Abstract
Aims
Assessment of cardiac anatomy and function by cardiovascular magnetic resonance (CMR) is accurate and reproducible and is commonly performed to clarify borderline results obtained by other techniques. Normal reference values are lacking in a large sample of young healthy adults. As CMR is increasingly solicited to discriminate normality from equivocal disease in this population, we sought to determine reliable reference values.
Methods and results
A sample of 434 Caucasian adults aged 26 ± 4 years (45% male) without cardiovascular disease or risk factors (including obesity and smoking) underwent CMR. Blood pressure, electrocardiogram, and plasma markers (lipid profile, fasting glucose, troponin, and Nt-pro-BNP) were within normal limits and typical of a low-cardiometabolic-risk profile. End-diastolic (ED), end-systolic (ES), and stroke volumes were greater in men for left and right atria and ventricles. Left ventricular (LV) mass was higher in men. ED wall thickness of all segments was greater in men, whereas ES wall thickening (segmental function) was similar in both genders. After normalization to body surface area, all gender differences remained. Left and right ventricular volumes were lower, and left atrial volumes were higher in older individuals. In contrast, LV mass was not associated with age.
Conclusion
This is the first large database of reference ranges for ventricular and atrial functions, volumes, and mass in young Caucasian men and women devoid of cardiovascular disease and risk factors. These data will contribute to improving the accuracy of CMR interpretation for clinical and research applications.
Keywords: normal values, mass, volume, systolic function, wall thickness/thickening
Introduction
Measurement of cardiac anatomy and assessment of heart function are among the most important clinical tasks in cardiology in order to distinguish between health and disease, and to determine accurate risk stratification and therapy.
Cardiovascular magnetic resonance (CMR) is precise and highly reproducible and has now become the gold standard for cardiac morphology and heart function. Several groups have previously presented CMR reference values for healthy people using fast gradient echo or steady-state free precession (SSFP) sequences,1–15 which were gathered in a recent review.16 However, sample sizes are often limited, and data focused on young adults are scarce. Precise morphologic measures in this range of ages are essential, especially in the context of most acquired cardiac diseases, but also in milder forms of congenital heart disease that might not have been diagnosed during infancy (such as atrial septal defects or compaction disorders for instance), where only subtle changes in anatomy and function may occur in early stages. In most studies, ‘healthy’ status was defined loosely on clinical examination and electrocardiogram (ECG) and did not exclude smoking or obesity.
The aims of the current study are to (1) establish comprehensive, accurate, gender-specific CMR reference values for young healthy Caucasian men and women in whom cardiovascular disease and risk factors were specifically excluded and (2) evaluate if age, gender, and body surface area (BSA) have influence on these parameters during early years of adulthood.
Material and methods
Study population
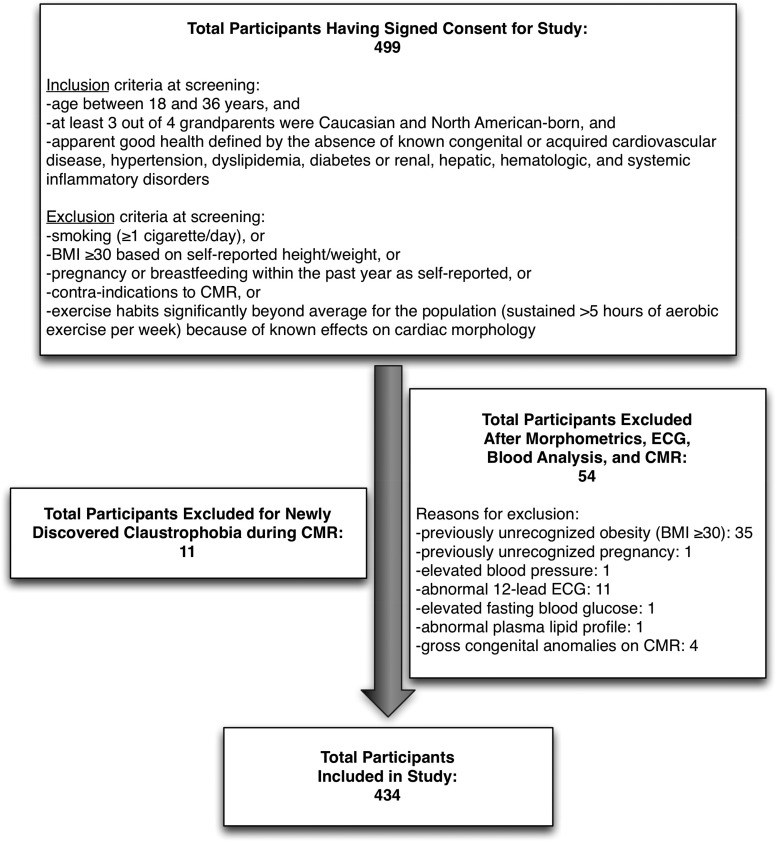
We prospectively recruited 434 Caucasian adults (196 men and 238 women) at the Quebec Heart and Lung Institute through phone, email, and word-of-mouth invitation (Figure 1). Eligibility criteria were age between 18 and 35 years (although CMR was performed in 3 at 36 years because of 1-month delay from consent to imaging), at least 3 out of 4 grandparents who were Caucasian and North American-born, and apparent good health defined by the absence of known congenital or acquired cardiovascular disease, hypertension, dyslipidemia, diabetes or renal, hepatic, haematologic, and systemic inflammatory disorders. Exclusion criteria were smoking (≥1 cigarette/day), BMI ≥30, pregnancy or breastfeeding within the past year, abnormal 12-lead ECG, elevated fasting glucose, abnormal plasma lipid profile (total, LDL-, or HDL-cholesterol, or triglycerides), elevated troponins or N-terminal pro-b-type natriuretic peptide (Nt-pro-BNP), and contra-indications to CMR. We also excluded those with exercise habits significantly beyond average for the population (sustained >5 h of aerobic exercise per week) because of known effects on cardiac morphology.
Figure 1.
Flow chart detailing consented participants with inclusion/exclusion criteria and reasons for exclusion.
CMR images were evaluated for gross congenital anomalies to exclude previously unknown congenital cardiac conditions. The institutional Ethics Committee approved the study, and all patients provided written informed consent.
CMR acquisition protocol
Imaging was performed with a 1.5-Tesla Philips Achieva scanner operating release 2.6 level 3 (Philips Healthcare, Best, The Netherlands). Cine imaging of cardiac morphology and function was performed by SSFP technique at 30 phases per cardiac cycle in held end expiration; 8–14 contiguous parallel short-axis (8 mm thickness, 0 mm gap) and 3 radial long-axis planes were performed covering the entire cardiac volume. Typical parameters included TR/TE 3.17/1.58 ms, flip angle 60°, and number of excitations of 1, yielding an in-plane spatial resolution of 1.6 × 2 mm and a mean temporal resolution of 33 ms.
CMR image analysis
Image analysis was performed offline in a core laboratory using a standardized approach by trained technicians [4 technicians with a total experience of 15 years (2–4 years each) dedicated reading in the laboratory] supervised by an experienced cardiologist (E.L.) following the American Heart Association (AHA) 17-segment model (cmr42 version 3.4.1, Circle Vascular Imaging, Canada).17 Cardiac volumes and function measurements were performed as previously described by our group and others, using the contiguous short-axis multi-slice acquisition with delineation of atria/ventricles confirmed in matched long-axis planes.4,18–20 Participants did not undergo gadolinium contrast injection.
Ventricles
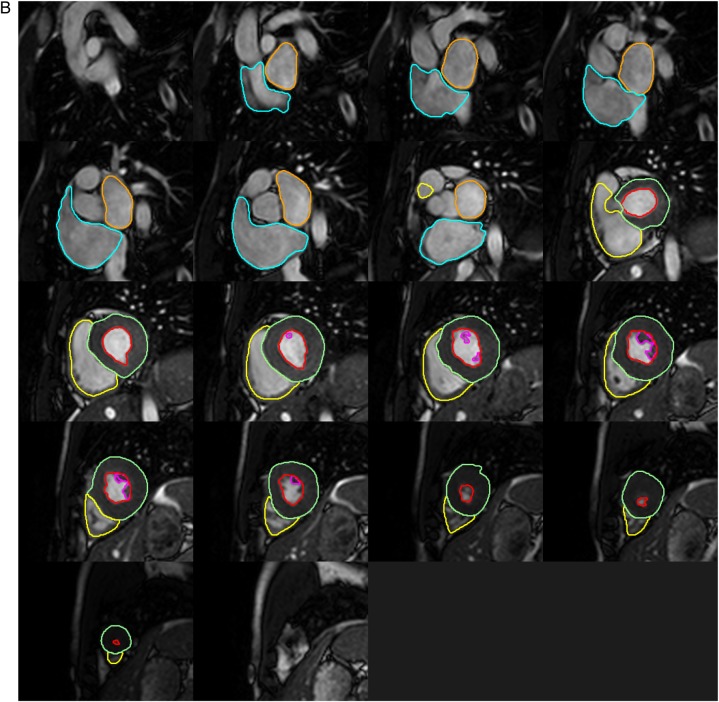
For ventricular volume analysis, the endocardial border was semi-automatically determined on the left ventricle (LV) for all 30 phases of the cardiac cycle and the cardiac phases that demonstrated the largest and smallest ventricular cavity volumes were defined as end-diastole (ED) and end-systole (ES), respectively (Figure 2). For the LV, the endocardial border was defined as the boundary between the myocardium and ventricular blood pool, from the most apical to the most basal slice. Manual correction of automated LV endocardial border and papillary muscles tracing was performed when necessary. Papillary muscles were included when measuring mass (equivalent to weighting the LV) and excluded when measuring volumes (equivalent to blood pool techniques).20,21 At the base of the heart, careful differentiation of ventricle from the atrium and aorta/pulmonary artery relied on examination of matching long-axis planes. If the basal slice contained both ventricular and atrial wall, the contours were drawn up to the junction of the atrium and the ventricle and the appropriate volume attributed to the ventricle. Similarly, if the aortic valve appeared in the basal slice, blood volume up to the aortic valve was included in the LV volume. For the right ventricle (RV), endocardial border was manually traced both in ED and ES from the most apical to the most basal slice. Trabeculations and moderator band of the RV were ignored, and a smooth endocardial border was drawn. The moderator band was included in blood pool.20 In basal slices, the RV outflow tract was accounted for in the RV volume, with a particular attention paid to include only the portion of volume below the level of the pulmonary valve.
Figure 2.
Detailed contouring of the four cardiac chambers in contiguous SSFP short-axis slices at end-diastole (A) and end-systole (B). Red: LV endocardial border, green: LV epicardial border, purple: LV papillary muscle border, yellow: RV endocardial border, orange: LA endocardial border, blue: RA endocardial border.
For LV mass measurement, the epicardial border was semi-automatically traced followed by manual correction to follow the middle of the chemical shift artefact line when necessary.20 Epicardial fat was excluded from the epicardial border.
The LV and RV ED volumes (LV-EDV, RV-EDV), ES volumes (LV-ESV, RV-ESV), stroke volumes (LV-SV, RV-SV), ejection fraction (LV-EF, RV-EF), and LV mass were computed using Simpson's rule. The LV-EDV, RV-EDV, LV-ESV, RV-ESV, and LV mass were normalized to BSA calculated by the Dubois formula.22 Segmental wall thickness was measured at ED by the centreline method (mean of 20–30 chords/segment), following the AHA 17-segment model definition. Segmental systolic wall thickening (segmental function) was defined for each individual segment as the difference between wall thickness at ED and thickness at ES (wall thickening) divided by wall thickness at ED to provide per cent thickening. As is customary, segment 17 (apex) was excluded from functional analysis. We uncovered high intra/inter-observers variability for RV mass in our preliminary analysis and elected not to report such unreliable measurements.
Atria
Atria images were obtained during the same acquisition as ventricles (full coverage of the cardiac silhouette in short axis); hence, slice thickness and gap were the same. In order to better discriminate between atria and ventricles, the long-axis planes were used to place a boundary at the level of the atrio-ventricular valve annulus. LA and RA endocardial borders were manually traced in atrial ED and ES phases on contiguous short-axis slices as detailed previously, ED referring to the atrial diastole (maximum volume). The atrial appendage was included in the total LA and RA volumes, but the pulmonary veins were excluded. Volumes were calculated from Simpson's rule.23
Statistical analysis
Continuous variables were tested for normality by the Shapiro–Wilk test and reported as means ± SDs. Categorical variables were expressed as a percentage and compared with the χ2 test. Gender differences were compared using Student's t-tests. Differences between basal, mid-ventricular, and apical LV thickness and thickening were assessed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. Intra- and inter-observer agreement (on 50 and 25 patients, respectively) was evaluated by absolute intraclass correlation coefficient (ICC) and by the Bland–Altman method. Association between morphological parameters indexed to BSA and age was assessed with linear regressions. All multivariable linear regression analyses included age, gender, and BSA. Statistical analyses were performed with Stata 13.0 (StataCorp LP, College Station, TX, USA). Statistical significance was set at P < 0.05.
Results
Characteristics of the population
The mean age of the 434 young adults (45% male) was 26.2 ± 4.5 years. The sample characteristics are presented in Table 1.
Table 1.
Population characteristics
| Total (n = 434) | Men (n = 196) | Women (n = 238) | P-value | |
|---|---|---|---|---|
| Age (years) (range 18–36) | 26.2 ± 4.5 | 26.7 ± 4.3 | 25.8 ± 4.6 | 0.03 |
| Height (cm) | 171 ± 9 | 178 ± 6 | 165 ± 6 | <0.001 |
| Weight (kg) | 67.0 ± 12.4 | 75.7 ± 10.4 | 59.8 ± 8.8 | <0.001 |
| BSA (m2) | 1.8 ± 0.2 | 1.9 ± 0.1 | 1.7 ± 0.1 | <0.001 |
| Body mass index (kg/m2) | 22.9 ± 2.9 | 23.9 ± 2.8 | 22.0 ± 2.7 | <0.001 |
| Heart rate (bmp) | 61 ± 10 | 58 ± 9 | 63 ± 10 | <0.001 |
| Systolic BP (mmHg) | 116 ± 10 | 122 ± 9 | 111 ± 9 | <0.001 |
| Diastolic BP (mmHg) | 72 ± 8 | 73 ± 7 | 70 ± 8 | <0.001 |
| Nt-pro-BNP (pmol/L) | 385 ± 227 | 370 ± 181 | 396 ± 256 | NS |
| Fasting glucose (mmol/L) | 4.6 ± 0.4 | 4.7 ± 0.4 | 4.4 ± 0.4 | <0.001 |
| Total cholesterol (mmol/L) | 4.4 ± 0.8 | 4.4 ± 0.8 | 4.5 ± 0.8 | NS |
| Triglycerides (mmol/L) | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.4 | NS |
| HDL-C (mmol/L) | 1.6 ± 0.4 | 1.3 ± 0.3 | 1.8 ± 0.4 | <0.001 |
| LDL-C (mmol/L) | 2.4 ± 0.7 | 2.6 ± 0.7 | 2.3 ± 0.7 | <0.001 |
| Total cholesterol/HDL-C ratio | 3.0 ± 0.8 | 3.4 ± 0.9 | 2.7 ± 0.6 | <0.001 |
Data are presented as means ± SD. P-value is for t-test between genders.
BP, blood pressure; Nt-pro-BNP, N-terminal pro-b-type natriuretic peptide; NS, non-significant.
Ventricle volumes, function, and mass
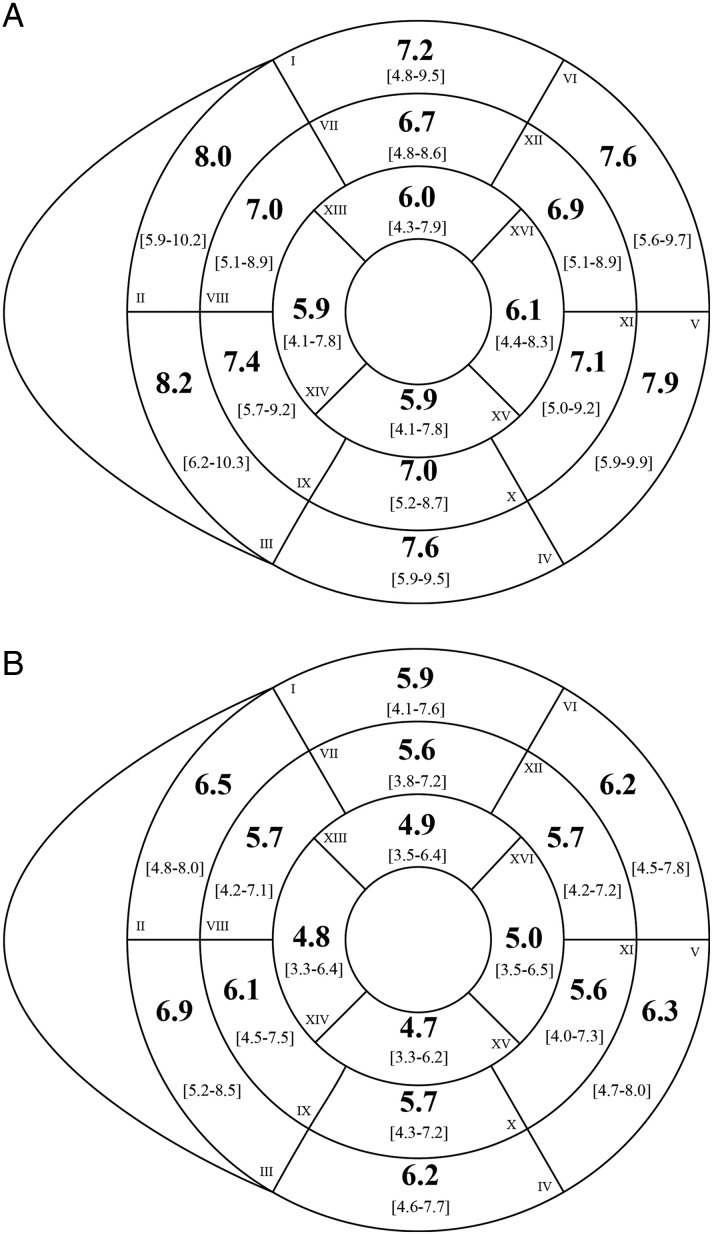
The values for LV volumes, function, and mass are presented in Table 2. LV-EDV, LV-ESV and LV-SV values were lower for women than men. LV-EF was similar in both sexes. LV mass was greater in men than in women. After normalizing to BSA, volumes and mass were still greater in men. LV wall thickness and thickening are presented in Figure 3 and Table 3. LV wall thickness was less in women than men (Figure 3 and Table 3). Mean values for systolic wall thickening (segmental function) were 56 ± 17% for basal segments, 72 ± 19% for mid-ventricular segments, and 78 ± 34% for apical segments, with no difference between genders. When progressing from the base to the apex, there was a gradual decrease in the thickness of myocardial segments, along with a gradual increase in systolic wall thickening (ANOVA P < 0.01, Table 3).
Table 2.
Global left ventricle parameters
| Men |
Women |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 5th | 95th | Mean ± SD | 5th | 95th | ||
| Absolute values | |||||||
| LV-EDV (mL) | 172 ± 30 | 128 | 226 | 128 ± 18 | 101 | 159 | <0.001 |
| LV-ESV (mL) | 61 ± 15 | 39 | 88 | 43 ± 9 | 29 | 60 | <0.001 |
| LV-SV (mL) | 112 ± 19 | 80 | 147 | 84 ± 13 | 63 | 105 | <0.001 |
| LV-EF (%) | 65 ± 5 | 56 | 74 | 65 ± 5 | 58 | 74 | NS |
| LV mass (g) | 125 ± 25 | 85 | 175 | 82 ± 15 | 59 | 108 | <0.001 |
| LV mass/EDV (g/mL) | 0.73 ± 0.12 | 0.56 | 0.92 | 0.64 ± 0.10 | 0.49 | 0.80 | <0.001 |
| Normalized to BSA | |||||||
| LV-EDV/BSA (mL/m2) | 89 ± 13 | 70 | 112 | 77 ± 8 | 64 | 92 | <0.001 |
| LV-ESV/BSA (mL/m2) | 31 ± 7 | 20 | 44 | 26 ± 5 | 18 | 34 | <0.001 |
| LV-SV/BSA (mL/m2) | 58 ± 8 | 43 | 73 | 51 ± 6 | 39 | 63 | <0.001 |
| LV mass/BSA (g/m2) | 64 ± 11 | 47 | 84 | 49 ± 8 | 36 | 64 | <0.001 |
Data are presented as means ± SD (5th and 95th percentiles). P-value is for t-test between genders.
NS, non-significant.
Figure 3.
Segmental left ventricular wall thickness at end-diastole for men (A) and women (B). Thickness is reported per standardized AHA segment in mm (bold) with 5th and 95th percentile limits (beneath in brackets). Basal slice: I, anterior; II, anteroseptal; III, inferoseptal; IV, inferior; V, inferolateral; VI, anterolateral. Mid-cavity slice: VII, anterior; VIII, anteroseptal; IX, inferoseptal; X, inferior; XI, inferolateral; XII, anterolateral. Apical slice: XIII, anterior; XIV, septal; XV, inferior; XVI, lateral.
Table 3.
Segmental left ventricle end-diastolic wall thickness and end-systolic wall thickening (function)
| Men |
Women |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 5th | 95th | Mean ± SD | 5th | 95th | ||
| End-diastolic thickness: absolute values | |||||||
| Basal (mm) | 7.7 ± 1.2 | 6.0 | 9.6 | 6.3 ± 0.9 | 4.8 | 7.8 | <0.001 |
| Mid (mm) | 7.0 ± 1.1* | 5.4 | 8.8 | 5.7 ± 0.8* | 4.4 | 7.2 | <0.001 |
| Apical (mm) | 5.9 ± 1.1*§ | 4.3 | 7.9 | 4.9 ± 0.8*§ | 3.5 | 6.3 | <0.001 |
| End-diastolic thickness: normalized to BSA | |||||||
| Basal (mm/m2) | 4.0 ± 0.6 | 3.1 | 5.0 | 3.9 ± 0.6 | 2.9 | 4.9 | <0.01 |
| Mid (mm/m2) | 3.6 ± 0.6* | 2.8 | 4.6 | 3.5 ± 0.6* | 2.6 | 4.4 | <0.01 |
| Apical (mm/m2) | 3.1 ± 0.5*§ | 2.3 | 4.1 | 3.0 ± 0.5*§ | 2.1 | 3.9 | <0.01 |
| End-systolic thickening | |||||||
| Basal (%) | 56 ± 18 | 33 | 90 | 57 ± 17 | 30 | 86 | NS |
| Mid (%) | 71 ± 18* | 47 | 105 | 73 ± 20* | 45 | 113 | NS |
| Apical (%) | 80 ± 32*§ | 35 | 131 | 76 ± 34* | 30 | 136 | NS |
Data are presented as means ± SD (5th and 95th percentiles). P-value is for t-test between genders.
NS, non-significant.
*P < 0.05 vs. basal segments.
§P < 0.05 vs. mid segments.
The values for RV volumes and function are reported in Table 4. Similarly to LV parameters, the values for RV-EDV, RV-ESV and RV-SV were lower for women than men. RV-EF was identical between genders. Volumes remained greater in men after normalizing to BSA.
Table 4.
RV parameters
| Men |
Women |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 5th | 95th | Mean ± SD | 5th | 95th | ||
| Absolute values | |||||||
| RV-EDV (mL) | 196 ± 36 | 143 | 264 | 141 ± 24 | 106 | 185 | <0.001 |
| RV-ESV (mL) | 75 ± 19 | 47 | 108 | 54 ± 13 | 36 | 76 | <0.001 |
| RV-SV (mL) | 121 ± 26 | 80 | 169 | 87 ± 18 | 58 | 119 | <0.001 |
| RV-EF (%) | 62 ± 7 | 49 | 72 | 62 ± 7 | 50 | 71 | NS |
| RV-EDV/LV-EDV | 1.14 ± 0.12 | 0.96 | 1.35 | 1.10 ± 0.10 | 0.88 | 1.29 | <0.001 |
| Normalized to BSA | |||||||
| RV-EDV/BSA (mL/m2) | 101 ± 16 | 78 | 133 | 85 ± 12 | 68 | 104 | <0.001 |
| RV-ESV/BSA (mL/m2) | 39 ± 9 | 26 | 56 | 32 ± 7 | 23 | 45 | <0.001 |
| RV-SV/BSA (mL/m2) | 62 ± 12 | 43 | 80 | 53 ± 9 | 38 | 67 | <0.001 |
Data are presented as means ± SD (5th and 95th percentiles). P-value is for t-test between genders.
NS, non-significant.
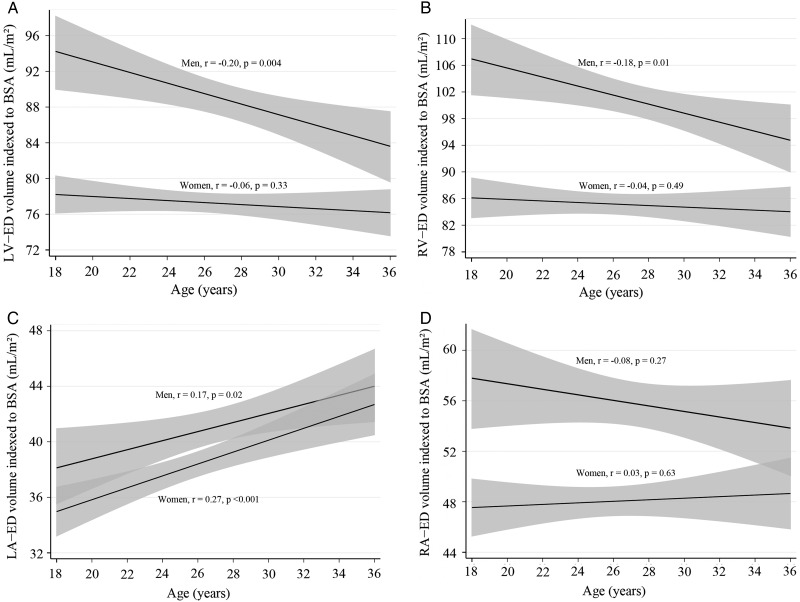
In multivariable analysis, gender and BSA were independently associated with LV and RV parameters (P < 0.001), except for LV-EF and RV-EF. Age was found to have an independent influence on most ventricular measurements (all P < 0.002), except for LV- and RV-SV, and LV mass. The associations with age remained significant even after adjustment for systolic, diastolic, or mean arterial pressure levels. In further analysis taking into account gender, age was no longer independently associated with LV- and RV-EDV, RV-ESV, and RV-EF in women. Evolution of LV- and RV-EDV (normalized to BSA) with age is illustrated for men and women in Figure 4.
Figure 4.
Evolution of end-diastolic chamber volumes with age for young adult men and women. Linear regressions with 95% confidence intervals of volumes normalized to BSA for LV-EDV (A), RV-EDV (B), LA-EDV (C), and RA-EDV (D).
Atrial volumes and function
The values for LA and RA volumes and function are presented in Tables 5 and 6, respectively. Men had greater LA- and RA-EDV, RA-ESV, and RA-SV than women. However, the values for LA- and RA-EF were lower in men compared with women. After BSA normalization, LA- and RA-EDV, and RA-ESV remained significantly greater in men than women.
Table 5.
Left atrium parameters
| Men |
Women |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 5th | 95th | Mean ± SD | 5th | 95th | ||
| Absolute values | |||||||
| LA-EDV (mL) | 79 ± 19 | 53 | 114 | 64 ± 14 | 42 | 88 | <0.001 |
| LA-ESV (mL) | 32 ± 9 | 19 | 49 | 24 ± 7 | 15 | 37 | <0.001 |
| LA-SV (mL) | 47 ± 13 | 27 | 71 | 39 ± 10 | 24 | 56 | <0.001 |
| LA-EF (%) | 59 ± 8 | 45 | 70 | 61 ± 7 | 49 | 72 | <0.001 |
| Normalized to BSA | |||||||
| LA-EDV/BSA (mL/m2) | 41 ± 8 | 28 | 56 | 38 ± 7 | 27 | 51 | <0.001 |
| LA-ESV/BSA (mL/m2) | 17 ± 4 | 10 | 24 | 15 ± 4 | 9 | 22 | <0.001 |
| LA-SV/BSA (mL/m2) | 24 ± 6 | 15 | 34 | 24 ± 5 | 15 | 32 | NS |
Data are presented as means ± SD (5th and 95th percentiles). P-value is for t-test between genders.
NS, non-significant.
Table 6.
Right atrium parameters
| Men |
Women |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 5th | 95th | Mean ± SD | 5th | 95th | ||
| Absolute values | |||||||
| RA-EDV (mL) | 108 ± 26 | 71 | 154 | 80 ± 17 | 52 | 111 | <0.001 |
| RA-ESV (mL) | 50 ± 17 | 25 | 80 | 33 ± 11 | 18 | 53 | <0.001 |
| RA-SV (mL) | 58 ± 16 | 32 | 87 | 47 ± 12 | 28 | 68 | <0.001 |
| RA-EF (%) | 54 ± 10 | 37 | 69 | 59 ± 9 | 42 | 75 | <0.001 |
| Normalized to BSA | |||||||
| RA-EDV/BSA (mL/m2) | 56 ± 12 | 37 | 78 | 48 ± 9 | 34 | 63 | <0.001 |
| RA-ESV/BSA (mL/m2) | 26 ± 8 | 14 | 39 | 20 ± 6 | 12 | 31 | <0.001 |
| RA-SV/BSA (mL/m2) | 30 ± 8 | 17 | 42 | 28 ± 7 | 17 | 39 | <0.01 |
Data are presented as means ± SD (5th and 95th percentiles). P-value is for t-test between genders.
In multivariable analysis, BSA was independently associated with all atrial parameters (all P < 0.01), except for LA-EF. Gender was independently associated with LA-EF (P < 0.03) as well as RA-EDV and RA-ESV (P < 0.01) but not with LA volumes. Age was found to have an independent influence on LA parameters (P < 0.01), except for LA-EF. The associations with age remained significant even after adjustment for systolic, diastolic, or mean arterial pressure levels. Evolution of LA-EDV and RA-EDV (normalized to BSA) with age is illustrated for men and women in Figure 4. Age was not independently associated with any RA parameter.
Intra- and inter-observer variabilities
Intra-observer agreement by ICC was 0.98 for LV-EDV (mean difference 2.5 mL, limits of agreement −0.7 to 5.6 mL), 0.96 for RV-EDV (2.3 mL, −3.4 to 8.1 mL), 0.95 for LA-EDV (0.7 mL, −2.1 to 4.3 mL), and 0.94 for RA-EDV (0.8 mL, −1.7 to 4.8 mL), all P < 0.001. Inter-observer agreement was 0.93 for LV-EDV (4.2 mL, −8.5 to 16.8 mL), 0.92 for RV-EDV (6.5 mL, −14.8 to 24.6 mL), 0.89 for LA-EDV (4.3 mL, −5.1 to 14.1 mL), and 0.90 for RA-EDV (3.8 mL, −3.2 to 10.8 mL), all P < 0.01.
Discussion
Several groups have worked to establish reference values using the SSFP technique.4,5,9,11,13–15 We observed some differences between our findings and those from Maceira et al., which are currently widely employed as a clinical standard.9,11 In the current study, we delineated the endocardium by semi-automated circular contouring with inclusion of trabeculations in ventricular volumes. This method is reported to be more reproducible than manual delineation of trabeculations but results in reduced LV mass and increased LV volumes.24 This may explain why we observe lower LV mass in both genders and higher volumes in men of our sample in comparison with Maceira et al. who used a semi-automated method that included portions of trabeculations. Nevertheless, we identify lower LV volumes in women, even after normalization to BSA. We also measure greater RV volumes in both genders, no doubt due to the inclusion of trabeculations and the moderator band in the blood pool in order to reduce variability. The differences between our findings and those of Maceira et al. are particularly pronounced when comparing limit values, which are more useful in clinical practice than are mean values.
One of the main advantages of our study is that the large size of our cohort allowed us to describe limit values using the 5th and 95th percentiles. Due to the smaller size of prior cohorts, previous studies described limit values on the basis of descriptions or graphic representations of 95% confidence interval to the mean. However, the latter methods may be misleading, since they actually reflect the reliability of the mean's estimation rather than the true range of values, and are strongly dependent on sample size.
We observed greater variability in atrial measurements compared with ventricles. This is undoubtedly related to the difficulty in identifying a definite border between the connections of pulmonary arteries and vena cava to the atria, adding two extra difficulties to delineating the RA and four extra difficulties to delineating the LA.
Influence of body size and ethnicity
In addition to the impact of a greater sample size, discrepancies compared with previous studies may be explained by the exclusive presence of Caucasian individuals having at least three out of four North American-born grandparents. Indeed, most previous studies have not taken into account ethnic origins, while ethnicity influences BSA,7 itself a strong independent predictor of ventricular volumes and mass.9,11 Furthermore, genetic factors may induce significant variability in cardiac morphology independent of BSA.25,26 Thus, ethnicity should be taken into account when reporting and developing normal reference values.
Influence of sex status
Although previous studies have described higher ventricular mass and volumes in men compared with women, these differences did not always persist after normalization to BSA.4,5,9,11 Similar discrepancies have been reported for atria as well.5,8 As described in recent large scale CMR studies,15,27 our data are consistent with the presence of sex-related differences, even after adjustment for age and BSA (with the exception of the LA). As previously reported by Dawson et al.,28 we also observe differences in ED wall thickness in men vs. women, even after normalization to BSA. Thus, the greater LV mass observed in men is not exclusively explained by larger ventricle volume. However, gender dimorphism disappears when considering systolic wall thickening, where segmental function remains comparable in women vs. men, contrary to what was previously reported by Ubachs et al.29
Influence of age
Our results indicate that age is independently associated with all LV and RV volumes, with the exception of SV. These findings are consistent with the majority of previous studies,2,3,6,9,11,13 even if the age range of our population was narrow. Although age is no longer independently associated with LV-EDV, RV-EDV, and RV-EF when examining women only, we cannot discount the possibility that such sex-specific differences in the effect of age may result from either a lack of statistical power in this narrow range of ages or by the overriding influence of BSA. In contrast to volume measurements, LV mass is not associated with age in our sample. Of note, results from previous studies were not always consistent, as some have reported that age had either no1,9,14,15,30 or minimal influence31,32 on LV mass, whereas others found a relationship variably limited to either women33 or men.5
We also observed that LA volume was associated with age, even after adjustment for gender and BSA. These results are consistent with previous observations,34 including in children and adolescents,35 but to our knowledge, this is the first time that this association is described in young adult women and men.
Strengths and limitations
Normality was defined by the absence of cardiovascular disease, and for the first time major risk factors including smoking and obesity. However, normality has no absolute definition, and despite our best efforts, there is no fail-safe method to ensure exclusion of all volunteers with mild subclinical disease. A second relative limitation is that it is not possible to extend our results to populations other than Caucasians, and similar studies should be performed for each specific ethnic group. By the same token, this may constitute one of the greatest strengths of our work since variability related to ethnicity was strongly reduced. Our results are derived from a young adult cohort and cannot be recommended for older individuals. Finally, the subjects were invited to participate through phone, email, and word of mouth, which is expected to bias towards a healthy sample.
Conclusion
This study, the largest to date, provides sex-specific normal reference values for both left and right ventricular and atrial volumes, LV mass, and functions in healthy young Caucasian adults by SSFP CMR. As CMR is increasingly solicited to discriminate normality from equivocal disease in young otherwise healthy adults, such reliable reference values should prove valuable to better define the limits of normality for research and clinical practice alike.
Conflict of interest: None declared.
Funding
This study was funded in part by the Heart and Stroke Foundation of Canada (Québec), the Fonds de recherche du Québec—Santé (FRQ-S), and the Canadian Institutes for Health Research (CIHR). F.L.V. is supported by a clinical and research fellowship from the Fédération Française de Cardiologie. E.L. is a research scholar of the FRQ-S and Laval University Chair of Research & Innovation in Cardiovascular Imaging.
References
- 1.Sandstede J, Lipke C, Beer M, Hofmann S, Pabst T, Kenn W et al. . Age- and gender-specific differences in left and right ventricular cardiac function and mass determined by cine magnetic resonance imaging. Eur Radiol 2000;10:438–42. [DOI] [PubMed] [Google Scholar]
- 2.Marcus JT, DeWaal LK, Gotte MJ, van der Geest RJ, Heethaar RM, Van Rossum AC. MRI-derived left ventricular function parameters and mass in healthy young adults: relation with gender and body size. Int J Card Imaging 1999;15:411–9. [DOI] [PubMed] [Google Scholar]
- 3.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson 1999;1:7–21. [DOI] [PubMed] [Google Scholar]
- 4.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 2003;17:323–9. [DOI] [PubMed] [Google Scholar]
- 5.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 2005;7:775–82. [DOI] [PubMed] [Google Scholar]
- 6.Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV et al. . Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol 2002;39:1055–60. [DOI] [PubMed] [Google Scholar]
- 7.Tandri H, Daya SK, Nasir K, Bomma C, Lima JA, Calkins H et al. . Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol 2006;98:1660–4. [DOI] [PubMed] [Google Scholar]
- 8.Sievers B, Addo M, Breuckmann F, Barkhausen J, Erbel R. Reference right atrial function determined by steady-state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2007;9:807–14. [DOI] [PubMed] [Google Scholar]
- 9.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417–26. [DOI] [PubMed] [Google Scholar]
- 10.Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J 2006;27:2879–88. [DOI] [PubMed] [Google Scholar]
- 12.Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference right atrial dimensions and volume estimation by steady state free precession cardiovascular magnetic resonance. J Cardiovasc 2013;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clay S, Alfakih K, Radjenovic A, Jones T, Ridgway JP, Sinvananthan MU. Normal range of human left ventricular volumes and mass using steady state free precession MRI in the radial long axis orientation. MAGMA 2006;19:41–5. [DOI] [PubMed] [Google Scholar]
- 14.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M et al. . Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186(6 Suppl. 2):S357–65. [DOI] [PubMed] [Google Scholar]
- 15.Chuang ML, Gona P, Hautvast GL, Salton CJ, Breeuwer M, O'Donnell CJ et al. . CMR reference values for left ventricular volumes, mass, and ejection fraction using computer-aided analysis: the Framingham Heart study. J Magn Reson Imaging 2014;39:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R et al. . Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson 2015;17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al. . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 18.Larose E, Rodes-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM et al. . Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol 2010;55:2459–69. [DOI] [PubMed] [Google Scholar]
- 19.Larose E, Ganz P, Reynolds HG, Dorbala S, Di Carli MF, Brown KA et al. . Right ventricular dysfunction assessed by cardiovascular magnetic resonance imaging predicts poor prognosis late after myocardial infarction. J Am Coll Cardiol 2007;49:855–62. [DOI] [PubMed] [Google Scholar]
- 20.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG et al. . Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennell DJ. Ventricular volume and mass by CMR. J Cardiovasc Magn Reson 2002;4:507–13. [DOI] [PubMed] [Google Scholar]
- 22.Sawyer M, Ratain MJ. Body surface area as a determinant of pharmacokinetics and drug dosing. Invest New Drugs 2001;19:171–7. [DOI] [PubMed] [Google Scholar]
- 23.Hudsmith LE, Cheng AS, Tyler DJ, Shirodaria C, Lee J, Petersen SE et al. . Assessment of left atrial volumes at 1.5 Tesla and 3 Tesla using FLASH and SSFP cine imaging. J Cardiovasc Magn Reson 2007;9:673–9. [DOI] [PubMed] [Google Scholar]
- 24.Papavassiliu T, Kuhl HP, Schroder M, Suselbeck T, Bondarenko O, Bohm CK et al. . Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology 2005;236:57–64. [DOI] [PubMed] [Google Scholar]
- 25.Swan L, Birnie DH, Padmanabhan S, Inglis G, Connell JM, Hillis WS. The genetic determination of left ventricular mass in healthy adults. Eur Heart J 2003;24:577–82. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P, Middelberg RP, Andrew T, Johnson MR, Christley H, Brown MJ. Heritability of left ventricular mass in a large cohort of twins. J Hypertens 2006;24:321–4. [DOI] [PubMed] [Google Scholar]
- 27.Yeon SB, Salton CJ, Gona P, Chuang ML, Blease SJ, Han Y et al. . Impact of age, sex, and indexation method on MR left ventricular reference values in the framingham heart study offspring cohort. J Magn Reson Imaging 2015;41:1038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson DK, Maceira AM, Raj VJ, Graham C, Pennell DJ, Kilner PJ. Regional thicknesses and thickening of compacted and trabeculated myocardial layers of the normal left ventricle studied by cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2011;4:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ubachs J, Heibeerg E, Steding K, Arheden H. Normal values for wall thickening by magnetic resonance imaging. J Magn Reson Imaging 2009;11(Suppl. 1):132–3. [Google Scholar]
- 30.Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Fujimoto N, Palmer MD et al. . Effect of healthy aging on left ventricular relaxation and diastolic suction. Am J Physiol Heart Circ Physiol 2012;303:H315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS et al. . Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health study. Circulation 1995;91:1739–48. [DOI] [PubMed] [Google Scholar]
- 32.Byrd BF III, Wahr D, Wang YS, Bouchard A, Schiller NB. Left ventricular mass and volume/mass ratio determined by two-dimensional echocardiography in normal adults. J Am Coll Cardiol 1985;6:1021–5. [DOI] [PubMed] [Google Scholar]
- 33.Shub C, Klein AL, Zachariah PK, Bailey KR, Tajik AJ. Determination of left ventricular mass by echocardiography in a normal population: effect of age and sex in addition to body size. Mayo Clin Proc 1994;69:205–11. [DOI] [PubMed] [Google Scholar]
- 34.Nikitin NP, Witte KK, Thackray SD, Goodge LJ, Clark AL, Cleland JG. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr 2003;4:36–42. [DOI] [PubMed] [Google Scholar]
- 35.Sarikouch S, Koerperich H, Boethig D, Peters B, Lotz J, Gutberlet M et al. . Reference values for atrial size and function in children and young adults by cardiac MR: a study of the German competence network congenital heart defects. J Magn Reson Imaging 2011;33:1028–39. [DOI] [PubMed] [Google Scholar]