Abstract
Aims
Stent-frame morphology of the newer-generation, balloon-expandable transcatheter heart valve (THV), the SAPIEN 3 (S3), after transcatheter aortic valve implantation (TAVI) is unknown. We evaluated the THV stent-frame morphology post TAVI of the S3 using multi-slice computed tomography (MSCT) compared with the prior-generation THV, SAPIEN XT (S-XT).
Methods and results
A total of 94 consecutive participants of RESOLVE registry (NCT02318342) had MSCT after balloon-expandable TAVI (S3 = 39 and S-XT = 55). The morphology of the THV stent-frame was evaluated for expansion area and eccentricity at the THV-inflow, native annulus, mid-THV and THV-outflow levels. Mean %-expansion area for the S3 and the S-XT was 100.9 ± 5.7 and 96.1 ± 5.5%, respectively (P < 0.001). In the S3 group, the THV-inflow level had the largest value of %-expansion area, which decreased from THV-inflow to mid-THV level (105.2 ± 6.4 to 96.5 ± 5.9%, P < 0.001). However, in the S-XT group, %-expansion area increased from THV-inflow level to mid-THV level (93.2 ± 6.2 to 95.1 ± 6.1%, P = 0.0058). On nominal delivery balloon volume, the S3 in 88.5% of cases had overexpansion at the THV-inflow level. The observed degree of THV oversizing of the S3 was significantly lower than the S-XT (6.3 ± 8.6 vs. 11.8 ± 8.5%, P = 0.0027). Nonetheless, the incidence of post-procedural paravalvular aortic regurgitation (PVR) ≥ mild following the S3 TAVI was also significantly lower than the S-XT TAVI (17.9 vs. 43.6%, P = 0.014).
Conclusion
The newer-generation, balloon-expandable device, the S3, has a flared inflow morphology, whereas the prior-generation device, the S-XT, has relatively constrained inflow morphology post TAVI. This may contribute to a lesser degree of PVR with the S3.
Keywords: TAVI, Morphology, MSCT, Transcatheter heart valve expansion, SAPIEN 3, SAPIEN XT
Introduction
With the increasing experience of operators and the improvement in transcatheter heart valve (THV) platforms, transcatheter aortic valve implantation (TAVI) has become an accepted alternative treatment option for high-risk patients and the standard of care for inoperable patients with symptomatic severe aortic stenosis.1,2
The SAPIEN 3 (S3; Edwards Lifesciences, Inc., Irvine, CA, USA) is the newer-generation, balloon-expandable THV that has some differences in stent-frame design compared with the prior-generation device, SAPIEN XT (S-XT; Edwards Lifesciences, Inc.). The S3 consists of a cobalt–chromium frame with large cell design and no commissural posts. On the other hand, the S-XT consists of a cobalt–chromium frame with smaller cell design and has three commissural posts. The different stent-frame geometry in the S3, with larger cells and wide strut angles, contributes to its high radial strength. Furthermore, the S3 has an additional outer polyethylene terephthalate cuff to enhance paravalvular leak sealing.3 Some retrospective analyses have suggested that the S3 design iteration facilitates lower rates of paravalvular aortic regurgitation (PVR) than the S-XT4,5 and could allow more challenging anatomies to be treated.6
Despite these device improvements, optimal annulus sizing, appropriate selection of device size, and understanding the morphology of the deployed THV remain critically important to reduce the incidence of complications, not only PVR but also aortic root injury.5,7,8 Better characterization of the native aortic valve morphology may be of great value to select the THV type and size.9 However, comparative data on the morphology of THV stent-frame expansion after TAVI between the newer-generation S3 and prior-generation the S-XT are currently absent. Therefore, in order to better understand how device selection and sizing may differ with the newer iteration, the objective of our study was to compare the morphology of the deployed S3 stent frame with that of the S-XT using multi-slice computed tomography (MSCT).
Methods
Study population and TAVI procedure
We analysed a total of 94 consecutive participants of the RESOLVE registry (the assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and Its Treatment with Anticoagulation: NCT02318342). Per study protocol, these patients had post-procedural MSCT after balloon-expandable TAVI between December 2014 and October 2015 at our institute (Cedars-Sinai Medical Center, Los Angeles, CA, USA). For the registry, approval by the institutional review board was obtained before study initiation. This study complies with the Declaration of Helsinki, and all patients provided written informed consent. The ethics committee of our institution approved the study protocol. TAVI was performed under general anaesthesia with fluoroscopic and TEE guidance. In our study, the S3 was implanted in 39 patients and the S-XT in 55 patients. THVs sized 23, 26 and 29 mm were used in both the S3 and S-XT groups. The S-XT was used for TAVI until it was superseded by the commercial availability of the S3. Annular dimensions used for THV sizing were based on three-dimensional MSCT measurements.10–12 The decision to proceed with TAVI was with the consensus of a dedicated heart team including experienced clinical and interventional cardiologists and cardiovascular surgeons.
MSCT image acquisition
Contrast-enhanced computed tomography (CT) examinations were performed if the renal function was considered satisfactory, as is routine clinical practice; this was generally if the serum creatinine was ≤2.0 mg/dL, using a second-generation dual-source CT system (SIEMENS SOMATOM Definition Flash; SIEMENS Healthcare, Erlangen, Germany). A commercially available contrast medium (Omnipaque, GE Healthcare, Little Chalfont, Buckinghamshire, UK) was used with 100 mL in each patient; bolus triggering in the ascending aorta was employed. CT was performed with a collimation of 128 × 0.625 mm, and maximum tube current ranged was automated for each patient using Caredose (SIEMENS Healthcare) with a fixed tube potential of 100–120 kV. Heart rate (HR) reduction with β-blockade was not performed, nor was additional dose modulation (other than Caredose). Acquisition was in the craniocaudal direction from the aortic arch to the diaphragm. Images were reconstructed at 0.6 mm slices with 0.3 mm overlap with iterative reconstruction for evaluation at 10% intervals within the 0–90% RR range. CT DICOM data were transmitted to a dedicated core laboratory.
THV analysis of MSCT
In all deployed THVs, curved multiplanar reconstruction analyses were performed using 3mensio Valves software™ (version 7.2, Pie Medical Imaging B.V., Maastricht, The Netherlands). A line was generated through the centre point of the proximal ascending aorta, THV and LVOT. Geometry of the THV stent frame was evaluated for orthogonal major and minor diameters, expansion area at the four levels of THV: (i) THV inflow, (ii) native annulus, (iii) mid-THV, and (iv) THV outflow. The THV-inflow level was defined as a plane perpendicular to the curved multiplanar reconstruction line through the centre of the THV stent frame that touched the nadir of the THV struts. The native annulus level at THV was defined based on the distance from the sinotubular junction (STJ) to the native annulus as that noted on the pre-procedural MSCT measurement in the same cardiac phase (generally a systolic phase at 20% of the cardiac cycle). Deployed THV depth was measured as the distance from THV-inflow to this native annulus level (Figure 1).
Figure 1.
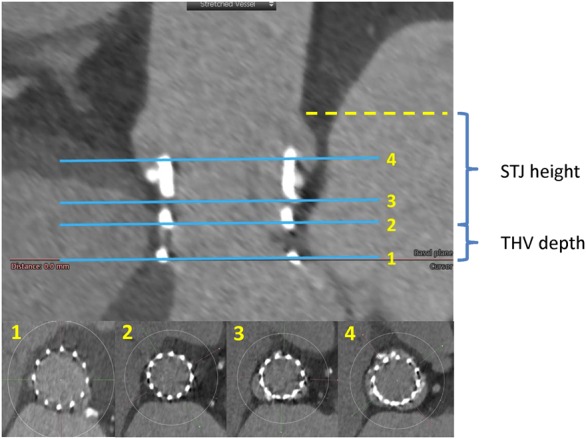
Multi-level measurements of the THV on MSCT. The THV expansion area was measured at four levels on MSCT: (1) THV inflow, (2) native annulus, (3) mid-THV, and (4) THV outflow. THV depth calculated as [distance from STJ to THV inflow]–[STJ height], i.e. distance from (1) to (2). STJ height was used pre-procedural MSCT measurement. THV, transcatheter heart valve; MSCT, multi-slice computed tomography .
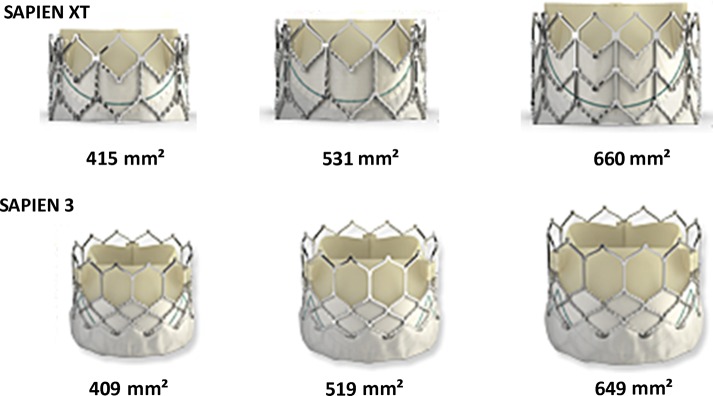
We measured external stent-frame dimensions at each level, including orthogonal major and minor diameters, area and perimeter. The THV expansion area was assessed by tracing the external margins of the stent frame. The nominal external valve areas of the S3 are 409 mm2 (23 mm), 519 mm2 (26 mm), and 649 mm2 (29 mm) according to the manufacturer; the corresponding expanded areas of the S-XT are 415 mm2 (23 mm), 531 mm2 (26 mm), and 660 mm2 (29 mm), respectively (Figure 2). The percentage of stent-frame expansion area (%-expansion area) was expressed in relation to labelled prosthesis size as: (observed THV external area/area derived from prosthesis labelled size) × 100. Overexpansion was defined as more than 100% expansion area, and underexpansion was defined as less than 100%.13 The THV stent frame was considered oversized when the THV expansion area was greater than the native annular area on pre-TAVI CT. Percentage of observed THV area oversizing was calculated as: (observed THV external area at the native annular level/native annular area − 1) × 100.14 Eccentricity index was defined as: (1 − short diameter/long diameter) × 100, with a THV considered circular when eccentricity index was <10%.15 Also, stent-frame fracture was evaluated on a volume-rendered (VR) view (Figure 3). These THV assessments were performed by two independent experienced observers in the core laboratory. Inter- and intra-observer variability was also assessed.
Figure 2.
Expected stent-frame areas for SAPIEN XT and SAPIEN 3. Appearance of SAPIEN 3 and SAPIEN XT THV, and expected stent-frame areas with nominal delivery balloon volume of the 23-, 26-, and 29-mm balloon-expandable THV are shown. THV, transcatheter heart valve.
Figure 3.
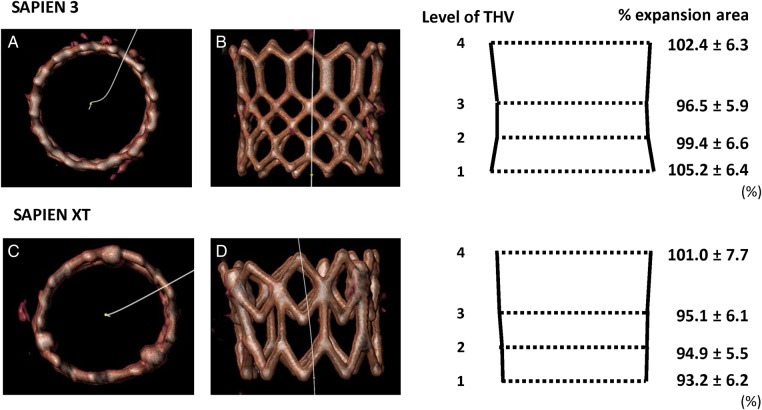
Evaluation of the THV stent frame after TAVI. VR view and schema are shown for the assessment of the stent-frame shape: (A and B) circular shape and flared THV inflow of the SAPIEN 3, (C and D) non-circular shape and constrained THV inflow of SAPIEN XT. THV, transcatheter heart valve; TAVI, transcatheter aortic valve implantation.
Echocardiographic assessment
All patients underwent transthoracic echocardiography (TTE) before TAVI and prior to discharge. The severity of pre-TAVI aortic stenosis was assessed by the mean transvalvular gradient, and aortic valve area (AVA) calculated with the continuity equation.16 The PVR severity post TAVI was evaluated using a multi-parametric approach on pre-discharge echocardiography and classified following the Valve Academic Research Consortium-2 recommendations as none-trace, mild, moderate, and severe.17
Statistical analysis
The population was divided into two groups according to the implanted THV type. Statistical analysis was done using SPSS Statistics 22.0 (SPSS, Inc., Chicago, IL) and JMP 11.0.2 software (SAS Institute, Inc., Cary, NC). Continuous variables are described by mean ± SD, and categorical variables are described by frequencies and percentages. Continuous parametric variables were compared using unpaired and paired Student's t-tests. Categorical variables were compared using the Fisher exact test. Inter- and intra-observer variability was evaluated by calculating the intraclass correlation coefficients (ICC) in 15 randomly selected cases with excellent agreement defined as an ICC of >0.8. Statistical significance was defined as P < 0.05.
Results
Baseline characteristics and pre-procedural measurements
Patient characteristics are summarized in Table 1. All patients were in NYHA functional class III or IV. Pre-procedural MSCT and echocardiographic characteristics are shown in Table 2. All MSCT scans used were contrast scans. There was no significant difference in these parameters between the S3 group and the S-XT group except the percentage of area oversizing by nominal THV area before TAVI (7.2 ± 9.0 vs. 18.1 ± 9.5%, P < 0.001).
Table 1.
Clinical characteristics
| Overall (n = 94) | SAPIEN 3 (n = 39) | SAPIEN XT (n = 55) | P-value | |
|---|---|---|---|---|
| Age, years | 83.1 ± 6.9 | 82.3 ± 7.3 | 83.6 ± 6.6 | 0.36 |
| Female | 32 (34.0) | 10 (25.6) | 22 (40.0) | 0.19 |
| Height, cm | 169.8 ± 10.0 | 171.8 ± 8.9 | 168.3 ± 10.6 | 0.11 |
| Weight, kg | 78.7 ± 16.7 | 81.4 ± 16.6 | 76.6 ± 16.6 | 0.19 |
| Body mass index, kg/m2 | 27.3 ± 5.0 | 27.7 ± 5.4 | 26.9 ± 4.8 | 0.50 |
| Diabetes | 24 (25.5) | 14 (35.9) | 10 (18.2) | 0.06 |
| Hypertension | 86 (91.5) | 37 (94.9) | 49 (89.1) | 0.46 |
| Chronic kidney disease | 14 (14.9) | 8 (20.5) | 6 (10.9) | 0.25 |
| peripheral artery disease | 20 (21.3) | 9 (23.1) | 11 (20.0) | 0.80 |
| Atrial fibrillation | 25 (26.6) | 10 (25.6) | 15 (27.3) | >0.99 |
| Coronary artery disease | 51 (54.3) | 23 (59.0) | 28 (50.9) | 0.53 |
| History of bypass surgery | 26 (27.7) | 14 (35.9) | 12 (21.8) | 0.16 |
| Previous permanent pacemaker | 13 (13.8) | 4 (10.3) | 9 (16.4) | 0.55 |
| STS score, % | 6.4 ± 2.5 | 5.2 ± 1.3 | 7.6 ± 2.7 | <0.001 |
| Euro SCORE II, % | 10.9 ± 6.8 | 9.6 ± 6.2 | 12.3 ± 7.2 | 0.13 |
Values are mean ± SD or n (%). Chronic kidney disease was defined as GFR < 60 (mL/min/1.73 m2).
NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; Euro SCORE II, European System for Cardiac Operative Risk Evaluation.
Table 2.
Pre-procedural imaging and procedural characteristics
| Overall (n = 94) | SAPIEN 3 (n = 39) | SAPIEN XT (n = 55) | P-value | |
|---|---|---|---|---|
| CT variables | ||||
| Annulus mean diameter, mm | 24.7 ± 2.0 | 25.1 ± 1.6 | 24.5 ± 2.2 | 0.13 |
| Annulus eccentricity, % | 18.1 ± 5.9 | 18.2 ± 6.4 | 18.1 ± 5.6 | 0.97 |
| Annulus area, mm2 | 478.3 ± 74.6 | 495.9 ± 59.9 | 466.2 ± 81.6 | 0.07 |
| Annulus perimeter, mm | 78.5 ± 6.1 | 80.0 ± 5.0 | 77.5 ± 6.6 | 0.05 |
| Aortic valve calcium volume, mm3 | 250.9 ± 216.5 | 252.6 ± 257.1 | 249.7 ± 185.0 | 0.92 |
| Oversizing (by nominal THV area), % | 13.6 ± 10.7 | 7.2 ± 9.0 | 18.1 ± 9.5 | <0.001 |
| Echocardiographic variables | ||||
| Pre EF, % | 58.9 ± 12.9 | 58.8 ± 11.3 | 59.1 ± 14.0 | 0.91 |
| Mean PG, mmHg | 46.4 ± 12.4 | 45.8 ± 10.9 | 46.8 ± 13.4 | 0.71 |
| Pre AVA, cm2 | 0.62 ± 0.14 | 0.60 ± 0.14 | 0.64 ± 0.14 | 0.21 |
| Procedure details | ||||
| THV size | ||||
| 23 mm | 20 (21.3) | 5 (12.8) | 15 (27.3) | 0.13 |
| 26 mm | 44 (46.8) | 22 (56.4) | 22 (40.0) | 0.14 |
| 29 mm | 30 (31.9) | 12 (30.8) | 18 (32.7) | >0.99 |
| Implantation access | ||||
| Transfemoral | 91 (96.8) | 39 (100) | 52 (94.5) | 0.26 |
| Other | 3 (3.2) | 0 (0.0) | 3 (5.5) | 0.26 |
| Pre-dilatation | 46 (48.9) | 24 (61.5) | 22 (40.0) | 0.06 |
| Post-dilatation | 2 (2.1) | 2 (5.1) | 0 (0.0) | 0.17 |
| New pacemaker implantsa | 11 (13.6) | 7 (20.0) | 4 (8.7) | 0.19 |
| Delivery balloon volume | ||||
| Nominal volume | 59 (62.8) | 26 (66.7) | 33 (60.0) | 0.53 |
| Over-filling volume | 17 (18.1) | 7 (17.9) | 10 (18.2) | >0.99 |
| Under-filling volume | 18 (19.1) | 6 (15.4) | 12 (21.8) | 0.60 |
Values are mean ± SD or n (%).
EF, ejection fraction; AVA, aortic valve area; PG, pressure gradient.
aNew pacemaker implants: excludes patients who had previous pacemaker/ICD (S3 = 4, S-XT = 9).
Procedural details
TAVI procedure details are also presented in Table 2. The majority of patients (96.8%) underwent transfemoral TAVI. There was no significant difference in any of these parameters between the S3 group and the S-XT group. On the balloon-expandable THV delivery system, we used nominal delivery balloon volume, supra-nominal volume (‘over-filling’, i.e. ≥1 mL extra volume) or sub-nominal volume (‘under-filling’, i.e. ≥1 mL less volume); these were 26 nominal (66.7%), 7 over-filling (17.9%), and 6 under-filling (15.4%) for the S3 and 33 nominal (60.0%), 10 over-filling (18.2%) and 12 under-filling (21.8%) for the S-XT. No case of aortic injury was seen in either type of THV implanted. Seven patients (20.0%) required permanent pacemaker implantation following the S3 TAVI and 4 patients (8.7%) following the S-XT (P = 0.19).
THV measurements
A total of 94 patients had MSCT at an average of 7.6 ± 6.7 months after TAVI. There were no cases of THV stent-frame fracture identified by MSCT. Deployed THV depth was not different between the S3 group and the S-XT group (4.1 ± 2.2 vs. 3.8 ± 1.9 mm, P = 0.46). Measurements of the deployed THV stent frame on MSCT are shown in Table 3. Inter- and intra-observer reproducibility at THV-inflow levels was satisfactory (inter-observer: ICC = 0.948, P < 0.001; intra-observer: ICC = 0.990, P < 0.001, respectively). There was a significant difference in the achieved THV stent-frame expansion between the S3 and the S-XT. Overall, mean %-expansion area of the S3 and the S-XT was 100.9 ± 5.7 vs. 96.1 ± 5.5%, P < 0.001. Particularly, at the THV-inflow and the native annular levels, the S3 had a greater %-expansion area in comparison with the S-XT (THV-inflow level: 105.2 ± 6.4 vs. 93.2 ± 6.2%, P < 0.001; native annular level: 99.4 ± 6.6 vs. 94.9 ± 5.5%, P < 0.001) Moreover, in the S3 group, the THV-inflow level had the largest value of %-expansion area, which decreased from inflow to mid-THV level (105.2 ± 6.4 to 96.5 ± 5.9%, P < 0.001). However, in the S-XT group, at the THV-inflow level, there was the smallest value of %-expansion area, which increased from inflow level to mid-THV level (93.2 ± 6.2 to 95.1 ± 6.1%, P < 0.01). Mean eccentricity index was not different between the S3 and the S-XT (overall levels: 3.62 ± 2.0 vs. 3.59 ± 1.8%, P = 0.95). Percentage of observed THV area oversizing in the S3 group was significantly lower than the S-XT group (6.3 ± 8.6 vs. 11.8 ± 8.5%, P = 0.0027).
Table 3.
Multi-level THV measurements on MSCT after TAVI
| Overall (n = 94) | SAPIEN 3 (n = 39) | SAPIEN XT (n = 55) | P-value | |
|---|---|---|---|---|
| Observed THV area oversizing, % (in relation to native annulus area) | 9.5 ± 8.9 | 6.3 ± 8.6 | 11.8 ± 8.5 | 0.0027 |
| Observed THV area expansion, % (in relation to prosthesis labelled area) | ||||
| Overall levels | 98.1 ± 6.0 | 100.9 ± 5.7 | 96.1 ± 5.5 | <0.001 |
| 1. THV inflow | 98.2 ± 8.6 | 105.2 ± 6.4 | 93.2 ± 6.2 | <0.001 |
| 2. Native annulus | 96.8 ± 6.4 | 99.4 ± 6.6 | 94.9 ± 5.5 | <0.001 |
| 3. Mid-THV | 95.7 ± 6.0 | 96.5 ± 5.9* | 95.1 ± 6.1** | 0.28 |
| 4. THV outflow | 101.6 ± 7.1 | 102.4 ± 6.3 | 101.0 ± 7.7 | 0.36 |
| Observed THV eccentricity index, % | ||||
| Overall levels | 3.60 ± 1.9 | 3.62 ± 2.0 | 3.59 ± 1.8 | 0.95 |
| 1. THV inflow | 4.25 ± 2.9 | 3.81 ± 2.8 | 4.56 ± 2.9 | 0.21 |
| 2. Native annulus | 3.72 ± 2.5 | 4.23 ± 2.7 | 3.35 ± 2.2 | 0.09 |
| 3. Mid-THV | 3.19 ± 2.3 | 3.46 ± 2.5 | 3.00 ± 2.1 | 0.33 |
| 4. THV outflow | 3.24 ± 2.7 | 2.95 ± 2.5 | 3.45 ± 2.8 | 0.37 |
| THV depth, mm | 3.9 ± 2.0 | 4.1 ± 2.2 | 3.8 ± 1.9 | 0.46 |
Values are mean±SD.
THV, transcatheter heart valve; TAVI, transcatheter aortic valve implantation; MSCT, multi-slice computed tomography.
*P < 0.001 vs. the SAPIEN 3 THV inflow; **P = 0.0058 vs. the SAPIEN XT THV inflow.
Percentage of THV expansion area and delivery balloon volume
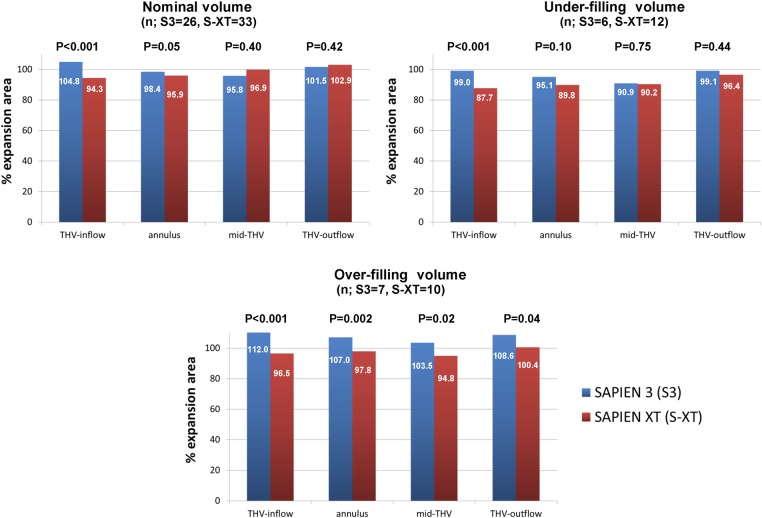
The %-expansion areas of THV in different delivery balloon volumes are shown in Table 4. On nominal delivery balloon volume, mean %-expansion area of the S3 and the S-XT was 100.1 ± 5.0 and 97.5 ± 4.1%, respectively (P = 0.029). In the S3 group, the THV-inflow level had greater expanded THV areas compared with at the mid-THV level (104.8 ± 5.7 vs. 95.8 ± 4.8%, P < 0.001). However, in the S-XT group, the THV-inflow level had lesser expanded THV areas compared with mid-THV level (94.3 ± 4.2 vs. 96.9 ± 5.0%, P = 0.0041). Particularly, at the THV-inflow and native annular levels, patients who had overexpansion area (by observed THV area) of the S3 and the S-XT were 23 (88.5%) vs. 3 (9.1%), P < 0.001 and 11 (42.3%) vs. 6 (18.2%), P = 0.05, respectively. However, at other levels (mid-THV and THV-outflow levels), the numbers of patients who had overexpansion area of the S3 and the S-XT were not significantly different (Figure 4). The S3 group was well expanded (by THV area) at the THV-inflow and native annular levels of measurement in the stent frame even using nominal or under-filling delivery balloon volume. On over-filling delivery balloon volume, the S3 had ∼8% greater expansion THV areas compared with its THV area using nominal volume (100.1 ± 5.0 vs. 107.8 ± 3.4, P < 0.001). Moreover, the S3 had a greater %-expansion area compared with the S-XT at each level. However, there were few cases with under-filling or over-filling delivery balloon volume (Table 4, Figure 5).
Table 4.
Per cent-expansion area and delivery balloon volume
| SAPIEN 3 (n = 39) |
SAPIEN XT (n = 55) |
|||||
|---|---|---|---|---|---|---|
| Under-filling (n = 6) | Nominal (n = 26) | Over-filling (n = 7) | Under-filling (n = 12) | Nominal (n = 33) | Over-filling (n = 10) | |
| Overall levels | 96.0 ± 3.2 | 100.1 ± 5.0 | 107.8 ± 3.4* | 91.0 ± 4.7 | 97.5 ± 4.1** | 97.4 ± 7.2 |
| 1. THV inflow | 99.0 ± 5.2 | 104.8 ± 5.7 | 112.0 ± 1.5 | 87.7 ± 5.7 | 94.3 ± 4.2 | 96.5 ± 8.5 |
| 2. Native annulus | 95.1 ± 7.8 | 98.4 ± 5.5 | 107.0 ± 3.3 | 89.8 ± 5.2 | 95.9 ± 4.2 | 97.8 ± 6.1 |
| 3. Mid-THV | 90.9 ± 3.5 | 95.8 ± 4.8*** | 103.5 ± 4.8 | 90.2 ± 5.1 | 96.9 ± 5.0**** | 94.8 ± 7.7 |
| 4. THV outflow | 99.1 ± 3.3 | 101.5 ± 6.3 | 108.6 ± 4.7 | 96.4 ± 8.0 | 102.9 ± 6.6 | 100.4 ± 8.8 |
Values are mean ± SD.
*P < 0.001 and **P = 0.029 vs. the SAPIEN 3 nominal, ***P < 0.001 vs. the SAPIEN 3 THV-inflow, ****P = 0.0041 vs. the SAPIEN XT THV-inflow.
Figure 4.
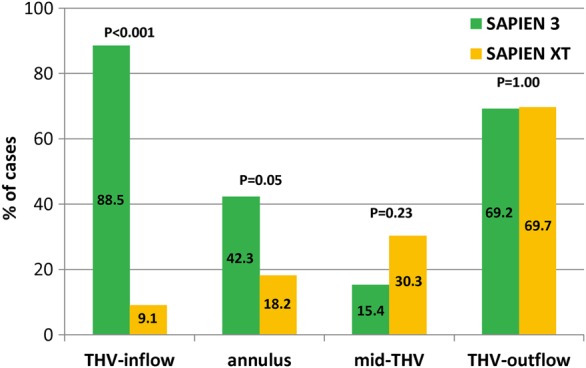
Percentage of cases with observed overexpansion by THV area measured on MSCT (using nominal delivery balloon volume). The numbers in the columns denote the percentage of cases. THV, transcatheter heart valve; MSCT, multi-slice computed tomography.
Figure 5.
Relationship between %-expansion area and delivery balloon volume; comparison of SAPIEN 3 and SAPIEN XT. Percentage of observed THV expansion area measured with CT according to the using delivery balloon volume (nominal, under-filling, over-filling). The numbers in the columns denote the percentage of THV expansion area. The S3 THV at the THV-inflow and the native annular levels had a greater expanded THV area compared with the S-XT THV. THV, transcatheter heart valve.
Parameters of the deployed THV and post-procedural PVR
The relationship between parameters of the deployed THV and post-procedural PVR is summarized in Table 5. Mild or more than mild PVR (≥mild) was present in 7 (17.9%) of patients in the S3 group and in 24 (43.6%) of patients in the S-XT group (P = 0.014). In both groups, eccentricity index was not significantly different between cases with PVR ≥ mild and PVR < mild. Although there was a non-significant trend, percentage of observed THV area oversizing with the S3 and the S-XT was 1.3 ± 7.3% in PVR ≥ mild vs. 7.4 ± 8.6% in PVR < mild, P = 0.09 and 9.6 ± 8.2% in PVR ≥ mild vs. 13.6 ± 8.5% in PVR < mild, P = 0.09, respectively. However, incidence of PVR ≥ mild was more frequently seen in undersizing cases with the S3 (57.1% in PVR ≥ mild vs. 15.6% in PVR < mild, P = 0.037) and also the S-XT with less ventricular deployment (3.1 ± 2.0 mm in PVR ≥ mild vs. 4.3 ± 1.7 mm in PVR < mild, P = 0.018).
Table 5.
Relationship between deployed THV and post-procedural PVR
| PVR ≥ mild | PVR < mild | P-value | |
|---|---|---|---|
| SAPIEN 3 | 7 (17.9) | 32 (82.1) | |
| Observed THV area oversizing, % | 1.3 ± 7.3 | 7.4 ± 8.6 | 0.09 |
| 0%> | 4 (57.1) | 5 (15.6) | 0.037 |
| 0–15% | 3 (42.9) | 21 (65.6) | 0.40 |
| 15%< | 0 (0.0) | 6 (18.8) | 0.57 |
| Observed THV eccentricity index, % | |||
| 1. THV inflow | 3.35 ± 2.8 | 3.92 ± 2.8 | 0.63 |
| 2. Native annulus | 4.33 ± 2.3 | 4.21 ± 2.8 | 0.91 |
| 3. Mid-THV | 4.06 ± 2.6 | 3.33 ± 2.6 | 0.50 |
| 4. THV outflow | 4.18 ± 2.5 | 2.68 ± 2.4 | 0.14 |
| THV depth, mm | 4.6 ± 2.8 | 4.0 ± 2.1 | 0.56 |
| SAPIEN XT | 24 (43.6)* | 31 (56.4) | |
| Observed THV area oversizing, % | 9.6 ± 8.2 | 13.6 ± 8.5 | 0.09 |
| 0%> | 2 (8.3) | 1 (3.2) | 0.58 |
| 0–15% | 18 (75.0) | 20 (64.5) | 0.56 |
| 15%< | 4 (16.7) | 10 (32.3) | 0.23 |
| Observed THV eccentricity index | |||
| 1. THV inflow | 5.02 ± 3.5 | 4.21 ± 2.4 | 0.32 |
| 2. Native annulus | 3.86 ± 2.6 | 2.96 ± 1.9 | 0.14 |
| 3. Mid-THV | 2.83 ± 2.4 | 3.13 ± 1.7 | 0.60 |
| 4. THV outflow | 3.98 ± 3.4 | 3.04 ± 2.1 | 0.22 |
| THV depth, mm | 3.1 ± 2.0 | 4.3 ± 1.7 | 0.018 |
Values are mean ± SD or n (%).
THV, transcatheter heart valve; PVR, paravalvular aortic regurgitation.
*P = 0.014 vs. the SAPIEN 3 group.
Discussion
The S3 is the latest U.S. Food and Drug Administration (FDA)-approved, balloon-expandable THV that replaced the prior-generation device, the S-XT. To the best of our knowledge, this is the first report systematically evaluating its deployed stent-frame morphology using MSCT. Comparing the S3 with the S-XT, our findings were as follows:
Stent-frame morphology: The S3 had a relatively larger or flared inflow in relation to the mid-THV level when compared with the S-XT, which had a relatively constrained inflow in relation to mid-THV level.
Eccentricity index was not different between the S3 and the S-XT.
Observed THV area oversizing and post-procedural PVR: The S3 group had a lesser degree of observed THV area oversizing in relation to native annular dimension than the S-XT group. Nonetheless, the incidence of post-procedural PVR ≥ mild following the S3 TAVI was significantly lower than the S-XT TAVI.
THV expansion area
Few data are available on the stent-frame morphology of deployed balloon-expandable THV. Willson et al.14 examined the geometry of the deployed THV stent frame by MSCT with the earlier-generation, balloon-expandable THV. They demonstrated complete and circular stent-frame expansion; however, there were small differences in expansion between THV levels, with expansion area being less at the inflow compared with at the outflow level. This may represent restriction by the aortic annulus, resistance to expansion by the covered skirt tissue within the THV inflow, or relative overexpansion of the uncovered outflow portion of the THV. Although our study showed similar data for the S-XT, the S3 had a different pattern of expansion; expansion area was larger at the THV inflow and THV outflow compared with that at the mid-portion. Interestingly, over-filling of the S3 achieved a similar morphology to nominal or under-filling (relative flaring of the inflow), whereas it caused a more cylindrical stent frame with the S-XT (inflow less constrained). These differences could be related to the absence of commissural posts with the S3 or to differences in the THV cell design between two valve types (Figure 3). It has been postulated that complete or underexpansion of THV may also be related to the annular size, extent of aortic valve calcification, THV stent recoil, or inadequate THV balloon expansion (under-filling or over-filling delivery balloon volume and short or long duration of balloon expansion).18,19 However, our study showed that aortic valve calcium distribution and LVOT calcium were not significantly different between the S3 and the S-XT groups (Tables 2 and 6), suggesting the critical difference to be device driven.
Table 6.
Pre-procedural distribution of aortic valvar complex calcium
| Overall (n = 94) | SAPIEN 3 (n = 39) | SAPIEN XT (n = 55) | P-value | |
|---|---|---|---|---|
| Aortic valve calcium volume,a mm3 | ||||
| RCC | 66.8 ± 78.1 | 63.8 ± 78.2 | 68.9 ± 78.6 | 0.76 |
| LCC | 66.0 ± 69.7 | 76.3 ± 89.1 | 58.7 ± 51.5 | 0.23 |
| NCC | 118.1 ± 111.2 | 112.6 ± 120.2 | 122.0 ± 105.4 | 0.69 |
| LVOT calcium | 22 (23.4) | 12 (30.8) | 10 (18.2) | 0.22 |
aContrast scan, HU-850 threshold for detection.
Values are mean ± SD or n (%).
RCC, right coronary cusp; LCC, left coronary cusp; NCC, non-coronary cusp; LVOT, left ventricular outflow tract.
The correlation between expansion area of the THV and delivery balloon volume on prior-generation, balloon-expandable THV (S-XT) has been described in one other study. Barbanti et al.18 analysed 134 patients with severe aortic stenosis who underwent TAVI with the S-XT. Nominally filled delivery balloon volume resulted in a prosthesis cross-sectional CT area that correlated well with the expected THV area. In our study, at the THV-inflow level, relative underexpansion of the THV was rarely observed in the S3 device using nominal delivery balloon volume. However, underexpansion was more frequently observed in the S-XT group even using nominal volume.
THV eccentricity
During the TAVI procedure, radial forces affect the aortic annulus geometry. It has been shown that the geometry of the aortic annulus changes from an elliptical shape before implantation to a more circular shape after implantation.20,21 Our study also demonstrates that implantation of balloon-expandable THV reduced the eccentricity of the aortic annulus, achieving circularity of the deployed stent-frame morphology in almost all the S3 and the S-XT THV.
Area oversizing and post-procedural PVR
Appropriate sizing for every THV is critically important to minimize the incidence of PVR. Leber et al.22 suggested that an oversizing ratio of 15–25% of the S-XT based on area appears to provide the best risk–benefit ratio in terms of PVR reduction and conduction disorders. However, Blanke et al.8 described contained rupture of the aortic root in balloon-expandable TAVI is associated with severe prosthesis oversizing of the S-XT (the threshold studied was >20%). In their studies, oversizing ratio was calculated using THV nominal area, whereas we calculated oversizing ratio with actual observed THV stent-frame expansion area from CT-derived measurements.
Despite less resultant observed stent-frame oversizing in relation to the native annulus, we observed lower rates of PVR with the S3 vs. the S-XT. Not only the external fabric cuff of the S3 but also its tendency to flare at the inflow may mitigate the PVR rate appropriately. However, this potential for inflow flaring with the S3 stent frame may carry some risks of aortic root injury and new permanent pacemaker implantation in the setting of oversizing. In view of this, there is evidence in the present study to support the differences in manufacturer's recommended sizing parameters for the S3 and S-XT, with less aggressive oversizing advised for the S3.
Study limitations
The present study is a non-randomized comparison of two different generations of balloon-expandable THV albeit with no difference in native aortic valve function, calcification, and dimension by rigorous baseline echocardiographic and MSCT analysis. Second, some stent frames have blooming artefacts on MSCT so that they might influence our stent-frame measurements. Finally, this study was a retrospective analysis of participants of the RESOLVE registry and is limited by the small single-centre sample of selected patients who underwent post-procedural MSCT at our institution, during the study period.
Conclusions
The THV stent-frame morphology post TAVI is significantly different between the newer-generation, balloon-expandable THV (S3) and its prior-generation counterpart. The S3 has a flared inflow morphology, whereas the prior generation, the S-XT, has a relatively constrained inflow morphology. This fundamental difference in THV stent-frame morphology may contribute to a lesser degree of PVR with the S3.
Conflict of interest: H.J. is a consultant for Edwards Lifesciences Corporation, St. Jude Medical, and Venus MedTech. R.R.M. has received grant support from Edwards Lifesciences Corporation and St. Jude Medical; is a consultant for Abbott Vascular, Cordis, and Medtronic; and holds equity in Entourage Medical.
Funding
This work was supported by Internal Departmental Resources. Funding to pay the Open Access publication charges for this article was provided by the first author paid personally.
References
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 2.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR et al. Two-year outcomes after transcatheter or surgical aorticvalve replacement. N Engl J Med 2012;388:1686–95. [DOI] [PubMed] [Google Scholar]
- 3.Binder RK, Rodés-Cabau J, Wood DA, Mok M, Leipsic J, De Larochellière R et al. Transcatheter aortic valve replacement with the SAPIEN 3; a new balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv 2013;6:293–300. [DOI] [PubMed] [Google Scholar]
- 4.Amat-Santos IJ, Dahou A, Webb J, Dvir D, Dumesnil JG, Allende R et al. Comparison of hemodynamic performance of the balloon-expandable SAPIEN 3 versus SAPIEN XT transcatheter valve. Am J Cardiol 2014;114:1075–82. [DOI] [PubMed] [Google Scholar]
- 5.Yang TH, Webb JG, Blanke P, Dvir D, Hansson NC, Nørgaard BL et al. Incidence and severity of paravalvular aortic regurgitation with multidetector computed tomography nominal area oversizing or undersizing after transcatheter heart valve replacement with the Sapien 3; a comparison with the Sapien XT. JACC Cardiovasc Interv 2015;8:462–71. [DOI] [PubMed] [Google Scholar]
- 6.Costopoulos C, Latib A, Maisano F, Testa L, Bedogni F, Buchanan L et al. Comparison of results of transcatheter aortic valve implantation in patients with severely stenotic bicuspid versus tricuspid or nonbicuspid valves. Am J Cardiol 2014;113:1390–3. [DOI] [PubMed] [Google Scholar]
- 7.Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G et al. Cross sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59:1275–86. [DOI] [PubMed] [Google Scholar]
- 8.Blanke P, Reinöhl J, Schlensak C, Siepe M, Pache G, Euringer W et al. Prosthesis oversizing in balloon-expandable transcatheter aortic valve implantation is associated with contained rupture of the aortic root. Circ Cardiovasc Interv 2012;5:540–8. [DOI] [PubMed] [Google Scholar]
- 9.Tops L, Wood DA, Delgado V, Schuijf JD, Mayo JR, Pasupati S et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging 2008;1:321–30. [DOI] [PubMed] [Google Scholar]
- 10.Kasel AM, Cassese S, Bleiziffer S, Amaki M, Hahn RT, Kastrati A et al. Standardized imaging for aortic annular sizing: implications for transcatheter valve selection. JACC Cardiovasc Imaging 2013;6:249–62. [DOI] [PubMed] [Google Scholar]
- 11.Willson AB, Webb JG, Freeman M, Wood DA, Gurvitch R, Thompson CR et al. Computed tomography-based sizing recommendations for transcatheter aortic valve replacement with balloon-expandable valves: comparison with transesophageal echocardiography and rationale for implementation in a prospective trial. J Cardiovasc Comput Tomogr 2012;6:406–14. [DOI] [PubMed] [Google Scholar]
- 12.Schmidkonz C, Marwan M, Klinghammer L, Mitschke M, Schuhbaeck A, Arnold M et al. Interobserver variability of CT angiography for evaluation of aortic annulus dimensions prior to transcatheter aortic valve implantation (TAVI). Eur J Radiol 2014;83:1672–8. [DOI] [PubMed] [Google Scholar]
- 13.Jilaihawi H, Chin D, Spyt T, Jeilan M, Vasa-Nicotera M, Mohamed N et al. Comparison of complete versus incomplete stent frame expansion after transcatheter aortic valve implantation with Medtronic CoreValve Bioprosthesis. Am J Cardiol 2011;107:1830–7. [DOI] [PubMed] [Google Scholar]
- 14.Willson AB, Webb JG, Labounty TM, Achenbach S, Moss R, Wheeler M et al. 3-Dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement; a multicenter retrospective analysis. J Am Coll Cardiol 2012;59:1287–94. [DOI] [PubMed] [Google Scholar]
- 15.Schultz CJ, Weustink A, Piazza N, Otten A, Mollet N, Krestin G et al. Geometry and degree of apposition of the CoreValve ReValving system with multislice computed tomography after implantation in patients with aortic stenosis. J Am Coll Cardiol 2009;54:911–8. [DOI] [PubMed] [Google Scholar]
- 16.Otto CM, Pearlman AS, Comess KA, Reamer RP, Janko CL, Huntsman LL. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J Am Coll Cardiol 1986;7:509–17. [DOI] [PubMed] [Google Scholar]
- 17.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH et al. Valve Academic Research Consortium-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438–54. [DOI] [PubMed] [Google Scholar]
- 18.Nombela-Franco L, Ribeiro HB, Urena M, Pasian S, Allende R, Doyle D et al. Incidence, predictive factors and haemodynamic consequences of acute stent recoil following transcatheter aortic valve implantation with a balloon-expandable valve. EuroIntervention 2014;9:1398–406. [DOI] [PubMed] [Google Scholar]
- 19.Barbanti M, Leipsic J, Binder R, Dvir D, Tan J, Freeman M et al. Underexpansion and ad hoc post-dilation in selected patients undergoing balloon-expandable transcatheter aortic valve replacement. J Am Coll Cardiol 2014;63:976–81. [DOI] [PubMed] [Google Scholar]
- 20.Delgado V, Ng AC, van de Veire NR, van der Kley F, Schuijf JD, Tops LF et al. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J 2010;31:1114–23. [DOI] [PubMed] [Google Scholar]
- 21.Schuhbaeck A, Weingartner C, Arnold M, Schmid J, Pflederer T, Marwan M et al. Aortic annulus eccentricity before and after transcatheter aortic valve implantation: comparison of balloon-expandable and self-expanding prostheses. Eur J Radiol 2015;84:1242–8. [DOI] [PubMed] [Google Scholar]
- 22.Leber AW, Eichinger W, Rieber J, Lieber M, Schleger S, Ebersberger U et al. MSCT guided sizing of the Edwards Sapien XT TAVI device: Impact of different degrees of oversizing on clinical outcome. Int J Cardiol 2013;168:2658–64. [DOI] [PubMed] [Google Scholar]