Abstract
Fanconi anemia (FA) is a rare inherited bone marrow failure syndrome associated with high risks of severe bone marrow failure (BMF), acute myeloid leukemia (AML), and solid tumors (ST). Bone marrow transplantation (BMT) provides a theoretical cure for hematologic risks (BMF, AML), but it introduces uncertain risks of transplantation-related mortality (TRM) and carcinogenicity. We developed a mathematical (Markov) decision model to estimate event-free survival (EFS) conditional on age based on per-year cause-specific hazard rates. We assumed that preemptive (PE) BMT eliminates the risks of BMF and AML, but it may introduce independent risks of TRM or influence the trajectory to ST. Our model suggested that the expected mean EFS in FA is higher for PE-BMT at young ages, with minimal risk of TRM and with little carcinogenicity. PE-BMT in adults decreased expected EFS because of the greater competing risk of ST in adulthood. Estimates of EFS conditioned on attained age may be used in shared decision-making when clinicians must counsel patients using limited data. Our methods may be used to model early transplantation in other blood disorders for which hematopoietic stem cell transplantation mitigates some but not all of the risks.
Keywords: Preemptive transplantation, Fanconi anemia, Markov model, Decision analysis
INTRODUCTION
Fanconi anemia (FA) is a primarily autosomal recessive, bone marrow failure syndrome caused by mutations in 1 of more than 19 genes in the FA/BRCA DNA damage response pathway, which makes individuals with FA prone to cancer. The syndrome is diagnosed at a median age of 7 years, often because of recognition of aplastic anemia and the presence of a characteristic constellation of developmental abnormalities, including short stature, café-au-lait spots, absent radii, and hypoplastic thumbs [1]. Patients with FA are at particularly high risk of severe bone marrow failure (BMF), acute myelogenous leukemia (AML), and solid tumors (ST), usually squamous cell carcinomas. The risk of each event is age dependent. In a competing risks analysis of first major events in FA, the rate of severe BMF peaked in childhood, rate of AML plateaued after adolescence, and rate of ST increased at a greater-than-linear rate in early adulthood [2]. Mild cytopenias may be managed conservatively with androgens and/or transfusions. Allogeneic hematopoietic stem cell transplantation (HSCT) is the recommended approach for patients with aplastic anemia (hemoglobin < 8g/dL, absolute neutrophil count <500/uL, or platelet count <30,000 uL), myelodysplastic syndrome, or AML [3].
Successful allogeneic HSCT may cure the hematologic manifestations of FA but introduces risks of transplantation-related mortality (TRM) and morbidity. Transplantation outcomes have improved in recent years, with a 5-year overall survival in patients with FA who received transplants from matched sibling donors for any indication (90% had aplastic anemia) increasing from 68% to 76% between 1972 and 1999 and 2000 and 2009 in the European registry. The risk of TRM depends on many donor and recipient characteristics. Generally, best results were in patients under age 10 who underwent transplantation for aplastic anemia with bone marrow from matched related donors [4].
Patients or parents of children with FA often face a dilemma about whether or not to undergo HSCT after development of BMF or AML. Families relied heavily in the past on the advice of physicians [5], and the decision was driven by attitudes about uncertainty [6]. Shared decision-making is useful in circumstances of uncertainty, when risks are imprecisely known and the best decision for an individual depends on his or her preferences [7]. In the present study, we used decision-analysis methods and developed a model to facilitate shared decision-making between families with FA and their doctors. This model considers bone marrow transplantation (BMT) before the development of any of the symptoms for which transplantation is indicated (termed preemptive [PE]), and it can be used to quantify the risks after transplantation conditioned on age at the time of the decision. Our model allows the user to evaluate a number of “what if” scenarios obtained by varying the model inputs. In this way, the model outputs can help provide data with which each family with FA can examine their level of risk aversion and risk acceptance regarding various transplantation options.
METHODS
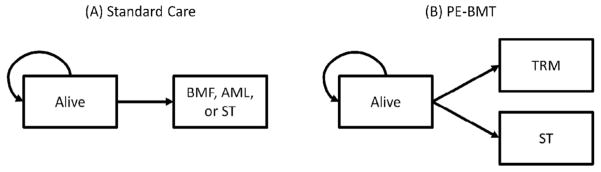
The structure of our model is based on the earlier model we created for patients with biallelic mutations in FANCD1/BRCA2 [8], allowing application to patients with FA of all ages and other genotypes. Specifically, we developed mathematical (Markov) models of event-free survival (EFS) [9] to reflect the natural history of FA, a strategy that we call standard care (Figure 1). Patients receiving standard care are at risk of BMF, AML, and ST. BMF in this context means marrow failure severe enough to lead to transplantation or death from aplastic anemia. We then developed a model for the competing strategy of PE-BMT in which the hematologic risks (BMF and AML) were eliminated by transplantation but an independent risk of TRM was introduced. The primary outcome was EFS, defined as freedom from BMF, AML, ST, or TRM. Note that our estimate of TRM means mortality, not morbidity. The models were analyzed using TreeAge Pro 2014 (TreeAge Software, Inc, Williamstown, MA). To obtain mathematically smooth outputs for tabulation and plotting, each model was run for 200 cycles with a cycle length of 3-months, all rates were transformed into 3-month probabilities, and we used a half-cycle correction [10].
Figure 1.
Models of event-free survival. (A) Standard care. (B) PE-BMT. BMF indicates bone marrow failure; AML, acute myeloid leukemia; ST, solid tumor; PE-BMT, preemptive bone marrow transplantation; TRM, treatment related mortality.
Data Sources
We implemented our model using estimates from the literature (Table 1). We incorporated the age-specific hazards of AML, BMF, and ST from an analysis of 4 cohorts: the North American Survey, the German FA Registry, the Israeli FA Registry, and the National Cancer Institute FA Cohort [2,11–13]. Linear extrapolation was used to compute hazards beyond the available data. We explored a range of ages at decision from birth to age 30 years.
Table 1.
Model Parameters and Ranges Tested in Sensitivity Analysis
| Parameter | Values (Range) | Source |
|---|---|---|
| Competing risks of BMF, AML, and ST | Age-specific hazard | Alter et al., 2010 [2] |
| Risk of PE-BMT TRM | 10% (0%–30%) | Peffault de Latour et al., 2013 [4] |
| Age at decision, yr | 7 (birth-30) | Peffault de Latour et al., 2013 [4] |
| Increased rate of ST following BMT | HR, 1.0 (1.0–4.4) | Rosenberg et al., 2005 [14] |
| Annual discount rate for future years* | 0% | Sonnenberg et al., 1993 [9] |
HR indicates hazard ratio.
The value of future years is not discounted.
We assumed that PE-BMT never failed to engraft, the recipient received bone marrow from an ideal matched related donor, the best available preparative regimen was used, and the risk of TRM occurred at a constant rate over the first year after PE-BMT. We defined TRM as the independent risk of mortality attributable to PE-BMT. The risk of TRM in practice depends on many factors, including transplantation regimen, age, prior transfusions, donor source, and source of stem cells. Experts may not agree on the precise estimate of TRM for any given patient. Our baseline assumption was the risk of TRM was 10%, but we modeled the decision over a range of TRM values between 0% and 30%, which we considered plausible given the statistical range of mortality reported in the European registry [4].
A retrospective study reported a 4.4-fold increase in the rate of ST in those who received a transplantation compared with those who did not receive a transplantation [14]. That study used data from more than 10 years ago, but no comparable studies have been published to assess the effect of contemporary transplantation regimens on the ST trajectory. We assumed for our base-case that PE-BMT did not increase the risk of ST over the baseline risk in FA, but we did explore plausible hazard ratios including values of 1.0 (no change), 2.0 (modest increase), and 4.4 (the point estimate from Rosenberg et al. [14]). We discounted the value of future years by 0% and did not incorporate decrements in quality of life (QoL), as the model was designed to be descriptive rather than prescriptive, and QoL data for patients with FA in this context are limited.
RESULTS
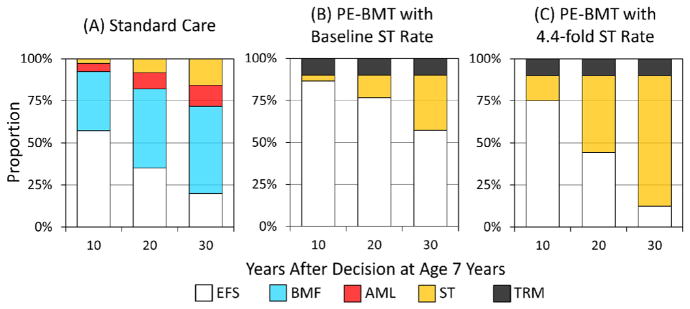
In a model cohort of children with FA whose decision to receive standard care rather than PE-BMT was made at age 7 years, the estimated 10-year EFS from that age forwards was 57%. Thirty-five percent of those with FA who receive standard care would develop BMF, 5% AML, and 3% ST (Figure 2A). In contrast, the estimated 10-year EFS would be 86% percent if the same cohort instead received PE-BMT with a 10% risk of TRM and subject to the same baseline rate of ST as patients with FA who did not undergo transplantation (Figure 2B). The crude proportion of the PE-BMT cohort that would develop ST was larger than that of the cohort who did not undergo transplantation, because patients whose BMF and AML were prevented by PE-BMT were more likely to survive event free long enough to develop ST. In contrast, 75% of a cohort receiving PE-BMT, in which there was a 4.4-fold increase in the rate of ST, would remain event free after 10 years (Figure 2C). Furthermore, the 20-year EFS after the decision increased from 35% under standard care to 77% for those receiving PE-BMT, with 10% TRM and baseline rate of ST, and to 44% if PE-BMT increased the rate of ST 4.4 fold. Finally, the 30-year EFS increased from 13% for those receiving standard care to 57% for those receiving PE-BMT with 10% TRM and no increase in the rate of ST, but it decreased to 12% if PE-BMT increased the rate of ST 4.4 fold.
Figure 2.
Outcomes after a decision at age 7 years. (A) Standard care. (B) PE-BMT with baseline ST rate. (C) PE-BMT with 4.4-fold increased ST rate. EFS, event-free survival; BMF, bone marrow failure; AML, acute myeloid leukemia; ST, solid tumor; PE-BMT, preemptive bone marrow transplantation; TRM, treatment related mortality.
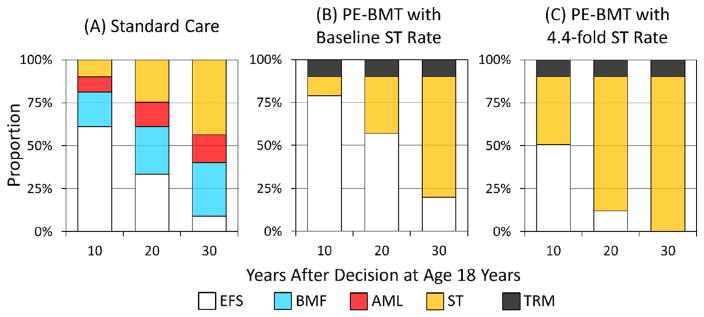
We then modeled the decision for a cohort of patients with FA who are age 18 years, the age at which patients are legally adults in the United States. The following results are conditioned on EFS to age 18 years. Ten-year EFS was 61% for those receiving standard care (Figure 3A). PE-BMT with 10% TRM and no increase in the rate of ST increased 10-year EFS to 79% (Figure 3B). However, if PE-BMT increased the rate of ST 4.4 fold, only 51% would remain event free after 10 years (Figure 3C). Furthermore, EFS at 20 years was 61% in those receiving standard care and 57% in those receiving PE-BMT with 10% TRM and a baseline rate of ST. Twenty-year EFS would decrease to 12% if PE-BMT increased the rate of ST 4.4 fold. Finally, EFS after 30 years increased from 9% for those receiving standard care to 20% for those receiving PE-BMT with a baseline rate of ST. However, if the already-high rate of ST was increased 4.4 fold, only .1% of patients would remain event free after 30 years.
Figure 3.
Outcomes after a decision at age 18 years. Abbreviations defined in Figures 1 and 2.
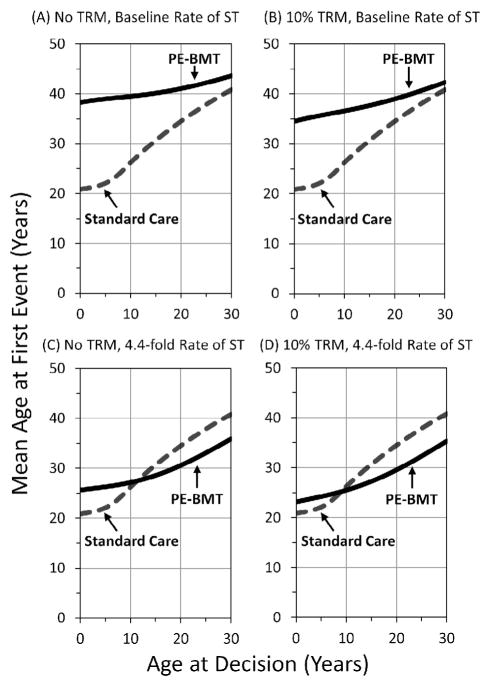
We calculated the mean age at first event, and we plotted these estimates versus the age at decision in Figure 4. The mean age at first event was 26 years in a cohort of children at age 10 years under standard care (Figure 4A). The mean age at first event would increase to nearly 40 years if PE-BMT were performed with no risk of TRM and there were no effect on the baseline risk of ST (Figure 4A). PE-BMT would increase the mean EFS by 14 years over standard care in this favorable scenario. Figure 4B depicts the change in the results if PE-BMT introduced a 10% risk of TRM, which would increase the mean age at first event to 36 years. The mean age at first event would increase by only 1 year over standard care, to age 27 years (Figure 4C), if PE-BMT did not carry a risk of TRM but increased the rate of ST 4.4 fold. PE-BMT with both a 10% risk of TRM and a 4.4-fold increased rate of TRM would decrease the mean age at first event to 25 years (Figure 4D).
Figure 4.
Mean age at first event by age at decision. (A) No TRM, baseline rate of ST. (B) TRM at 10%, baseline rate of ST. (C) No TRM, 4.4-fold increased rate of ST. (D) 10% TRM, 4.4-fold increased rate of ST. Abbreviations in previous figures.
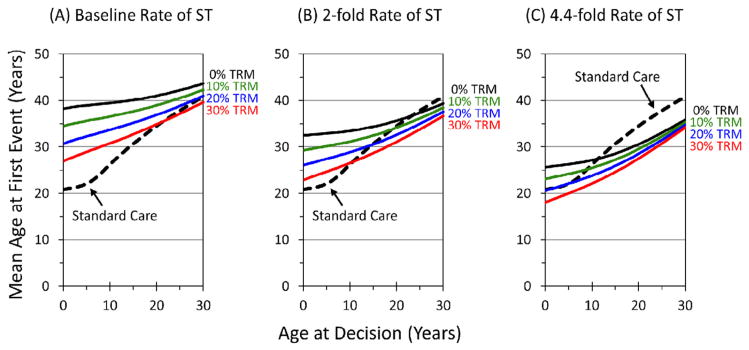
Our sensitivity analyses suggests the model results were sensitive to the risk of TRM, the rate of ST after transplantation, and the age at decision. Figure 5 provides estimates over a range of TRM and an ST rate that is unaffected (Figure 5A), increased 2 fold (Figure 5B), or increased 4.4 fold (Figure 5C). For example, if the rate of ST was increased 2 fold over the FA baseline, PE-BMT would provide no advantage on average in those older than age 18 years if the risk of TRM was 10%, and PE-BMT would provide no advantage on average for those older than 15 years of age with a 20% risk of TRM (Figure 5B). From these data, a strategy of transplantation at younger ages was superior, because transplantations performed at earlier ages preempt more of the lifetime risk of a major hematologic event.
Figure 5.
Mean age at first event by age at decision for a range of TRM and relative rates of ST following transplantation. Abbreviations in previous figures.
DISCUSSION
Our model suggests that PE-BMT in adults with FA would provide little advantage but PE-BMT in children would increase EFS so long as the risk of TRM is low and the regimen has little or no impact on the already high risk of developing ST. The results of our model were sensitive to increases in the rate of ST after transplantation, identifying the risk of ST after transplantation as an area that requires further research. The best-case scenario includes no risk of TRM and no increase over the FA baseline risk of ST. Removing the risks of BMF and AML at birth would increase the mean age at first event from 21 years to 38 years. However, a modest (2-fold) increase in the rate of ST after transplantation substantially decreased the expected long-term benefit of PE-BMT and identified the effect of BMT on ST risk as being of particular importance. Presently, it is unknown whether contemporary low-intensity transplantation regimens affect the trajectory to ST, although in the general population, HSCT is still associated with an increased risk of second cancers [15]. The last study of its kind in FA was published 10 years ago [14] and a contemporary study of the effect of HSCT in FA on ST risk needs to be conducted to inform current decision-making in FA. This is an important issue, as solid tumors in patients with FA (those who underwent transplantation or those who did not) are very difficult to treat, with a 5-year EFS of less than 50% [16].
We did not consider QoL in our analyses because the QoL in FA independent of transplantation status is poorly understood. Morbidities associated with transplantation may be due to the transplantation (eg, graft-versus-host disease, infection), but some morbidities overlap with those seen in patients with FA who do not undergo transplantation (eg, endocrine dysfunction) [17]. Research is needed to understand how the QoL of patients with FA changes over the life course and how transplantation-associated morbidities affect QoL in this context.
We purposefully explored a range of TRM because the risks in the literature vary widely and because experts may have variable prior estimates as to what the risks may be for a given patient and regimen. The fundamental problem is gauging future risks in the presence of uncertainty. No studies to date have quantified the risks patients with FA are willing to take, although risk aversion has been studied for other blood diseases. For example, in a recent study of sickle cell anemia patients, 56% of participants were willing to accept a 10% or greater risk of mortality for an opportunity for cure of the hematologic manifestations of their disease [18]. In the context of FA, patients and parents must decide whether an earlier risk of TRM might be worthwhile to avert the later risks of AML or myelodysplastic syndrome, the development of which may increase the risk of TRM in subsequent BMTs [4].
There are limitations to our decision-analysis approach. Importantly, the results here are neither prescriptive nor generalizable to all FA patients. For example, our findings were not intended to inform decision-making for children with biallelic mutations in FANCD1/BRCA2, which we reported elsewhere using similar methods [8]. Moreover, some patients prefer not to share in the decision-making process with their physicians, a preference that must be respected by clinicians [19]. Our use of retrospective data to estimate the risks is another limitation that introduces biases of ascertainment and enrollment. FA is phenotypically and genetically heterogeneous, and the risks we used may not reflect the natural history of FA for those with milder phenotypes. We also did not model some of the more complex and rare phenomena, such as somatic hematopoietic mosaicism or donor-cell leukemia. However, modeling of any complex process requires balancing parsimony, accuracy, and the limits of existing data [20].
A strength of our model is that uncertain estimates may be varied over a range of values to determine the sensitivity of a model to changes in its individual parts. Additional variables that could be added to the model include comorbidities in the recipient, such as renal disease, liver toxicity, or prior infection; use of unrelated or haploidentical donors; different preparative regimens; or different sources of stem cells, such as peripheral blood or cord blood. These factors will modify the risk of TRM or graft-versus-host disease. Rather than be explicit for each, we modeled the net effect of such factors implicitly by varying the TRM from 10% to 30%, as well as by increasing the baseline ST risk by 2 fold, or 4.4 fold. Our simple model for FA, which identifies a situation where PE-BMT in childhood might be considered, also shows that decision-making might be improved using newer data on whether PE-BMT might increase the risk of cancer.
Our methods are generalizable to other disorders for which HSCT may mitigate some but not all of the risks. For example, major hematologic risks in Diamond-Blackfan anemia are a development of AML, severe iron overload, or antibodies precluding transfusions [21,22]. AML is a frequent adverse outcome in severe congenital neutropenia, particularly in patients requiring high doses of granulocyte colony–stimulating factor, and also in Shwachman-Diamond syndrome [23]. The next step in the application of this model might involve using more recent data on transplantation outcomes from syndrome-specific registries or transplantation cohorts. Overall, models allow for a consistent evaluation of strategies and can serve to facilitate discussion when experts lack consensus.
Acknowledgments
Financial disclosure: This research was supported in part by the Intramural Research Program of the National Cancer Institute of the National Institutes of Health.
Conflict of interest statement: None.
Authorship statement: N.E.K., P.S.R., and B.P.A. designed the study; N.E.K. analyzed the data and wrote the paper, and all authors revised and checked the final version of the paper.
References
- 1.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24:101–122. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150:179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frohnmayer D, Frohnmayer L, Guinan E, Kennedy T, Larsen K, editors. Fanconi Anemia: Guidelines for Diagnosis and Management. 4. Eugene, OR: Fanconi Anemia Research Fund, Inc; 2014. [Google Scholar]
- 4.Peffault de Latour R, Porcher R, Dalle JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279–4286. doi: 10.1182/blood-2013-01-479733. [DOI] [PubMed] [Google Scholar]
- 5.Hutson SP, Han PK, Hamilton JG, et al. The use of haematopoietic stem cell transplantation in Fanconi anaemia patients: a survey of decision making among families in the US and Canada. Health Expect. 2015;18:929–941. doi: 10.1111/hex.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton JG, Hutson SP, Moser RP, et al. Sources of uncertainty and their association with medical decision making: exploring mechanisms in Fanconi anemia. Ann Behav Med. 2013;46:204–216. doi: 10.1007/s12160-013-9507-5. [DOI] [PubMed] [Google Scholar]
- 7.Legare F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood) 2013;32:276–284. doi: 10.1377/hlthaff.2012.1078. [DOI] [PubMed] [Google Scholar]
- 8.Khan NE, Rosenberg PS, Lehmann HP, Alter BP. Preemptive bone marrow transplantation for FANCD1/BRCA2. Biol Blood Marrow Transplant. 2015;21:1796–1801. doi: 10.1016/j.bbmt.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 10.Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. doi: 10.2165/00019053-199813040-00003. [DOI] [PubMed] [Google Scholar]
- 11.Tamary H, Nishri D, Yacobovich J, et al. Frequency and natural history of inherited bone marrow failure syndromes: the Israeli Inherited Bone Marrow Failure Registry. Haematologica. 2010;95:1300–1307. doi: 10.3324/haematol.2009.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93:511–517. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi Anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 15.Inamoto Y, Shah NN, Savani BN, et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50:1013–1023. doi: 10.1038/bmt.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutler DI, Patel KR, Auerbach AD, et al. Natural history and management of Fanconi anemia patients with head and neck cancer: a 10 year follow-up. LID. Laryngoscope. 2015;126:870–879. doi: 10.1002/lary.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with Fanconi Anemia. J Clin Endocrinol Metab. 2007;92:2624–2631. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- 18.Meier ER, Dioguardi JV, Kamani N. Current attitudes of parents and patients toward hematopoietic stem cell transplantation for sickle cell anemia. Pediatr Blood Cancer. 2015;62:1277–1284. doi: 10.1002/pbc.25446. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum L. The paternalism preference–choosing unshared decision making. N Engl J Med. 2015;373:589–592. doi: 10.1056/NEJMp1508418. [DOI] [PubMed] [Google Scholar]
- 20.Epstein JM. Why model? J Artif Soc Soc Simulat. 2008;11 [Google Scholar]
- 21.Vlachos A, Muir E. How I treat Diamond-Blackfan anemia. Blood. 2010;116:3715–3723. doi: 10.1182/blood-2010-02-251090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlachos A, Rosenberg PS, Atsidaftos E, Alter BP, Lipton JM. Incidence of neoplasia in Diamond Blackfan anemia: a report from the Diamond Blackfan Anemia Registry. Blood. 2012;119:3815–3819. doi: 10.1182/blood-2011-08-375972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg PS, Alter BP, Bolyard AA, et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 2006;107:4628–4635. doi: 10.1182/blood-2005-11-4370. [DOI] [PMC free article] [PubMed] [Google Scholar]