be multifactorial. PDGFR and c-KIT are strongly expressed in the ductal epithelium of eccrine glands21,22 which are found at the highest density in the palms and soles. Thus the possibility of eccrine excretion of the drug in high concentrations and resultant (direct) toxic damage to tissues, crossreaction and simultaneous inhibition of multiple receptors, leading to impairment in wound healing/repair mechanisms of endothelial cells and fibroblasts, especially at pressure and friction sites (palms/soles), has been entertained.23
Clinically, HFSR is characterized by tender, painful, bilaterally symmetrical erythematous lesions on the palms and soles, usually appearing within the first 2–5 weeks of treatment (Fig. 2). It generally starts with prodromal symptoms of tingling and numbness on the palms and soles, and sometimes a painful sensation induced upon touching hot objects. This is followed by three phases: the inflammatory phase, characterized by erythema, desquamation and blisters with a perilesional erythematous rim; the yperkeratotic phase, marked by the appearance of new lesions that become hyperkeratotic and development of pain in the older, hyperkeratotic areas; and the resolution phase, typified by clearing of the lesions as a result of dose modification or drug termination. Patients’ activities of daily living and HRQoL may be significantly impaired.5 Prophylactic management reduces the incidence of all-grade HFSR and delays its onset. The management strategies are outlined in Table 3, and include topical corticosteroids plus anaesthetics and oral analgesics.
Figure 2.
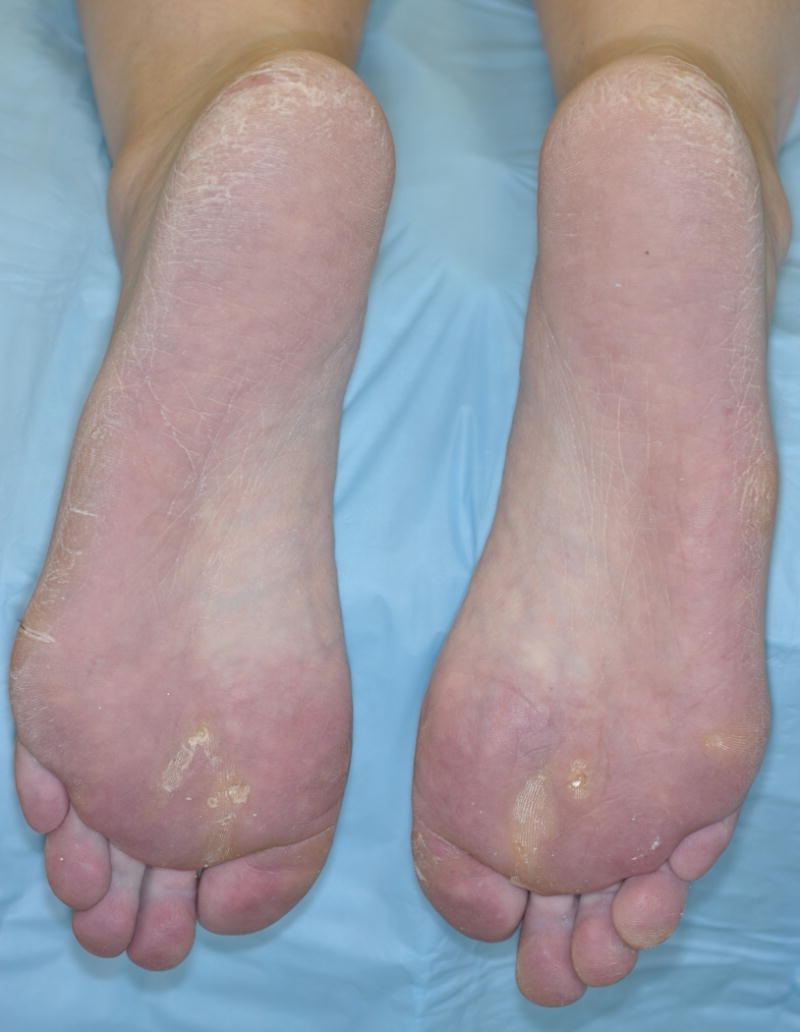
Grade 2 hand–foot skin reaction in a 42-year-old woman with non-small cell lung cancer, 5 months after treatment with cabozantinib. Note the hyperkeratotic over the medial forefoot (lesser metatarsal head region), erythema over the weight-bearing areas of the foot (lateral aspect of the sole) and toes and marked sparing of the instep and proximal phalangeal areas.
Table 3.
Prophylactic measures and management strategies for HSFR.
| Prophylactic measures |
| Full body examination to look for hyperkeratotic regions on palms and soles, and removal of calluses |
| Use of |
| Moisturizing creams containing keratolytics, such as ammonium lactate 12% (both before and during therapy) or urea 10–50%, or salicylic acid cream 3–6% twice daily |
| Thick cotton gloves and/or socks and slippers |
| Avoidance of: |
| Hot water when taking a shower/bath, or when washing dishes |
| Trauma and friction during the first 2–4 weeks |
| Vigorous exercise, especially during the first month of therapy |
| Tight-fitting shoes (evaluation by orthotist should be carried out if necessary) |
| Excessive pressure when applying lotions |
| Management strategies |
| *Treat the patient according to the NCI-CTCAE grading of HFSR |
| Grade 0. Use pre-emptive therapy as described above |
| Grade 1. Use urea 20% cream twice daily and clobetasol 0.05% cream once daily. Reassess after 2 weeks (either by healthcare professional or patient self-report); if reactions worsen or do not improve, continue anticancer therapy and follow grade 2 recommendations for management of HFSR |
| Grade 2. Urea 20% cream twice daily and clobetasol 0.05% cream once daily. Pain control with NSAIDs/GABA agonists/narcotics. Reassess after 2 weeks (either by healthcare professional or patient self-report); if reactions worsen or do not improve, continue anticancer therapy and follow grade 3 recommendations for management of HFSR |
| Grade 3. Interrupt treatment until severity decreases to grade 0–1. Continue treatment of skin reaction with the following clobetasol 0.05% cream twice daily and pain control with NSAIDs/GABA agonists/narcotics. Reassess after 2 weeks; if reactions worsen or do not improve, dose interruption or discontinuation as per package insert may be necessary |
HSFR, hand–foot skin reaction; GABA, gamma-aminobutyric acid; NCI-CTCAE, National Cancer Institute–Common Terminology Criteria for Adverse Events; NSAID, nonsteroidal anti-inflammatory drug.
Definitions for the severity grades of HFSR are provided in the Methods section.
Risk factors for HSFR include the presence of calluses and other hyperkeratotic areas on the body, poorly fitting shoes and/or repeated work-related friction to the hands/feet.24 A risk-scoring index has also been developed to identify patients at high risk of developing HFSR from sorafenib before each treatment cycle.20 The variables comprising this scoring system include female sex, pretreatment white blood cell counts, performance status, liver metastases and number of organs affected.
There is a paucity of information available to oncologists regarding the incorporation of newly approved agents into clinical practice, including their potential AEs. A survey of medical oncologists, assessing their clinical experiences and comfort levels with 20 recently approved targeted agents in the USA, showed that 14% had prescribed cabozantinib for MTC, and that only 11% felt very confident about prescribing the medication.25 This is in contrast to vandetanib, which was prescribed by 30% of the oncologists for MTC, with 16% feeling comfortable prescribing the drug. With expanding use of cabozantinib because of the comprehensive inhibition of targets (VEGFR plus MET), improved survival rates and lack of cardiovascular morbidity, oncologists are more likely to confront HFSR.
Our study has several limitations. First, although our analysis included a relatively large sample of patients, the overall number of studies and the numbers of trials for any individual cancer were low. Second, the meta-analysis was performed at the study level, as individual subject data was not available. Third, the risk analysis in our study considered only one RCT (patients with MTC). Fourth, the dose may have been expressed differently in the literature (salt weight vs. freebase weight); however, in such cases the formulation was the same. Fifth, the familiarity with HFSR of oncologist from different institutions and specialties may vary, introducing interobserver bias. Lastly, clinical trials have an arbitrary cutoff threshold for reporting AEs (e.g. > 5% or > 10%) in their safety data, and events below these thresholds are not reported. Therefore, our findings might be an underestimation of the actual burden.
Conclusion
Our study draws attention to the high incidence and increased risk of developing HFSR with cabozantinib, which possibly occurs in a dose-dependent fashion. The AE profile of cabozantinib is consistent with that of other MKIs and is manageable with supportive care interventions or dose modifications.2,4 However, the frequency with which dose alterations occur appears to be high, prompting plans to use lower starting doses in the future.2 It is important that oncologists are aware of cabozantinib-induced HFSR, a dose-limiting dermatological AE and its management, which is crucial for optimal clinical outcomes and good HRQoL in patients. Further research is required to enhance our current understanding and develop evidence-based management strategies and risk prediction algorithms for HFSR.
What’s already known about this topic?
HFSR is a dose-limiting AE that may be encountered in patients with cancer during treatment with VEGFR inhibitors.
An increased risk of HFSR has been reported with VEGFR pathway inhibitors, such as sorafenib, sunitinib, axitinib, pazopanib and regorafenib, but the incidence with cabozantinib is not known.
What does this study add?
The incidence and increased risk of developing all-grade and high-grade HFSR with cabozantinib is high.
Successful management of this untoward condition is critical to prevent impairment in patients’ quality of life.
CPD questions.
Learning objective
To help with understanding the important aspects pertaining to hand–foot skin reaction (HFSR) in patients with cancer.
Question 1
Which of the following is not an adverse event of cytotoxic chemotherapeutic agents or targeted therapies?
Hand, foot and mouth disease (HFMD).
Hand–foot syndrome (HFS).
Hand–foot–skin reaction (HFSR).
Hand–foot–genital syndrome (HFGS).
Palmar–plantar erythrodysaesthesia (PPE).
Question 2
The Common Terminology Criteria for Adverse Events (CTCAE), developed by the National Cancer Institute (NCI), is the most widely used adverse event severity grading system used by oncologists. At which of the following CTCAE grades of hand–foot–skin reaction (HSFR) is interruption of cancer treatment recommended?
Grade 1.
Grade 2.
Grade 3.
Grade 0.
Grade 6.
Question 3
Which of the following strategies has not shown benefit in the prevention of hand–foot skin reaction (HSFR)?
Topical emollient creams.
Psoralen ultraviolet therapy (PUVA).
Protective footwear (e.g. orthopaedic soles, inserts).
Podiatric examination/intervention.
Topical keratolytic creams (e.g. urea).
Question 4
In the pathogenesis of hand–foot skin reaction (HSFR), which of the following molecular target(s) is most commonly implicated?
VEGF.
PDGFR/VEGFR.
EGFR.
HER2.
mTOR.
Question 5
Which of the following agents is typically associated with the development of hand–foot skin reaction (HSFR)?
Imatinib.
Cetuximab.
Regorafenib.
Trametinib.
Temsirolimus.
Answer 1
Which of the following are not adverse events of cytotoxic chemotherapeutic agents or targeted therapies?
Correct. HFMD is caused by an enteroviruses (Coxsackievirus A16, Enterovirus 71) and is not an adverse event of cancer therapies. Palmar–plantar erythrodysaesthesia (PPE) is the terminology used in the CTCAE grading system to denote HFS (cytotoxic chemotherapy) and HFSR (targeted therapies).
Incorrect.
Incorrect.
Correct. HFGS is a rare genetic condition caused by mutations in the HOXA13 gene.
Incorrect.
Answer 2
The Common Terminology Criteria for Adverse Events (CTCAE), developed by the National Cancer Institute (NCI), is the most widely used adverse event severity grading system used by oncologists. At which of the following CTCAE grades of hand–foot–skin reaction (HSFR) is interruption of cancer treatment recommended?
Incorrect.
Incorrect.
Correct. For grade 3 HFSR, it is recommended that treatment be interrupted for a minimum of 7 days and until the adverse event reverts to grade 0–1. Treatment is restarted at a reduced dose after such interruptions. Subsequently, if the severity of HFSR remains at grade 0–1 at the reduced dose for a minimum of 7 days, the initial dose should be instituted again. If grade 3 HFSR occurs, clinical judgment and patient preference dictate whether the dose is re-escaled. A similar approach is followed when deciding whether to discontinue therapy after the third episode of grade 3 HFSR. There are no grade 0 or 6 definitions for HFSR in the CTCAE.
Incorrect.
Incorrect.
Answer 3
Which of the following strategies has not shown benefit in the prevention of hand–foot skin reaction?
Incorrect.
Correct. In a small case-series, PUVA therapy showed benefit in the treatment of HSFR induced by imatinib or sunitinib HFSR, but there are no reports of its (beneficial) use in the prevention of HFSR.
Incorrect.
Incorrect.
Incorrect.
Answer 4
In the pathogenesis of hand–foot skin reaction (HSFR), which of the following molecular target(s) is most commonly implicated?
Incorrect.
Correct. HFSR is not generally seen with inhibitors of the VEGF (e.g. bevacizumab), EGFR (e.g. cetuximab), HER2 (e.g. trastuzumab) and mTOR (e.g. temsirolimus). The multikinase inhibitors, sorafenib, sunitinib, pazopanib, axitinib, regorafenib and cabozantinib target PDGFR/VEGFR, and are typically associated with the development of HFSR.
Incorrect.
Incorrect.
Incorrect.
Answer 5
Which of the following agents is typically associated with the development of hand–foot skin reaction (HSFR)?
Incorrect.
Incorrect.
Correct. Regorafenib is a multikinase inhibitor currently approved for use in the treatment of metastatic colorectal cancer and locally advanced gastrointestinal stromal tumours. The overall incidences of all-grade and high-grade HFSR with regorafenib are 60.5% and 20.4%, respectively.
Incorrect.
Incorrect.
Footnotes
Conflict of interest: SW has a speaking arrangement/consulting/advisory role with Novartis, Bayer-Onyx and Pfizer. MEL has a speaking, consultant or advisory role with Advancell, Amgen, AstraZeneca, Augmentium, Aveo, Bayer, Berg Pharma, Biopharm Communications, Boehringer Ingelheim, Brickell Biotech, Bristol-Myers Squibb, Clinical Assistance Programs, Clinical Care Options, EMD Serono, Envision Communications, Foamix, Galderma, Genentech, GlaxoSmithKline, Helsinn, Institute for Medical Education and Research, Integro-MC, Lindi Skin, Medscape, Medtrend International, Merck, Nerre Therapeutics, Novartis, Novocure, Oncology Specialty Group, OSI Pharmaceuticals, Permanyer, Physicians Education Resource, Pierre Fabre, Pfizer, Reata Pharmaceuticals, Roche, Sandoz, Sanofi Aventis and Threshold Pharmaceuticals. The other authors declare that they have no conflicts of interest.
References
- 1.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 2.Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon MS, Vogelzang NJ, Schoffski P, et al. Activity of cabozantinib (XL184) in soft tissue and bone: Results of a phase II randomized discontinuation trial (RDT) in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2011;29:3010. [Google Scholar]
- 4.Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–19. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nardone B, Hensley JR, Kulik L, et al. The effect of hand-foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. J Drugs Dermatol. 2012;11:e61–5. [PubMed] [Google Scholar]
- 6.Wen PY, Prados M, Schiff D, et al. Phase II study of XL184 (BMS 907351), an inhibitor of MET, VEGFR2, and RET, in patients (pts) with progressive glioblastoma (GB) ASCO Meeting Abstracts. 2010;28:2006. [Google Scholar]
- 7.Winer EP, Tolaney S, Nechushtan H, et al. Activity of cabozantinib (XL184) in metastatic breast cancer (MBC): Results from a phase II randomized discontinuation trial (RDT) ASCO Meeting Abstracts. 2012;30:535. [Google Scholar]
- 8.Hellerstedt BA, Edelman G, Vogelzang NJ, et al. Activity of cabozantinib (XL184) in metastatic NSCLC: Results from a phase II randomized discontinuation trial (RDT) ASCO Meeting Abstracts. 2012;30:7514. [Google Scholar]
- 9.Verslype C, Cohn AL, Kelley RK, et al. Activity of cabozantinib (XL184) in hepatocellular carcinoma: Results from a phase II randomized discontinuation trial (RDT) ASCO Meeting Abstracts. 2012;30:4007. [Google Scholar]
- 10.Buckanovich RJ, Berger R, Sella A, et al. Activity of cabozantinib (XL184) in advanced ovarian cancer patients (pts): Results from a phase II randomized discontinuation trial (RDT) ASCO Meeting Abstracts. 2011;29:5008. [Google Scholar]
- 11.Gordon MS, Kluger HM, Shapiro G, et al. Activity of cabozantinib (XL184) in metastatic melanoma: Results from a phase II randomized discontinuation trial (RDT) ASCO Meeting Abstracts. 2012;30:8531. [Google Scholar]
- 12.Gild ML, Bullock M, Robinson BG, et al. Multikinase inhibitors: a new option for the treatment of thyroid cancer. Nat Rev Endocrinol. 2011;7:617–24. doi: 10.1038/nrendo.2011.141. [DOI] [PubMed] [Google Scholar]
- 13.Balagula Y, Wu S, Su X, et al. The risk of hand foot skin reaction to pazopanib, a novel multikinase inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs. 2012;30:1773–81. doi: 10.1007/s10637-011-9652-2. [DOI] [PubMed] [Google Scholar]
- 14.Chu D, Lacouture ME, Weiner E, et al. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer. 2009;7:11–19. doi: 10.3816/CGC.2009.n.002. [DOI] [PubMed] [Google Scholar]
- 15.Fischer A, Wu S, Ho AL, et al. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs. 2013;31:787–97. doi: 10.1007/s10637-013-9927-x. [DOI] [PubMed] [Google Scholar]
- 16.Chu D, Lacouture ME, Fillos T, et al. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol. 2008;47:176–86. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 17.Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs. 2013;31:1078–86. doi: 10.1007/s10637-013-9977-0. [DOI] [PubMed] [Google Scholar]
- 18.Cascone T, Heymach JV. Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J Clin Oncol. 2012;30:441–4. doi: 10.1200/JCO.2011.38.7621. [DOI] [PubMed] [Google Scholar]
- 19.Legendre C, Hori T, Loyer P, et al. Drug-metabolising enzymes are down-regulated by hypoxia in differentiated human hepatoma HepaRG cells: HIF-1alpha involvement in CYP3A4 repression. Eur J Cancer. 2009;45:2882–92. doi: 10.1016/j.ejca.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Dranitsaris G, Vincent MD, Yu J, et al. Development and validation of a prediction index for hand-foot skin reaction in cancer patients receiving sorafenib. Ann Oncol. 2012;23:2103–8. doi: 10.1093/annonc/mdr580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lammie A, Drobnjak M, Gerald W, et al. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–25. doi: 10.1177/42.11.7523489. [DOI] [PubMed] [Google Scholar]
- 22.Ponten F, Ren Z, Nister M, et al. Epithelial-stromal interactions in basal cell cancer: the PDGF system. J Invest Dermatol. 1994;102:304–9. doi: 10.1111/1523-1747.ep12371787. [DOI] [PubMed] [Google Scholar]
- 23.Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–61. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 24.Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71:787–94. doi: 10.1016/j.jaad.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Love N, Anderson KC, Flaherty K, et al. Medical oncologists’ clinical experiences and comfort levels with 20 recently approved agents. ASCO Meeting Abstracts. 2013;31:e17570. [Google Scholar]


