ABSTRACT
In 2011, one of the world's largest outbreaks of hemolytic-uremic syndrome (HUS) occurred, caused by a rare Escherichia coli serotype, O104:H4, that shared the virulence profiles of Shiga toxin-producing E. coli (STEC)/enterohemorrhagic E. coli (EHEC) and enteroaggregative E. coli (EAEC). The persistence and fitness factors of the highly virulent EHEC/EAEC O104:H4 strain, grown either in food or in vitro, were compared with those of E. coli O157 outbreak-associated strains. The log reduction rates of the different EHEC strains during the maturation of fermented sausages were not significantly different. Both the O157:NM and O104:H4 serotypes could be shown by qualitative enrichment to be present after 60 days of sausage storage. Moreover, the EHEC/EAEC O104:H4 strain appeared to be more viable than E. coli O157:H7 under conditions of decreased pH and in the presence of sodium nitrite. Analysis of specific EHEC strains in experiments with an EHEC inoculation cocktail showed a dominance of EHEC/EAEC O104:H4, which could be isolated from fermented sausages for 60 days. Inhibitory activities of EHEC/EAEC O104:H4 toward several E. coli strains, including serotype O157 strains, could be determined. Our study suggests that EHEC/EAEC O104:H4 is well adapted to the multiple adverse conditions occurring in fermented raw sausages. Therefore, it is strongly recommended that STEC strain cocktails composed of several serotypes, instead of E. coli O157:H7 alone, be used in food risk assessments. The enhanced persistence of EHEC/EAEC O104:H4 as a result of its robustness, as well as the production of bacteriocins, may account for its extraordinary virulence potential.
IMPORTANCE In 2011, a severe outbreak caused by an EHEC/EAEC serovar O104:H4 strain led to many HUS sequelae. In this study, the persistence of the O104:H4 strain was compared with those of other outbreak-relevant STEC strains under conditions of fermented raw sausage production. Both O157:NM and O104:H4 strains could survive longer during the production of fermented sausages than E. coli O157:H7 strains. E. coli O104:H4 was also shown to be well adapted to the multiple adverse conditions encountered in fermented sausages, and the secretion of a bacteriocin may explain the competitive advantage of this strain in an EHEC strain cocktail. Consequently, this study strongly suggests that enhanced survival and persistence, and the presumptive production of a bacteriocin, may explain the increased virulence of the O104:H4 outbreak strain. Furthermore, this strain appears to be capable of surviving in a meat product, suggesting that meat should not be excluded as a source of potential E. coli O104:H4 infection.
INTRODUCTION
The Shiga toxin-producing Escherichia coli (STEC) strains, which include enterohemorrhagic E. coli (EHEC) strains, are pathogenic E. coli strains with the potential to cause severe enteric and systemic disease in humans. These bacteria have accounted for various foodborne infections over recent decades. The pathogenesis of EHEC disease depends on the production of Shiga toxin (Shiga toxin 1 [Stx1] and/or Stx2) in combination with other virulence factors. EHEC strains typically harbor virulence genes for attachment (e.g., intimin genes) or potentiating toxin genes (e.g., subtilase cytotoxin) (1). Non-sorbitol-fermenting (NSF) E. coli O157:H7 is recognized as one of the most important foodborne pathotypes occurring worldwide and is associated with human diseases including diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome (HUS). Moreover, sorbitol-fermenting (SF) E. coli O157:NM (nonmotile) strains have emerged as important pathogens, because they are associated with a higher incidence of progression to HUS than NSF E. coli O157:H7 strains, especially in continental Europe (2–4). The proportion of STEC infections in Europe caused by non-O157 serotypes increased from 45.1% to 51.1% in 2013 (5). Even though the clinical manifestations of non-O157 STEC infections may differ, they can be as virulent as O157:H7 infections.
This was shown to be the case in one of the world's largest outbreaks of HUS, in Germany in 2011. The causative agent was identified as a rare E. coli serotype, O104:H4, that exhibited the virulence profiles of both typical Shiga toxin-producing E. coli (EHEC) and enteroaggregative E. coli (EAEC) (6). The phenotypes expressed by the O104:H4 German outbreak strain included the production of Stx2, aggregative adherence to epithelial cells, and the production of extended-spectrum β-lactamase (ESBL) (6, 7). The data suggest that besides enhanced adherence to intestinal epithelial cells, which might facilitate the systemic absorption of Shiga toxin, resistance to antibiotics was also responsible for the apparently augmented virulence potential of the O104:H4 outbreak strain (6). Consumption of sprouted fenugreek seeds was identified as the most likely source of infection for primary outbreak cases (8). Particularly later in the outbreak, person-to-person transmission and foodborne outbreaks associated with infected food handlers also took place (9).
Humans have been shown to be a reservoir both for EAEC strains, including O104:H4 strains, and for SF E. coli O157:NM, in addition to other possible, but still unknown, reservoirs (6, 10–13). Several studies have also provided evidence of a reservoir for SF E. coli O157:NM in cattle (14–17). Recently, genes characteristic of the O104:H4 German outbreak strain were found in cattle herd samples from one abattoir located near the outbreak epicenter (18). Consequently, like other STEC/EHEC serotypes, both E. coli O104:H4 and E. coli O157:NM might enter the food chain via cattle as the potential reservoir. After a number of foodborne STEC outbreaks that involved fermented sausages, the European Union considered that minced and/or fermented beef and its products represent a hazard to public health with regard to these pathogens (19). Commonly, the incorporation of sequential or concurrent hurdles consisting of different preservation strategies, such as the addition of preservatives (nitrite or sodium ascorbate), the growth of competitive microbiota, acidification, smoking, and drying, result in safe and stable fermented-sausage products (20). However, a delayed start of fermentation and short curing periods at cold temperatures were identified as the main factors enabling EHEC survival in fermented sausage. These factors were linked to an outbreak of E. coli O157:H7 in southern Sweden, where contaminated beef was suspected to be the source of infection (21). Even the issue of cross-contamination, e.g., by asymptomatic infected persons (22), should not be ignored; cross-contamination may also contribute to the presence of STEC/EHEC, including E. coli O104:H4 and O157:NM, in fermented sausage or generally, in all foods. Many studies have dealt with the survival and inactivation of E. coli O157:H7 in different food matrices, but little research has been carried out so far on the persistence of non-O157 STEC in foods. Rode et al. (23) described the sorbitol-fermenting O157:NM outbreak strain as the STEC strain that showed the greatest ability to survive the conditions in fermented sausages. Only a few studies investigated the survival of the EHEC/EAEC O104:H4 outbreak strain, and these focused mainly on plant foods (24–27). Moreover, the European Food Safety Authority (EFSA) recently expressed the need for further controlled studies that fully quantify the survival characteristics of EAEC in wet and dry substrates under laboratory and natural conditions (28).
In order to gain basic knowledge about the survival and fitness of the EHEC/EAEC O104:H4 strain in food, compared to those of the E. coli O157:H7 and sorbitol-fermenting E. coli O157:NM outbreak-associated strains, challenge studies were carried out in fermented raw sausages as well in in vitro assays under conditions similar to those occurring in the sausage environment. These conditions included decreased pH, the presence of sodium nitrite, and moderate fermentation temperatures. Moreover, the most resistant EHEC isolate that survives the conditions in fermented raw sausages was determined in multistrain cocktail inoculum experiments.
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
Two EHEC strains linked to recent outbreaks in Germany were used in the present study. The E. coli O104:H4 (MRI collection number E965) and O157:NM (MRI collection number E963) isolates, recovered from HUS patients in 2011 and 2009, respectively, were kindly provided by the Bavarian Health and Food Safety Authority (Oberschleißheim, Germany). E. coli O157:H7 EDL933 (ATCC 43895; MRI collection number E135) was used as the reference strain (29). Selected strains of Enterobacteriaceae from the MRI strain collection (n = 25) served as indicator strains in inhibition assays with E. coli O104:H4 (Table 1).
TABLE 1.
Inhibitory activity of E. coli O104:H4 in competition assays with selected Enterobacteriaceae indicator strains
| Indicator strain | Serotype | Collection no. | Source | Inhibition zonea |
|---|---|---|---|---|
| Escherichia coli | O157:H7 | E135 (EDL933; ATCC 43895) | Meat (burger) | +++ |
| O157:H7 | E118 | Bovine feces | ++ | |
| O157:H7 | E141 | Apple cider | ++ | |
| O157:NM | E963 | Human feces (HUS) | ++ | |
| O157:NM | E148 | Human feces (HUS) | ++ | |
| O26:H11 | E157 | Human feces (HUS) | ++ | |
| O26:H11 | E165 | Calf feces | + | |
| O26:H11 | E972 | Wild boar meat | ++ | |
| E162 | Ice cream | +++ | ||
| E163 | Smear cheese | + | ||
| E164 | Raw milk | +++ | ||
| Salmonella enterica subsp. enterica | Typhimurium | S509 | Swab (meat plant) | − |
| Typhimurium | S692 | Bovine feces | − | |
| Typhimurium | S702 (ATCC 14028) | Heart/liver tissues (chicken) | − | |
| Enteritidis | S522 | Mettwurst sausage | − | |
| Enteritidis | S575 | Lymphatic tissue (pig) | − | |
| Enteritidis | S630 | Meat juice | − | |
| Citrobacter freundii | Ci5 | Bovine feces | − | |
| Ci24 | Chicken meat | − | ||
| Ci29 | Dry cured ham | − | ||
| Hafnia spp. | NE146 | Teewurst | − | |
| NE137 | Swab (meat plant) | − | ||
| NE142 | Meat | − | ||
| Klebsiella oxytoca | Kl31 | Ground meat | − | |
| NE226 | Human feces | − |
Results of three experiments in a duplicate setup are presented. Diameters of inhibition zones were calculated and are indicated as +++ (>15 mm), ++ (15 to 10 mm), or + (10 to 5 mm); −, no inhibition activity.
Stock cultures were maintained in cryobeads (Pro-Lab Diagnostics Microbank bacterial and fungal preservation system; bestbiondx, Cologne, Germany) at −70°C. Bacteria were revived in 9 ml of brain heart infusion (BHI; Oxoid, Wesel, Germany) or Trypticase soy broth plus 0.5% yeast extract (TSBY) for 16 h at 37°C. For inoculation experiments with either a single strain or a cocktail of strains, cell concentrations were determined in a Thoma counting chamber and were then diluted to an inoculation level of 3 log10 CFU/g. This was confirmed by plating on sorbitol-MacConkey (SMAC) agar (Oxoid, Wesel, Germany). Colonies were enumerated after the incubation of plates for 24 h at 37°C.
Sausage preparation.
Fresh raw materials of high quality were used in order to keep background microbiota at a minimum. Batters for the production of pure beef salami were prepared from lean beef (70%) and abdominal fat (30%) according to a standard recipe (30). Sliced frozen meat and fat ingredients were chopped in a rotating bowl of a meat cutter (ETK 20/1 [10 liters]; E.-Müller, Saarbrücken, Germany) with the addition of sodium ascorbate (0.05%), brine salt (Südsalz GmbH, Heilbronn, Germany) with sodium nitrite (150 ppm), glucose (0.5%), saccharose (0.5%), and pepper (0.3%). Freeze-dried BITEC LS-1 starter culture, comprising Lactobacillus curvatus, Staphylococcus carnosus, and Kocuria varians (Gewürzmüller GmbH, Korntal-Münchingen, Germany), was added at approximately 6 log10 CFU/g to the sausage batter. For challenge experiments, single EHEC strains were either mixed alone into different salami batters or were combined as an EHEC cocktail inoculum that contained equal numbers of each strain (1:1:1) and a final concentration of 3 log10 CFU/g for inoculation. Batters were stuffed into 60-mm synthetic casings (Naturin Viscofan GmbH, Weinheim, Germany). Sausages were adjusted to room temperature for 4 to 5 h before fermentation and ripening in a climate-controlled cabinet (Karl Weiss, Giessen, Germany). The production process included fermentation for 72 h in total with 24 h each at 22°C (relative humidity [RH], 93 to 94%), 21°C (RH, 90 to 92%), and 20°C (RH, 90%). After maturation at 18°C for 72 h (RH, 88%) and 48 h (RH, 85%), sausages remained for an additional 9 days at 17°C (RH, 83 to 85%). According to the standard manufacturer's procedure, the salami products were vacuum packed 18 days after preparation and were stored at ambient temperature. For each inoculation experiment with a single strain or the triple-EHEC-strain cocktail, three salami batches were produced on different days, together with an uninoculated control batch. Three technical replicates were used for chemical and microbiological analyses. Samples were tested on the production day (day 0), during ripening and maturation (days 1, 2, 3, 7, and 14), after vacuum packaging and storage (days 21, 28, and 42), and at the end of the product's shelf life (day 60).
Chemical and microbiological analyses.
Control batches were tested in triplicate for pH and water activity (aw) on each sampling day (see above). For this purpose, a 25-g sample was homogenized in 225 ml deionized water in a laboratory paddle blender (Stomacher 400 Circulator; Seward), and the pH of the homogenate was measured with a pH meter (WTW pH 526). The aw of sausages was determined with a cryometer (Nagy AWK-20). For comparison, commercial beef salami products made with a similar recipe and similar production periods (n = 5) were also tested for pH and aw.
For microbiological analyses, 25 g of a sausage sample or a commercial beef salami product was added to 225 ml buffered peptone water (BPW) and was macerated for 5 min in a stomacher. The homogenate was serially diluted and was spread plated, using an automatic spiral plater (Eddy Jet; IUL Instruments, Königswinter, Germany), onto plate count (PC) agar (Oxoid, Wesel, Germany) for total mesophilic aerobic bacteria (MAB), onto de Man, Rogosa, and Sharpe (MRS) agar (Oxoid, Wesel, Germany) for lactic acid bacteria (LAB), and onto SMAC agar (Oxoid, Wesel, Germany) for EHEC counts. Colonies were enumerated after incubation for 48 h at 30°C (PC agar and MRS agar) and for 24 h at 37°C (SMAC agar). For counts lower than the limit of quantitative detection (10 CFU/g), EHEC strains were recovered by selective or nonselective enrichment in modified Trypticase soy broth supplemented with novobiocin (mTSB-N) (Oxoid, Wesel, Germany) or BPW, respectively. For this purpose, a 25-g sample of raw material (beef and fat) or sausage was homogenized in 225 ml mTSB or BPW and was incubated with shaking at 37°C for 18 h. One loop of enrichment broth was then streaked out onto SMAC agar in order to determine the presence of viable EHEC cells. As a confirmation, multiplex real-time PCR with the CFX96 Touch real-time PCR detection system (Bio-Rad Laboratories GmbH, Munich, Germany) was also used to detect EHEC bacteria, as described by Pavlovic et al. (31) (see below).
In inoculation experiments with the cocktail of EHEC strains, biochemical and molecular traits were used to distinguish between the different EHEC strains. The total EHEC count was determined on SMAC agar, which allowed identification of the colorless colonies as the non-sorbitol-fermenting E. coli O157:H7 strain. Red colonies, on the other hand, were picked and were resuspended in 1 ml BPW. After transfer to Brilliance ESBL agar (Oxoid, Wesel, Germany) with a sterile toothpick and incubation for 24 h at 37°C, the isolates that were able to grow on this medium were identified as E. coli O104:H4, while those that did not were characterized as E. coli O157:NM. At least five colonies from SMAC agar plates from which each of the three EHEC strains could be identified were confirmed as belonging to the respective types by multiplex real-time PCR, which targeted the virulence genes stx1, stx2, and eae (31). For DNA extraction, a single colony was boiled in 100 μl Limulus amoebocyte lysate (LAL) reagent water (VWR, Ismaning, Germany) for 15 min and was centrifuged for 3 min at 9,000 × g. The supernatant was stored at −20°C until use. Each PCR mixture (25 μl) contained 2× master mix (DyNAmo Flash Probe qPCR kit; Biozym, Hessisch Oldendorf, Germany) with primers and probes at the concentrations described by Pavlovic et al. (31), as well as 5 μl of template DNA. Thermal cycling and detection were performed using the CFX96 Touch real-time PCR detection system (Bio-Rad, Munich, Germany). Differences in the virulence gene profiles of the different EHEC strains resulted in positive signals for all three virulence genes (stx1, stx2, and eae) (E. coli O157:H7), for stx2 and eae only (E. coli O157:NM), or for eae only (E. coli O104:H4).
In vitro survival assays.
The conditions found in raw sausage—decreased pH, the presence of sodium nitrite, and moderate fermentation temperatures—were simulated in vitro. For this purpose, assays with E. coli O104:H4 and O157:H7 were performed in TSBY adjusted to pH 5.1 with 20% (vol/vol) lactic acid (Sigma-Aldrich, Taufkirchen, Germany) either in the absence or in the presence of 150 mg/liter sodium nitrite (Merck, Darmstadt, Germany). TSBY without any additions was used as a control. Three broth batches—TSBY, TSBY at pH 5.1, and TSBY at pH 5.1 containing 150 mg/liter sodium nitrite—were inoculated with 7 log10 CFU/ml of one of the EHEC strains as determined by a Thoma cell counting chamber (see above). After 0, 18, 42, and 66 h of incubation at 22°C, cell viabilities were assessed using the LIVE/DEAD BacLight viability kit (Invitrogen, Life Technologies GmbH, Darmstadt, Germany). Stock solutions of the two nucleic acid stains used for this method, green fluorescent SYTO 9 dye and red fluorescent propidium iodide, were prepared in 5 ml distilled water (dH2O) and were stored in aliquots at 4°C according to the manufacturer's instructions. Ten milliliters of bacterial suspensions was centrifuged for 15 min at 3,000 × g, and the pellet was washed twice in 10 ml dH2O and was then resuspended in 1 ml dH2O. Fifteen microliters of the staining stock solution and 15 μl of the bacterial suspension were mixed and were incubated in the dark for 15 min. For fluorescence detection, 5 μl of the stained mixture was placed on a microscope slide under a coverslip, spread with immersion oil, and analyzed using a fluorescence microscope at a magnification of ×100 (Olympus BX60 microscope; BH2-RFL-T3 power supply; U-MWB filter module; band pass 450- to 480-nm exciter filter). The respective numbers of dead, viable, and damaged cells were determined by counting red, green, and orange cells, respectively, in the field of view for 1 min in triplicate, and the percentages of live and dead cells were calculated. All experiments were repeated three times.
Inhibition assays.
The inhibitory spectrum of E. coli O104:H4 was determined according to the method of Bigwood et al. (32). Briefly, E. coli O104:H4 was grown as streaks across the center of TSA (TSB containing 1.5% [wt/vol] agar) plates, and the plates were incubated for 16 h at 37°C. At the same time, indicator microorganisms (Table 1) were grown for 16 h at 37°C in TSB, and 0.1 ml of overnight cultures was inoculated into 10 ml TSB and was incubated for 4 h at 37°C. A 0.1-µl volume was then added to 4 ml soft TSA (0.7% agar) equilibrated at 47°C. The tube was briefly vortexed, and the agar was then poured as an overlay onto the TSA plate with E. coli O104:H4 growth. Plates were incubated overnight at 37°C before examination for inhibition zones of the bacterial lawn in the agar overlay. Inhibitory activity was regarded as strong (+++) if the diameter of the zone of clearing measured >15 mm, as moderate (++) if the diameter was 15 to 10 mm, and as weak (+) if the diameter was 10 to 5 mm.
The published genome of E. coli O104:H4 strain 2011C-3493 (33) was analyzed in silico with the BAGEL automated bacteriocin mining tool (http://bagel2.molgenrug.nl/). BAGEL is a Web server that identifies putative bacteriocin open reading frames (ORFs) in a DNA sequence using novel, knowledge-based bacteriocin databases and motif databases (34).
Statistical analysis.
Colony counts were log-transformed, and samples with counts below the quantitative limit of detection (LOD) (<1 log10 CFU/g) that were positive after an enrichment procedure were arbitrarily assigned a value of 0.7 log10 CFU/g (⩠5 CFU/g). Samples that were negative in the enrichment procedure were assigned the value of zero. Log10 reductions in EHEC counts during sausage production were calculated by subtracting the log10 CFU of EHEC per gram of fermented sausage (day 3, 7, or 14) from the log10 CFU of EHEC per gram of sausage batter on the production day (day 0). Median and mean values, as well as standard deviations, were calculated. EHEC fractions in the inoculation experiments with triple-strain cocktails were determined by dividing the count of non-sorbitol-fermenting bacteria on SMAC agar or Brilliance ESBL agar by the total-colony count on SMAC agar (and multiplying by 100 to determine the percentage). One-way analysis of variance (ANOVA) with SigmaPlot, version 11.0, was used to analyze differences in EHEC reduction rates during salami fermentation and differences in cell viability between strains in correlation with incubation conditions at a significance level (P) of <0.05 (Tukey test) or <0.001 (Holm-Sidak method).
RESULTS
Reductions in EHEC counts during sausage production.
All samples of raw material from beef and fat were free of STEC at the beginning of the experiment, as determined after enrichment for STEC. The noninoculated sausages remained negative for STEC throughout storage. The pH of beef salami decreased from 5.54 ± 0.10 to 5.00 ± 0.14 within 7 days and then increased during storage until a pH of 5.24 ± 0.12 was obtained at day 60 (Table 2). In contrast, the aw gradually decreased from 0.959 ± 0.002 to 0.876 ± 0.016 within 60 days (Table 2). During fermentation and ripening, the products experienced a weight loss of 28.2% ± 1.7% in total. The commercial beef salami products, used as controls (n = 5), exhibited pH values ranging from 4.50 to 4.73 and aw values of 0.874 to 0.886 on the day of purchase, which was approximately 3 to 4 weeks postproduction (data not shown).
TABLE 2.
Chemical parameters and bacterial cell counts of total mesophilic aerobic bacteria, lactic acid bacteria, and EHEC during the fermentation and storage of beef salamia
| Day | Chemical parameter |
Bacterial cell countb (log10 CFU/g) |
||||||
|---|---|---|---|---|---|---|---|---|
| pH | aw | MAB | LAB |
E. coli |
||||
| O157:H7 | O157:NM | O104:H4 | EHEC cocktail | |||||
| 0 | 5.54 ± 0.10 | 0.959 ± 0.002 | 7.19 ± 0.09 | 6.95 ± 0.21 | 3.55 ± 0.26 | 3.60 ± 0.09 | 3.06 ± 0.30 | 3.32 ± 0.36 |
| 1 | 5.56 ± 0.09 | 0.957 ± 0.006 | 7.91 ± 0.20 | 7.85 ± 0.19 | 3.10 ± 0.30 | 3.26 ± 0.11 | 3.10 ± 0.27 | 2.92 ± 0.09 |
| 3 | 5.12 ± 0.14 | 0.953 ± 0.003 | 8.77 ± 0.22 | 8.83 ± 0.15 | 2.47 ± 0.25 | 2.58 ± 0.12 | 2.37 ± 0.30 | 2.61 ± 0.36 |
| 7 | 5.00 ± 0.14 | 0.942 ± 0.005 | 8.79 ± 0.13 | 8.87 ± 0.12 | 2.15 ± 0.18 | 2.45 ± 0.20 | 2.28 ± 0.25 | 2.36 ± 0.32 |
| 14 | 5.03 ± 0.11 | 0.909 ± 0.01 | 8.86 ± 0.16 | 8.83 ± 0.12 | 1.84 ± 0.32 | 1.92 ± 0.39 | 1.65 ± 0.66 | 2.26 ± 0.35 |
| 21 | 5.05 ± 0.09 | 0.893 ± 0.015 | 8.76 ± 0.13 | 8.79 ± 0.15 | 1.15 ± 0.40 | <1 | <1 | 1.30 ± 0.57 |
| 28 | 5.07 ± 0.11 | 0.891 ± 0.015 | 8.67 ± 0.18 | 8.71 ± 0.18 | <1 | <1 | <1 | <1 |
| 42 | 5.10 ± 0.10 | 0.886 ± 0.014 | 8.46 ± 0.20 | 8.60 ± 0.23 | <1 | <1 | <1 | <1 |
| 60 | 5.24 ± 0.12 | 0.876 ± 0.016 | 8.04 ± 0.51 | 8.08 ± 0.30 | <1 | <1 | <1 | <1 |
Results are means ± standard deviations for three experiments in a triplicate setup. MAB, total mesophilic aerobic bacteria; LAB, lactic acid bacteria.
The limit of quantitative detection was 1 log10 CFU/g.
Total counts of mesophilic aerobic bacteria (MAB) and lactic acid bacteria (LAB) were ca. 7 log10 CFU/g at day 0 (Table 2). The MAB and LAB counts then increased and reached almost 9 log10 CFU/g on day 3; they stayed at this level up to day 28 and then decreased to approximately 8 log10 CFU/g (Table 2). Counts of 8 log10 CFU/g and 7 log10 CFU/g were determined for MAB and LAB in the commercial beef salami products, respectively.
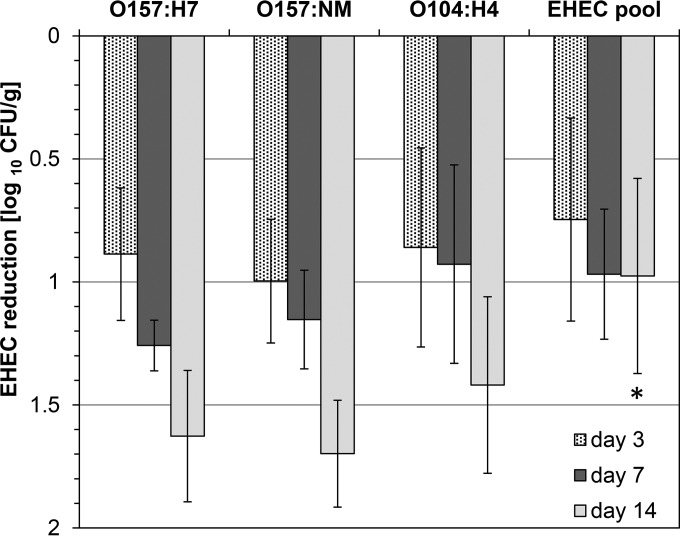
In inoculation experiments, EHEC counts decreased rapidly within 3 days of sausage fermentation by about 0.75 to 1 log10 unit, to reach counts between 2.4 log10 CFU/g and 2.6 log10 CFU/g (Table 2; Fig. 1). In total, EHEC loads were reduced on day 14 by ca. 1.7 log10 units (E. coli O157:H7/NM) or 1.4 log10 units (E. coli O104:H4), whereas EHEC counts in fermented sausages inoculated with the three-EHEC-strain cocktail were reduced by only 1 log10 unit (Fig. 1). The log reduction rates of the various single EHEC strains differed significantly from that of the EHEC strain cocktail on day 14 (P < 0.001) (Fig. 1). The limit of detection (LOD) by direct plating was reached on day 14 for E. coli O157:NM and E. coli O104:H4 and on day 21 for O157:H7 and the EHEC cocktail of strains (Table 2). After this time point, enrichment was used to detect the presence of EHEC. After 60 days, E. coli O157:NM and E. coli O104:H4 could be detected by mTSB-N enrichment in 10 of 12 and 6 of 12 samples, respectively, whereas E. coli O157:H7 was not detectable in any of the 18 samples tested (Table 3). More-efficient isolation of E. coli O104:H4 could be shown for the samples enriched with the nonselective medium BPW (69.7%) than for the mTSB-N enrichment broth samples (24.1%) from day 28 to day 60 (Table 3).
FIG 1.
Reduction of EHEC counts in single- and triple-strain inoculation experiments during the fermentation and maturation of raw fermented sausages up to day 14. EHEC cell counts were determined on SMAC agar. Values are presented as the means for three independent experiments with a triplicate setup and are expressed as log10 CFU per gram. EHEC log10 reductions were calculated by subtracting the EHEC count in fermented sausages on day 3, 7, or 14 from the EHEC count in the sausage batter on the day of production (day 0) (with both counts expressed in log10 CFU per gram). The asterisk indicates a significant difference (P < 0.001) by the Holm-Sidak method.
TABLE 3.
Qualitative detection of EHEC by selective or nonselective enrichment during the storage of beef salami
| Day | No. of samples positive for the indicated organism(s)/total no. of samplesa in: |
||||
|---|---|---|---|---|---|
| mTSB-N |
BPW |
||||
| O157:H7 | O157:NM | O104:H4 | O104:H4 | EHEC cocktail | |
| 21 | ND | 11/11 | 9/12 | ND | ND |
| 28 | 15/15 | 16/16 | 5/18 | 9/9 | 12/12 |
| 42 | 7/18 | 18/18 | 2/18 | 11/12 | 12/12 |
| 60 | 0/18 | 10/12 | 6/12 | 3/12 | 9/12 |
mTSB-N, modified Trypticase soy broth (selective enrichment); BPW, buffered peptone water (nonselective enrichment). The results of three independent experiments are presented. ND, not done (>1 log10 CFU/g).
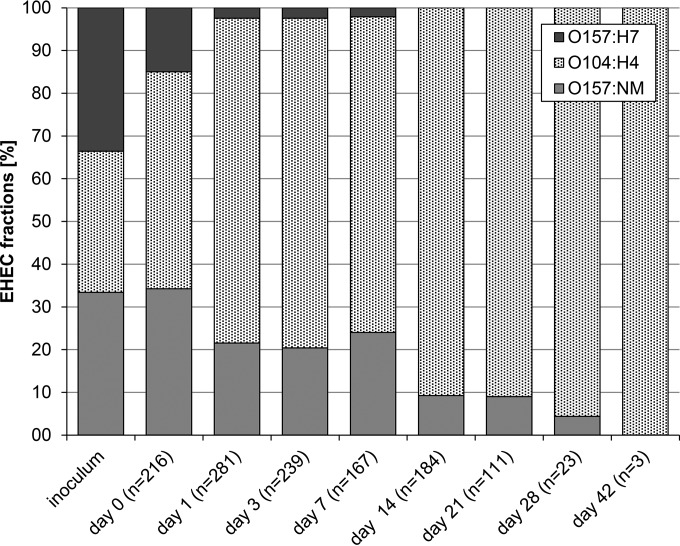
Sorbitol fermentation, β-lactamase production, and molecular detection of the virulence markers stx1, stx2, and eae were used to monitor strain persistence during the production and storage of fermented sausages that were inoculated with the cocktail of three different strains. The experiments were designed so that each of the EHEC strains occurred in the inoculum cocktail at the same level. After inoculation and adaption for 30 min in the food matrix, E. coli O157:H7, O104:H4, and O157:NM strains from the cocktail could be isolated on SMAC agar at incidences of 15%, 51%, and 34%, respectively, on day 0 (Fig. 2). During sausage fermentation, the proportional distribution of the individual strains initially inoculated as a cocktail shifted toward a remarkable dominance of E. coli O104:H4, with a decrease in the occurrence of the E. coli O157 serotypes. Only 2% of EHEC isolates on SMAC agar were NSF O157:H7 strains on days 1 to 7, and after 14 days, E. coli O157:H7 was undetectable (Fig. 2). Levels of E. coli O157:NM decreased from 34% to 4% within 28 days, and this serotype could not be detected on day 42. Quantitative detection was accomplished in individual samples on day 42, but only the E. coli O104:H4 strain could be identified (Fig. 2). BPW enrichment on day 60 produced positive results for 9 of 12 samples. Phenotypic and molecular analyses of the EHEC isolates from these samples matched only with the corresponding characteristics of the E. coli O104:H4 strain.
FIG 2.
Percentages of occurrence of E. coli O157:H7, O104:H4, and O157:NM in the EHEC cocktail used as an inoculum in fermented sausages. Homogenized sausage samples were spread on SMAC agar for quantitative enumeration of EHEC, and isolates were further characterized. Red sorbitol-fermenting isolates were cultivated on ESBL agar for the screening of β-lactamase activity. Further molecular traits (the presence or absence of the virulence markers stx1, stx2, and eae) were used for identification of the different EHEC strains. Values are averages for three independent experiments; n, number of isolates.
Fitness of E. coli O104:H4 under food-related laboratory conditions.
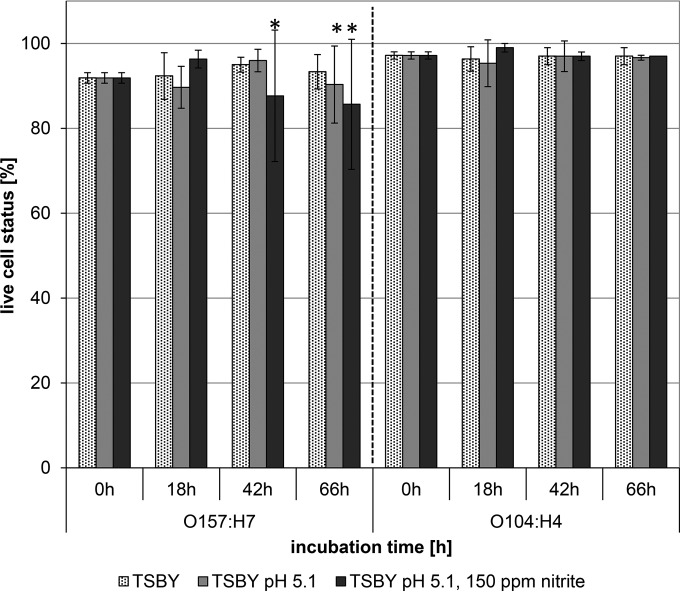
Raw sausage conditions—decreased pH, the presence of sodium nitrite, and moderate fermentation temperatures—were simulated in vitro. Cell viability was analyzed after 0, 18, 42, and 66 h of incubation at 22°C. E. coli O104:H4 appeared to be more viable than E. coli O157:H7, as indicated by larger proportions of live cells in all samples (97% ± 0.8% versus 93% ± 3.2% [P < 0.001]). In control (TSBY) and acidified (TSBY at pH 5.1) broth media, the viabilities of E. coli strain O157:H7 and O104:H4 cells were similar over time (P > 0.05). The addition of sodium nitrite led to significant differences in viability between E. coli O157:H7 and O104:H4 cells (P < 0.05) (Fig. 3). After 66 h of incubation, only 86% of E. coli O157:H7 cells were alive, while 97% of E. coli O104:H4 cells were alive (Fig. 3).
FIG 3.
Live-cell status of E. coli O157:H7 and E. coli O104:H4 under raw sausage conditions of decreased pH and the presence of sodium nitrite in vitro. Assays were performed either in Trypticase soy broth plus 0.5% yeast extract (TSBY) as a control, in TSBY adjusted to pH 5.1 with 20% (vol/vol) lactic acid (TSBY pH 5.1), or in TSBY at pH 5.1 with the addition of 150 mg/liter sodium nitrite (TSBY pH 5.1, 150 ppm NaNO2). After 0, 18, 42, and 66 h of incubation at 22°C, cell viabilities were assessed using the LIVE/DEAD BacLight viability kit according to the manufacturer's instructions. The numbers of dead, viable, and damaged cells were determined by counting red, green, and orange cells in a triplicate setup, and the percentages of live, dead, and damaged cells were calculated. Values are averages for three independent experiments. *, P < 0.05.
Bacteriocin production by E. coli O104:H4.
The inhibitory range of E. coli O104:H4 (E965) was determined with selected indicator strains of Enterobacteriaceae isolated from different sources (Table 1). The antibacterial substance produced had a narrow spectrum of activity. Inhibitory activities ranging from weak to strong could be observed only against strains of the E. coli species. Very strong activity was demonstrated against E. coli O157:H7 (ATCC 43895). The growth of other species of Enterobacteriaceae tested (Salmonella enterica subsp. enterica, Citrobacter freundii, Klebsiella oxytoca, and Hafnia spp.) was not affected by the presence of E. coli O104:H4 (E965).
In silico analysis of the published genome of E. coli O104:H4 strain 2011C-3493 (33) with the BAGEL bacteriocin mining tool indicated the existence of potential bacteriocin genes. Two genome areas of interest showed the presence of potential microcin and bottromycin gene loci. A GenBank database search indicated that these genes matched with genes encoding the bacteriocin microcin I47 (MccI47) (results not shown).
DISCUSSION
The beef salami manufactured in this study was representative of products found on the retail market, since the MAB/LAB counts and sausage aw values that were measured were comparable to those of freshly produced sausages. The commercial products, however, were determined to be slightly more acidic. These differences may be attributed to the use of a different starter culture, the presence of more fermentable sugar and acidifiers (e.g., lactic acid), or the use of smoke in the commercially obtained products.
During the fermentation of beef salami, the reduction rates for E. coli O157:H7, O157:NM, and O104:H4 strains and the EHEC cocktail with all three strains (0.89, 1.0, 0.86, and 0.75 log10 unit, respectively) did not differ significantly after 3 days of sausage fermentation. Rode et al. (23) showed that the average reduction in the counts of 11 different STEC strains, mainly outbreak strains, was approximately 0.8 log10 unit after 5 days in dry fermented sausage, with no statistically significant difference in the average reduction between isolates. Overall, the reductions in the counts of the three EHEC isolates during fermentation and maturation up to day 14 ranged from 1.4 to 1.7 log10 units, which were also comparable to the reductions in STEC/EHEC counts observed in other studies on dry fermented sausages (35–39). In our study, no strain-dependent differences in the reduction kinetics of the EHEC strains tested were evident, indicating that the combination of increasing acidity and reduced aw and redox potential (Eh), together with the presence of preservatives, such as nitrite, and competitive microbiota, was effective at reducing the EHEC load in fermented sausages and, conversely, preventing EHEC growth. Glass et al., comparing the survival of non-O157 STEC strains (O26, O45, O103, O111, O121, and O145 serovars) with that of E. coli O157:H7 strains during pepperoni production, showed that O103 and O157 strains exhibited the highest survival rates (40). Consequently, processes suitable for controlling E. coli O157 would also be applicable to the inactivation of other STEC strains tested in the study (40). Luchansky et al. (41) investigated the thermal stability of E. coli O104:H4 and other STEC strains in ground beef and showed that the cooking times and temperatures effective for inactivating serotype O157:H7 in ground beef were equally effective against the seven non-O157:H7 Shiga toxin-producing strains, including the O104:H4 strain. Our results indicate that E. coli O157:NM and E. coli O104:H4 are more resistant to raw sausage conditions than E. coli O157:H7, since the strains of the former two serovars could be detected by qualitative methods during the whole evaluation period, up to the end of the shelf life on day 60.
According to Rode et al. (23), the sorbitol-fermenting O157:NM outbreak strain showed the greatest ability to survive conditions in fermented sausage. Moreover, Alvarez-Ordóñez et al. (42) confirmed strong acid stress resistance especially for O157:NM strains. These strains are associated with a higher incidence of progression to HUS than E. coli O157:H7 strains and have emerged as important pathogens since their first isolation in Germany in 1998 (2–4). It is assumed that such increased resistance might be a key factor for the successful persistence of this clonal type of EHEC and for the serious pathogenicity of this strain in the host (42). Enhanced persistence may also account for the extraordinary virulence potential of the O104:H4 outbreak strain, as indicated by the large number of HUS cases and deaths in 2011. Markland et al. (24) detected low populations of E. coli O104:H4 strains on basil plants 10 days postinoculation, while E. coli O157:H7 was not found. This finding addresses the hypothesis that certain E. coli strains have evolved toward enhanced fitness in adverse environments and thus may indicate that these strains are better adapted to harsh environments (43). The results of our in vitro survival assays revealed significant differences in cell viability between E. coli O157:H7 and E. coli O104:H4, with 86% and 97% live cells in the presence of acidic nitrite, respectively, differences that also point to enhanced fitness of E. coli O104:H4 under food-related laboratory conditions.
The adaptation of E. coli O104:H4 to multiple adverse conditions occurring in the food matrix has been documented by only a few studies (25–27). A recent study showed increased resistance of the O104:H4 strain to high pressure after precultivation of the strain in nutrient broth at pH 5 and inactivation in carrot juice (pH 5.1) (27). A comparison between the growth kinetics values observed for E. coli O104:H4 in foods and those predicted for E. coli O157:H7 by using the U.S. Department of Agriculture Pathogen Modeling Program indicated that E. coli O104:H4 grows faster than E. coli O157:H7 in broth and in alfalfa and broccoli sprouts at 15°C (25). Yoo et al. (26) compared the growth characteristics of unstressed and stressed O157 or non-O157 STEC strains, including E. coli O104:H4 strains, in fresh produce. Their results suggested that sublethal osmotic, acid, or starvation stress may enhance the growth of non-O157 STEC strains on lettuce or cantaloupe, leading to a greater safety risk. Therefore, it is highly recommended to include non-O157 STEC strains in food risk assessments that previously addressed only E. coli O157:H7 and to use a STEC strain cocktail composed of several serotypes, including O104:H4. In order to account for differences in survival among strains, challenge studies should generally be conducted using three to five bacterial strains, either individually or in combination (44). However, results might be biased, reflecting the characteristics of the most resistant serotype in the strain cocktail (45). In our study, challenge experiments in fermented sausages were also performed with an EHEC strain cocktail consisting of equivalent cell counts of E. coli O157:H7, O157:NM, and O104:H4. The reduction rate of the EHEC strain cocktail up to day 14 was only 1 log10 unit, in contrast to counts of single EHEC strains that showed 1.4 to 1.7 log10 (P < 0.001) reduction rates. Differences of <0.5 log10 unit in counts at specific sampling points might result from analytic variabilities due to sampling and measurement errors. A difference of >0.5 log10 unit, however, is considered an appropriate criterion for relevant changes in counts (44). By comparing EHEC counts from strain cocktails with the single EHEC strain counts, differences of >0.5 log10 unit were detected for O157:H7 and O157:NM. The cell counts of the EHEC cocktail and E. coli O104:H4 differed by only 0.4 log10 unit, reflecting similar behaviors. Indeed, analysis of the composition of EHEC strains in the cocktail showed that E. coli O104:H4 was the dominant representative in all samples of fermented sausage over 42 days of quantitative detection. The dominance of a certain strain in an inoculation cocktail could be also shown by Kagkli et al. (46), who inoculated five strains of Listeria monocytogenes individually and as a cocktail in cheese. The strains did not show the same behavior when inoculated individually as when pooled. Specifically, one serotype 4b strain prevailed over the others, and strain interactions in the inoculation cocktail were more or less obvious (46). Levels of E. coli O157:H7 were already dramatically reduced shortly after inoculation into the fermented sausage. After 14 days, E. coli O157:H7 was undetectable, in contrast to the successful detection of O157:H7 until day 21 in single-strain inoculation experiments. The combination of biochemical trait comparisons and multiplex PCR allowed reliable differentiation of all EHEC strains isolated in this study.
Antagonism between strains in the EHEC cocktail may rely on the production of a bacteriocin(s) or other antimicrobial compounds. The inhibitory activity of E. coli O104:H4 (E965) was verified in a inhibition assay against several strains of Enterobacteriaceae from different sources, including E. coli, Salmonella enterica subsp. enterica, Citrobacter freundii, Klebsiella oxytoca, and Hafnia spp. Zones of inhibition were limited to the E. coli strains tested as sensitive indicator microorganisms, with noticeably strong activity occurring against an O157:H7 strain (EDL933). Bacteriocins are antimicrobial peptides that are generally active against bacteria closely related to the producer. E. coli is known to produce two types of bacteriocins, classified by their molecular masses into colicins (25 to 80 kDa) and microcins (10 kDa). The absence of colicin production distinguishes the outbreak strain of 2011 from the serotype O104:H4 (HUSEC41) strain isolated in the year 2001 (47). In silico analysis of the published genome of E. coli O104:H4 strain 2011C-3493 with the BAGEL automated bacteriocin mining tool, however, revealed the existence of presumptive bacteriocin genes with homology to microcin I47 (MccI47). MccI47 is part of the MccH47 genetic system, which contains all genes necessary for peptide production, posttranslational maturation, secretion, and immunity, as well as genes for a second antibacterial activity of microcin I47, the production of which is detected only under conditions of iron deprivation (48). A microcin gene cluster was previously identified in the E. coli O104:H4 German outbreak strain and is absent from the genome of its close relative E. coli strain 55989 (49). By using the newly developed GIST (genomic island identification by signals of transcription) method, Huang et al. (50) detected a genomic island, which included the microcin H47 system, in the 2011 German E. coli O104:H4 outbreak strain. Hence, it is assumed that the antibacterial activity of E. coli O104:H4 (E965) is mediated by microcins. Studies are in progress to identify the gene or gene cluster conferring potential bactericidal activity by constructing a genome library of E. coli O104:H4 (E965). Ongoing studies also seek to confirm gene function by transposon mutagenesis and to characterize the bacteriocin expressed. Previous studies have suggested that microcins act as fitness factors, which aid in successful competition with other bacteria during intestinal colonization, and contribute to the virulence of E. coli (51–53). Therefore, our results suggested that the secretion of bacteriocins detected in E. coli O104:H4 (E965) could be conducive to the high virulence of the O104:H4 outbreak strain.
This study demonstrated that the EHEC O157:NM and O104:H4 outbreak-associated strains survived longer than E. coli O157:H7 during the production and storage of fermented raw sausages. The results suggested, furthermore, that E. coli O104:H4 is well adapted to multiple adverse conditions encountered in fermented sausages or in food-related laboratory environments. Furthermore, secretion of potential bacteriocins by E. coli O104:H4 was indicated by the antagonism of this strain toward others in an EHEC strain cocktail, and this was verified in inhibition assays with several E. coli strains, including serotype O157 strains. Consequently, this study strongly suggests enhanced persistence and the possible production of bacteriocins as potential factors which, among others, could account for the increased virulence of the O104:H4 outbreak strain in Germany in 2011.
ACKNOWLEDGMENTS
This work was supported by the Federal Ministry of Education and Health within the Security Research Program in the “SiLeBAT” collaboration project (grant 13N11205).
We thank Regina Conrad (Bavarian Health and Food Safety Authority) for kindly providing EHEC outbreak strains, and we are grateful to Liane Weber, Gina Krappmann, Jörgen Dresel, and Helga Loske for technical assistance. Special thanks also go to Lisa Kaiser, Anna Feulner, and Ina Weber for their contributions.
REFERENCES
- 1.Karch H. 2001. The role of virulence factors in enterohemorrhagic Escherichia coli (EHEC)-associated hemolytic-uremic syndrome. Semin Thromb Hemost 27:207–213. doi: 10.1055/s-2001-15250. [DOI] [PubMed] [Google Scholar]
- 2.Ammon A, Petersen LR, Karch H. 1999. A large outbreak of hemolytic uremic syndrome caused by an unusual sorbitol-fermenting strain of Escherichia coli O157:H−. J Infect Dis 179:1274–1277. doi: 10.1086/314715. [DOI] [PubMed] [Google Scholar]
- 3.Karch H, Bielaszewska M. 2001. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157:H− strains: epidemiology, phenotypic and molecular characteristics, and microbiological diagnosis. J Clin Microbiol 39:2043–2049. doi: 10.1128/JCM.39.6.2043-2049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosser T, Dransfield T, Allison L, Hanson M, Holden N, Evans J, Naylor S, La Ragione R, Low JC, Gally DL. 2008. Pathogenic potential of emergent sorbitol-fermenting Escherichia coli O157:NM. Infect Immun 76:5598–5607. doi: 10.1128/IAI.01180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Food Safety Authority (EFSA). 2015. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J 13(1):3991. doi: 10.2903/j.efsa.2015.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielaszewska M, Mellmann A, Zhang W, Köck R, Fruth A, Bauwens A, Peters G, Karch H. 2011. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis 11:671–676. doi: 10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- 7.Tran SL, Billoud L, Lewis SB, Phillips AD, Schuller S. 2014. Shiga toxin production and translocation during microaerobic human colonic infection with Shiga toxin-producing E. coli O157:H7 and O104:H4. Cell Microbiol 16:1255–1266. doi: 10.1111/cmi.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchholz U, Bernard H, Werber D, Bohmer MM, Remschmidt C, Wilking H, Delere Y, an der Heiden M, Adlhoch C, Dreesman J, Ehlers J, Ethelberg S, Faber M, Frank C, Fricke G, Greiner M, Hohle M, Ivarsson S, Jark U, Kirchner M, Koch J, Krause G, Luber P, Rosner B, Stark K, Kuhne M. 2011. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med 365:1763–1770. doi: 10.1056/NEJMoa1106482. [DOI] [PubMed] [Google Scholar]
- 9.Hauri AM, Gotsch U, Strotmann I, Krahn J, Bettge-Weller G, Westbrock HJ, Bellinger O, Uphoff H. 2011. Secondary transmissions during the outbreak of Shiga toxin-producing Escherichia coli O104 in Hesse, Germany, 2011. Euro Surveill 16(31):pii=19937 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19937. [PubMed] [Google Scholar]
- 10.Cassar CA, Ottaway M, Paiba GA, Futter R, Newbould S, Woodward MJ. 2004. Absence of enteroaggregative Escherichia coli in farmed animals in Great Britain. Vet Rec 154:237–239. doi: 10.1136/vr.154.8.237. [DOI] [PubMed] [Google Scholar]
- 11.Nataro JP. 1998. Enteroaggregative Escherichia coli (EAEC). Alpe Adria Microbiol J 7:265–273. [Google Scholar]
- 12.Uber AP, Trabulsi LR, Irino K, Beutin L, Ghilardi ACR, Gomes TAT, Liberatore AMA, de Castro AF, Elias WP. 2006. Enteroaggregative Escherichia coli from humans and animals differ in major phenotypical traits and virulence genes. FEMS Microbiol Lett 256:251–257. doi: 10.1111/j.1574-6968.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 13.Werber D, Bielaszewska M, Frank C, Stark K, Karch H. 2011. Watch out for the even eviler cousin—sorbitol-fermenting E. coli O157. Lancet 377:298–299. doi: 10.1016/S0140-6736(11)60090-1. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Choi SJ. 2006. Isolation and characteristics of sorbitol-fermenting Escherichia coli O157 strains from cattle. Microbes Infect 8:2021–2026. doi: 10.1016/j.micinf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Sallam KI, Mohammed MA, Ahdy AM, Tamura T. 2013. Prevalence, genetic characterization and virulence genes of sorbitol-fermenting Escherichia coli O157:H− and E. coli O157:H7 isolated from retail beef. Int J Food Microbiol 165:295–301. doi: 10.1016/j.ijfoodmicro.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 16.King LA, Loukiadis E, Mariani-Kurkdjian P, Haeghebaert S, Weill FX, Baliere C, Ganet S, Gouali M, Vaillant V, Pihier N, Callon H, Novo R, Gaillot O, Thevenot-Sergentet D, Bingen E, Chaud P, de Valk H. 2014. Foodborne transmission of sorbitol-fermenting Escherichia coli O157:H7 via ground beef: an outbreak in northern France, 2011. Clin Microbiol Infect 20:O1136–O1144. doi: 10.1111/1469-0691.12736. [DOI] [PubMed] [Google Scholar]
- 17.Narváez-Bravo C, Echeverry A, Miller MF, Rodas-Gonzalez A, Brashears MT, Aslam M, Brashears MM. 2015. Virulence characterization and molecular subtyping of typical and atypical Escherichia coli O157:H7 and O157:H− isolated from fecal samples and beef carcasses in Mexico. J Food Prot 78:264–272. doi: 10.4315/0362-028X.JFP-14-348. [DOI] [PubMed] [Google Scholar]
- 18.Cabal A, Geue L, Gomez-Barrero S, Barth S, Barcena C, Hamm K, Porrero MC, Valverde A, Canton R, Menge C, Gortazar C, Dominguez L, Alvarez J. 2015. Detection of virulence-associated genes characteristic of intestinal Escherichia coli pathotypes, including the enterohemorrhagic/ enteroaggregative O104:H4, in bovines from Germany and Spain. Microbiol Immunol 59:433–442. doi: 10.1111/1348-0421.12275. [DOI] [PubMed] [Google Scholar]
- 19.Scientific Committee on Veterinary Measures relating to Public Health. 2003. Opinion of the Scientific Committee on Veterinary Measures relating to Public Health on verotoxigenic E. coli (VTEC) in foodstuffs. Health and Consumer Protection Directorate-General, European Commission, Brussels, Belgium. [Google Scholar]
- 20.Leistner L. 2000. Basic aspects of food preservation by hurdle technology. Int J Food Microbiol 55:181–186. doi: 10.1016/S0168-1605(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 21.Sartz L, De Jong B, Hjertqvist M, Plym-Forshell L, Asterlund R, Loefdahl S, Osterman B, Stahl A, Eriksson E, Hansson HB, Karpman D. 2008. An outbreak of Escherichia coli O157:H7 infection in southern Sweden associated with consumption of fermented sausage; aspects of sausage production that increase the risk of contamination. Epidemiol Infect 136:370–380. doi: 10.1017/S0950268807008473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gareis M, Pichner R, Brey N, Steinrueck H. 2000. Shedding of verotoxigenic E. coli by healthy staff of a food producing company. Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz 43:781–787. doi: 10.1007/s001030050357. [DOI] [Google Scholar]
- 23.Rode TM, Holck A, Axelsson L, Høy M, Heir E. 2012. Shiga toxigenic Escherichia coli show strain dependent reductions under dry-fermented sausage production and post-processing conditions. Int J Food Microbiol 155:227–233. doi: 10.1016/j.ijfoodmicro.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Markland SM, Shortlidge KL, Hoover DG, Yaron S, Patel J, Singh A, Sharma M, Kniel KE. 2013. Survival of pathogenic Escherichia coli on basil, lettuce, and spinach. Zoonoses Public Health 60:563–571. doi: 10.1111/zph.12033. [DOI] [PubMed] [Google Scholar]
- 25.Juneja VK, Mukhopadhyay S, Ukuku D, Hwang CA, Wu VC, Thippareddi H. 2014. Interactive effects of temperature, pH, and water activity on the growth kinetics of Shiga toxin-producing Escherichia coli O104:H4. J Food Prot 77:706–712. doi: 10.4315/0362-028X.JFP-13-387. [DOI] [PubMed] [Google Scholar]
- 26.Yoo BK, Liu Y, Juneja V, Huang L, Hwang C-A. 2015. Growth characteristics of Shiga toxin-producing Escherichia coli (STEC) stressed by chlorine, sodium chloride, acid, and starvation on lettuce and cantaloupe. Food Control 55:97–102. doi: 10.1016/j.foodcont.2015.02.040. [DOI] [Google Scholar]
- 27.Reineke K, Sevenich R, Hertwig C, Janssen T, Frohling A, Knorr D, Wieler LH, Schluter O. 2015. Comparative study on the high pressure inactivation behavior of the Shiga toxin-producing Escherichia coli O104:H4 and O157:H7 outbreak strains and a non-pathogenic surrogate. Food Microbiol 46:184–194. doi: 10.1016/j.fm.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 28.EFSA Panel on Biological Hazards. 2015. Public health risks associated with enteroaggregative Escherichia coli (EAEC) as a food-borne pathogen. EFSA J 13(12):4330. doi: 10.2903/j.efsa.2015.4330. [DOI] [Google Scholar]
- 29.Fellner L, Huptas C, Simon S, Mühlig A, Scherer S, Neuhaus K. 2016. Draft genome sequences of three European laboratory derivatives from enterohemorrhagic Escherichia coli O157:H7 strain EDL933, including two plasmids. Genome Announc 4(2):e01331-15. doi: 10.1128/genomeA.01331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch H, Fuchs M. 2009. Die Fabrikation feiner Fleisch- und Wurstwaren, 23rd ed, p 143–152 Deutscher Fachverlag GmbH, Frankfurt am Main, Germany. [Google Scholar]
- 31.Pavlovic M, Huber I, Skala H, Konrad R, Schmidt H, Sing A, Busch U. 2010. Development of a multiplex real-time polymerase chain reaction for simultaneous detection of enterohemorrhagic Escherichia coli and enteropathogenic Escherichia coli strains. Foodborne Pathog Dis 7:801–808. doi: 10.1089/fpd.2009.0457. [DOI] [PubMed] [Google Scholar]
- 32.Bigwood T, Hudson JA, Cooney J, McIntyre L, Billington C, Heinemann JA, Wall F. 2012. Inhibition of Listeria monocytogenes by Enterococcus mundtii isolated from soil. Food Microbiol 32:354–360. doi: 10.1016/j.fm.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed SA, Awosika J, Baldwin C, Bishop-Lilly KA, Biswas B, Broomall S, Chain PSG, Chertkov O, Chokoshvili O, Coyne S, Davenport K, Detter JC, Dorman W, Erkkila TH, Folster JP, Frey KG, George M, Gleasner C, Henry M, Hill KK, Hubbard K, Insalaco J, Johnson S, Kitzmiller A, Krepps M, Lo CC, Luu T, McNew LA, Minogue T, Munk CA, Osborne B, Patel M, Reitenga KG, Rosenzweig CN, Shea A, Shen XH, Strockbine N, Tarr C, Teshima H, van Gieson E, Verratti K, Wolcott M, Xie G, Sozhamannan S, Gibbons HS, Threat Characterization Consortium . 2012. Genomic comparison of Escherichia coli O104:H4 isolates from 2009 and 2011 reveals plasmid, and prophage heterogeneity, including Shiga toxin encoding phage stx2. PLoS One 7:e48228. doi: 10.1371/journal.pone.0048228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Jong A, van Hijum S, Bijlsma JJE, Kok J, Kuipers OP. 2006. BAGEL: a web-based bacteriocin genome mining tool. Nucleic Acids Res 34:W273–W279. doi: 10.1093/nar/gkl237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calicioglu M, Faith NG, Buege DR, Luchansky JB. 2002. Viability of Escherichia coli O157:H7 during manufacturing and storage of a fermented, semidry soudjouk-style sausage. J Food Prot 65:1541–1544. [DOI] [PubMed] [Google Scholar]
- 36.Heir E, Holck AL, Omer MK, Alvseike O, Høy M, Måge I, Axelsson L. 2010. Reduction of verotoxigenic Escherichia coli by process and recipe optimisation in dry-fermented sausages. Int J Food Microbiol 141:195–202. doi: 10.1016/j.ijfoodmicro.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Holck AL, Axelsson L, Rode TM, Høy M, Måge I, Alvseike O, L'Abée-Lund TM, Omer MK, Granum PE, Heir E. 2011. Reduction of verotoxigenic Escherichia coli in production of fermented sausages. Meat Sci 89:286–295. doi: 10.1016/j.meatsci.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Porto-Fett ACS, Call JE, Shoyer BE, Hill DE, Pshebniski C, Cocoma GJ, Luchansky JB. 2010. Evaluation of fermentation, drying, and/or high pressure processing on viability of Listeria monocytogenes, Escherichia coli O157:H7, Salmonella spp., and Trichinella spiralis in raw pork and Genoa salami. Int J Food Microbiol 140:61–75. doi: 10.1016/j.ijfoodmicro.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Hinkens JC, Faith NG, Lorang TD, Bailey P, Buege D, Kaspar CW, Luchansky JB. 1996. Validation of pepperoni processes for control of Escherichia coli O157:H7. J Food Prot 59:1260–1266. [DOI] [PubMed] [Google Scholar]
- 40.Glass KA, Kaspar CW, Sindelar JJ, Milkowski AL, Lotz BM, Kang J, Faith NG, Enache E, Kataoka AI, Henry C. 2012. Validation of pepperoni process for control of Shiga toxin-producing Escherichia coli. J Food Prot 75:838–846. doi: 10.4315/0362-028X.JFP-11-486. [DOI] [PubMed] [Google Scholar]
- 41.Luchansky JB, Porto-Fett AC, Shoyer BA, Phillips J, Eblen D, Evans P, Bauer N. 2013. Thermal inactivation of a single strain each of serotype O26:H11, O45:H2, O103:H2, O104:H4, O111:H−, O121:H19, O145:NM, and O157:H7 cells of Shiga toxin-producing Escherichia coli in wafers of ground beef. J Food Prot 76:1434–1437. doi: 10.4315/0362-028X.JFP-12-429. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez-Ordóñez A, Alvseike O, Omer MK, Heir E, Axelsson L, Holck A, Prieto M. 2013. Heterogeneity in resistance to food-related stresses and biofilm formation ability among verocytotoxigenic Escherichia coli strains. Int J Food Microbiol 161:220–230. doi: 10.1016/j.ijfoodmicro.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 43.van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. 2012. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci U S A 109:1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Advisory Committee on Microbiological Criteria for Foods. 2010. Parameters for determining inoculated pack/challenge study protocols. J Food Prot 73:140–202. [DOI] [PubMed] [Google Scholar]
- 45.Juneja VK, Marks HM, Huang LH. 2003. Growth and heat resistance kinetic variation among various isolates of Salmonella and its application to risk assessment. Risk Anal 23:199–213. doi: 10.1111/1539-6924.00300. [DOI] [PubMed] [Google Scholar]
- 46.Kagkli DM, Iliopoulos V, Stergiou V, Lazaridou A, Nychas GJ. 2009. Differential Listeria monocytogenes strain survival and growth in Katiki, a traditional Greek soft cheese, at different storage temperatures. Appl Environ Microbiol 75:3621–3626. doi: 10.1128/AEM.01571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert Koch Institut (RKI). 2011. Bakteriologische Untersuchungen im Rahmen des Ausbruchs mit E. coli O104:H4. Epidemiol Bull 35:325–329. https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2011/Ausgaben/35_11.pdf?__blob=publicationFile. [Google Scholar]
- 48.Azpiroz MF, Bascuas T, Lavina M. 2011. Microcin H47 system: an Escherichia coli small genomic island with novel features. PLoS One 6:e26179. doi: 10.1371/journal.pone.0026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohde H, Qin JJ, Cui YJ, Li DF, Loman NJ, Hentschke M, Chen WT, Pu F, Peng YQ, Li JH, Xi F, Li SH, Li Y, Zhang ZX, Yang XW, Zhao MR, Wang P, Guan YL, Cen Z, Zhao XN, Christner M, Kobbe R, Loos S, Oh J, Yang L, Danchin A, Gao GF, Song YJ, Li YR, Yang HM, Wang J, Xu JG, Pallen MJ, Wang J, Aepfelbacher M, Yang RF; E. coli O104:H4 Genome Analysis Crowd-Sourcing Consortium . 2011. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med 365:718–724. doi: 10.1056/NEJMoa1107643. [DOI] [PubMed] [Google Scholar]
- 50.Huang QL, Cheng XJ, Cheung MK, Kiselev SS, Ozoline ON, Kwan HS. 2012. High-density transcriptional initiation signals underline genomic islands in bacteria. PLoS One 7:e33759. doi: 10.1371/journal.pone.0033759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, Dobrindt U. 2004. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol 186:5432–5441. doi: 10.1128/JB.186.16.5432-5441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budič M, Rijavec M, Petkovšek Z, Zgur-Bertok D. 2011. Escherichia coli bacteriocins: antimicrobial efficacy and prevalence among isolates from patients with bacteraemia. PLoS One 6:e28769. doi: 10.1371/journal.pone.0028769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azpiroz MF, Poey ME, Lavina M. 2009. Microcins and urovirulence in Escherichia coli. Microb Pathog 47:274–280. doi: 10.1016/j.micpath.2009.09.003. [DOI] [PubMed] [Google Scholar]