ABSTRACT
Modification of teichoic acid through the incorporation of d-alanine confers resistance in Gram-positive bacteria to antimicrobial peptides (AMPs). This process involves the products of the dltXABCD genes. These genes are widespread in Gram-positive bacteria, and they are also found in a few Gram-negative bacteria. Notably, these genes are present in all soft-rot enterobacteria (Pectobacterium and Dickeya) whose dltDXBAC operons have been sequenced. We studied the function and regulation of these genes in Dickeya dadantii. dltB expression was induced in the presence of the AMP polymyxin. It was not regulated by PhoP, which controls the expression of some genes involved in AMP resistance, but was regulated by ArcA, which has been identified as an activator of genes involved in AMP resistance. However, arcA was not the regulator responsible for polymyxin induction of these genes in this bacterium, which underlines the complexity of the mechanisms controlling AMP resistance in D. dadantii. Two other genes involved in resistance to AMPs have also been characterized, phoS and phoH. dltB, phoS, phoH, and arcA but not dltD mutants were more sensitive to polymyxin than the wild-type strain. Decreased fitness of the dltB, phoS, and phoH mutants in chicory leaves indicates that their products are important for resistance to plant AMPs.
IMPORTANCE Gram-negative bacteria can modify their lipopolysaccharides (LPSs) to resist antimicrobial peptides (AMPs). Soft-rot enterobacteria (Dickeya and Pectobacterium spp.) possess homologues of the dlt genes in their genomes which, in Gram-positive bacteria, are involved in resistance to AMPs. In this study, we show that these genes confer resistance to AMPs, probably by modifying LPSs, and that they are required for the fitness of the bacteria during plant infection. Two other new genes involved in resistance were also analyzed. These results show that bacterial resistance to AMPs can occur in bacteria through many different mechanisms that need to be characterized.
INTRODUCTION
Antimicrobial peptides (AMPs) are defense molecules produced by animals and plants and are part of their innate immune systems. These peptides show wide diversity in size, sequence, structure, and antimicrobial mechanisms (1). Most AMPs are positively charged, and their first interaction with bacteria involves the negatively charged components of the bacterial surface, namely, lipopolysaccharides (LPS) for Gram-negative bacteria and teichoic acids (TA) for Gram-positive bacteria. Bacteria have evolved inducible mechanisms for preventing these interactions by modifying their surface charge. In Gram-negative bacteria, this mechanism has been studied thoroughly in Salmonella enterica (2, 3). These modifications target mainly the lipid A domain of LPS. The principal modifications are the addition of 4-aminoarabinose (4-NAra) by the arnB operon products, addition of phosphoethanolamine by EptA, hydroxylation of lipid A acyl chains by LpxO, and acylation or deacylation of lipid A by PagP or PagL, respectively (3). The addition of 4-NAra and phosphoethanolamine is a modification commonly found in proteobacteria, whereas the others are specific to individual bacterial species. For example, resistance of the Vibrio cholerae O1 El Tor biotype to polymyxin is a result of the remodeling of lipid A by the addition of glycine or diglycine. The classical O1 biotype, which is sensitive to polymyxin, cannot perform this modification (4).
In Gram-positive bacteria, modifications conferring resistance to AMP occur in TA by the esterification of phosphate with alanine. This reaction requires the products of at least four of the proteins encoded by the dltXABCD operon (5). DltA catalyzes the adenylation of d-alanine and transfers the activated amino acid to the d-alanyl carrier protein DltC (6, 7). DltB is an inner-membrane protein. It has been suggested that it might be involved in the transport of alanine out of the cell through a lipid-linked intermediate, which has not yet been detected (8). The function of DltD is still less clear. It is anchored by a single transmembrane segment in the membrane, but several experiments identified different locations for the soluble part of the protein, either in the cytoplasm or outside the cell (8, 9). No role in TA alanylation has yet been ascribed to dltX.
Dickeya dadantii is a plant pathogenic bacterium that is responsible for the soft-rot disease of many plants of agricultural interest (10). It was also shown recently that these bacteria can kill some species of insects (11, 12). Like most Enterobacteriaceae, D. dadantii possesses genes that are required to modify LPSs in response to AMPs, such as the arnB operon and eptA. An arnB mutant is less pathogenic to the pea aphid Acyrthosiphon pisum, showing that modification of their LPSs by 4-NAra confers resistance to animal AMPs (13). In S. enterica, the expression of all the genes involved in resistance to AMPs is controlled directly or indirectly (through PmrA-PmrB) by the two-component regulator system PhoP-PhoQ (2). In D. dadantii, expression of arnB is induced by the AMPs polymyxin and protamine. PhoP-PhoQ is required for the induction of arnB by protamine but not for induction by polymyxin, indicating that in D. dadantii, at least two different regulatory systems are involved in sensing AMPs and regulating AMP resistance genes (13).
In addition to the genes involved in resistance to AMPs that are commonly found in Gram-negative bacteria, D. dadantii contains homologues to the dlt genes expressed by Gram-positive bacteria. These genes are found in a few proteobacteria, such as Bordetella pertussis (14) and Photorhabdus luminescens (15). However, they are present in all the sequenced genomes of Dickeya and Pectobacterium spp., which are different genera of plant pathogenic enterobacteria. We suspected that the presence of these genes is related to the necrotrophic lifestyle of these bacteria having to contend with plant AMPs. In this work, we studied the regulation of dlt gene expression in D. dadantii isolates and analyzed the role that these genes play during plant infection. Moreover, we identified new genes that are involved in resistance of D. dadantii to AMPs.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains, phages, plasmids, and oligonucleotides used in this study are described in Table 1. D. dadantii and Escherichia coli cells were grown at 30 and 37°C, respectively, in LB medium or M63 minimal medium supplemented with a carbon source (0.2% [wt/vol]). When required, antibiotics were added at the following concentrations: ampicillin, 100 mg · liter−1; kanamycin and chloramphenicol, 25 mg · liter−1; and gentamicin, 20 mg · liter−1. Media were solidified with 1.5% (wt/vol) agar. Transduction with phage ΦEC2 was performed according to Résibois et al. (16).
TABLE 1.
Bacterial strains and plasmids used in the study
| Strain, plasmid, or oligonucleotide | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Dickeya dadantii strain 3937 | ||
| A350a | rafR ganB | 42 |
| A3092 | gmd::uidA Kanr | 29 |
| A4194 | rafR ganB phoP::Cmr | 13 |
| A5256 | rafR ganB arnB::uidA Kanr | 13 |
| A5394 | rafR ganB dltB::uidA Kanr | 3 |
| A5537 | rafR ganB arcA::Cmr | This work |
| A5632 | rafR ganB phoH::uidA Kanr | This work |
| A5749 | rafR ganB phoS::lacZ Kanr | This work |
| A5823 | rafR ganB dltD::uidA Kanr | This work |
| Plasmids | ||
| pGEM-T | PRC cloning vector | Promega |
| pBBR1-MCS5 | Gmr | 43 |
| Oligonucleotides | ||
| phoSfor | GTAGCGCACATTCATCTTGCC | |
| phoSrev | GGATAAGCGCCACACCAGAC | |
| arcAfor | CCCAAAGCGCCCGAGAACC | |
| arcArev | CTGGCGCATGATAAGCGCGC | |
| phoHfor | TGTCGTTTTTGCAGTGAAGTGTCC | |
| phoHrev | ATTAACACCGCCATCAACCAGATG | |
| alldltfor | GGAAGCTTTACCGTCTGGTGTGGCGC | |
| alldltrev | CCGAATTCGATGTTCTTCCTAACCGCG |
Strain A350 is a rafR and ganB mutant of strain 3937 devoid of β-galactosidase activity.
Mutant construction.
To construct strain A5749 that contains a phoS-lacZ fusion, a 1.4-kb DNA fragment containing phoS was amplified with the primers phoSfor and phoSrev. The resulting fragment was inserted into the pGEM-T plasmid (Promega). A lacZ Kanr cassette was inserted into the unique BamHI site of phoS. To construct strain A5537 that contains an arcA Cmr mutation, an 800-bp DNA fragment containing arcA was amplified with the primers arcAfor and arcArev. The resulting fragment was inserted into the pGEM-T plasmid. A Cmr cassette was inserted into the unique EcoRI site of arcA. To construct strain A5632 that contains a phoH-uidA fusion, a 1.4-kb DNA fragment containing phoH was amplified with the primers phoHfor and phoHrev. The resulting fragment was inserted into the pGEM-T plasmid. A uidA Kanr cassette was inserted into the unique AgeI site of phoS. To construct strain A5823 that contains a dltD-uidA fusion, a 4.2-kb DNA fragment containing the dlt operon was amplified with the primers alldltfor and alldltrev. This fragment was digested with EcoRI and HindIII and inserted into the plasmid pUC19, which was digested by the same enzymes. The resulting plasmid was digested with EcoRI and NcoI, treated with Klenow enzyme, and ligated to give plasmid td10delNE1. A uidA Cmr cassette was inserted into the unique SalI site of this plasmid. All the constructs were recombined into the D. dadantii chromosome according to Roeder and Collmer (17). All recombinations were checked by PCR.
Molecular biology techniques.
To construct the D. dadantii gene library, chromosomal DNA was partially digested with Sau3A. Fragments larger than 4 kb were purified and inserted into the plasmid pBBR-MCS-5, digested with BamHI, and then dephosphorylated.
Enzymatic assays.
β-Glucuronidase assays were performed on toluenized extracts of cells grown to exponential phase using the method of Bardonnet and Blanco (18) with p-nitrophenyl-β-d-glucuronate as the substrate. β-Galactosidase assays were performed on toluenized extracts of cells that were grown to exponential phase using the method of Miller with o-nitrophenyl-β-d-galactose as the substrate.
Purification and detection of LPS.
LPSs were extracted following the isobutyric acid-1 M ammonium hydroxide method (19). They were further purified with an enzymatic treatment to remove DNA, RNA, and proteins, as previously described (20). Phospholipid and lipoprotein contaminants were also extracted with a mixture of solvents, chloroform/methanol/water (30:15:2.5). Lipid A was obtained by direct hydrolysis of the lyophilized bacteria (21). Briefly, 10 mg of lyophilized bacteria was suspended in 400 μl of isobutyric acid and 1 M ammonium hydroxide (5:3 [vol/vol]), heated for 2 h at 100°C with stirring, cooled to 4°C, and then centrifuged. The supernatant was diluted with water (1:1 [vol/vol]) and lyophilized. The material obtained was then washed twice with 400 μl of methanol and centrifuged (2,000 × g for 15 min). Finally, the insoluble lipid A was extracted once in a 100- to 200-μl volume of the mixture of solvents, as mentioned previously.
To analyze LPSs by Western blotting, bacterial components were separated using 12% SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane. To detect LPS, the membrane was incubated with anti-KdgM antibody (22) at a dilution of 1/10,000 in TBS-Tween (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20) followed by incubation with horseradish peroxidase (HRP)-coupled secondary antibody (Sigma) at a dilution of 1/20,000 in TBS-Tween.
Antimicrobial peptide resistance assays.
Stationary phase-grown cultures of D. dadantii strains were diluted 103-fold in LB medium. One milliliter of the dilution was placed in a 1.5-ml Eppendorf tube, and polymyxin was added. After a 1-h incubation at room temperature, bacteria were diluted and added onto LB plates. Colonies were counted after 2 days of growth at 30°C.
Coinoculation experiments.
To determine the competitivity index, the wild-type (WT) and mutant strains undergoing testing, marked with an antibiotic resistance gene, were grown overnight in M63 medium with glycerol. Bacteria were washed in M63 medium, and the optical density (OD) was adjusted to 1.0. Bacteria were mixed to a 1:1 ratio, and 10 μl of the mixture was inoculated into a hole in a chicory leaf that was made with a pipet tip. The hole was covered with mineral oil, and the leaf was incubated at 30°C with high humidity. After 24 h, the macerated tissue was collected, homogenized, diluted in M63 medium, and spread onto LB plates with or without antibiotic. After 48 h, the numbers of colonies were counted. The competitivity index is the bacterial ratio (number of mutant bacteria/number of WT bacteria) in the rotten tissue/(number of mutant bacteria/number of WT bacteria) in the inoculum.
RESULTS
D. dadantii encodes homologues of Gram-positive bacterial dlt genes.
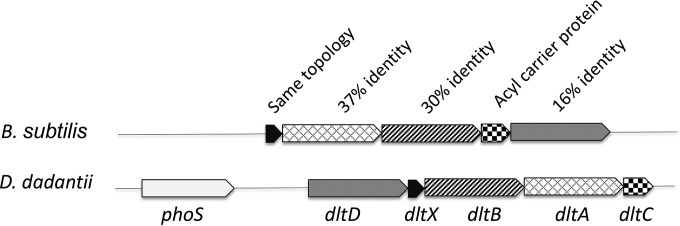
A group of four adjacent genes (ABF-16058, ABF-19383, ABF-19382, and ABF-19381) in the annotated genome of D. dadantii strain 3937 presents homology with Gram-positive dlt genes involved in the alanylation of TA and resistance to AMPs (Fig. 1). A homology search in GenBank revealed that the protein encoded by ABF-16058 is homologous to DltD of Lactobacillus brevis (E value, 9e–81, 16% identity), the protein encoded by ABF-19383 is homologous to Bacillus subtilis strain 168 DltB (E value, 6.5e–41, 30% identity), and the protein encoded by ABF-19382 is homologous to Bacillus subtilis strain 168 DltA (E value, 5e–87, 37% identity). No homology was detected between ABF-19381 and a Gram-positive bacterial DltC protein, but an Interproscan analysis found that it belongs to the same acyl carrier protein family as DltC proteins of Gram-positive bacteria. Although the functions of the products of these four genes are obviously different from those in Gram-positive bacteria since there is no TA in Gram-negative bacteria, we nevertheless conserved the same gene nomenclature. The four dltDBAC genes are present in a few proteobacteria; they can be found in all soft-rotting enterobacteria (Dickeya and Pectobacterium spp.) whose genomes have been sequenced, in Enterobacter, and in a few strains of Cedecea, Achromobacter, Photobacterium, and Bordetella. A fifth gene, dltX, is the first gene of the dltXABCD operon in Gram-positive bacteria. dltX encodes a small protein of 48 amino acids of unknown function. Topological prediction indicates that DltX might be anchored in the inner membrane with the C-terminal domain facing the outer medium. In D. dadantii, a small open reading frame (ORF) located between dltD and dltC encodes a 39-amino-acid protein with the DltX topology that might be functionally equivalent. The genetic organization of the five genes with no or very short intergenic regions (i.e., dltD, -X, -B, -A, and -C) suggests that they may form an operon. Thus, the D. dadantii dlt operon formed by the dltDXBAC genes contains genes similar to those found in Gram-positive bacteria (Fig. 1).
FIG 1.
Organization of dlt operons in Bacillus subtilis 168 and Dickeya dadantii 3937. Homology between equivalent proteins is indicated.
The gene immediately upstream of dltD, ABF-16056, is oriented in the same direction as the dlt genes and separated by 572 bp that do not contain an additional ORF. It was characterized in another study as PhoP regulated (23). Thus, similar to many PhoP-regulated genes, it might be involved in the resistance to AMP. We named it phoS. It encodes a protein of 133 amino acids that possesses a lipoprotein signal sequence. The second amino acid of the processed protein is a threonine, suggesting that PhoS is anchored in the outer membrane. This protein has no homologue of known function. phoS is not present in all Dickeya strains, and when it is present, it is not in the same genetic context as is D. dadantii strain 3937. The gene phoS can be found in other bacteria, such as Pantoea ananatis, Xenorhabdus bovienii, Providencia stuartii, and Klebsiella oxytoca. However, in any given species, its presence is strain dependent. Cooccurrence of phoS with dlt genes is rare. The lower G+C content of the phoS gene (38%) compared to that of the D. dadantii genome (57%) suggests that it might have been acquired recently.
Regulation of the dltDXBAC and phoS genes.
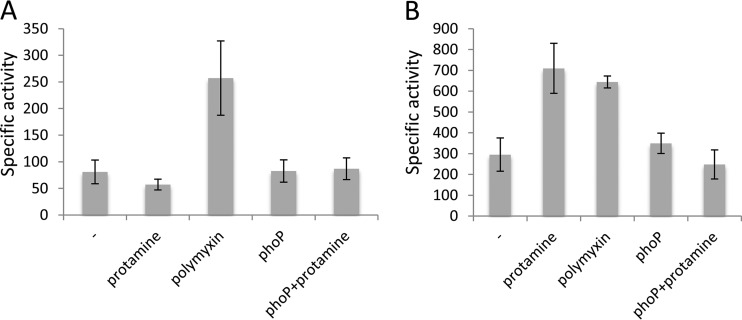
Genes involved in resistance to AMPs are often induced by AMPs (13). To analyze the transcription of dltDXBAC and phoS, we constructed transcriptional fusions of dltB and phoS. Expression of these fusions was assayed in the presence of two AMPs, polymyxin and protamine, an inducer of genes regulated by PhoP (13). Expression of dltB was induced 3-fold by polymyxin but not by protamine. Its expression was not modified in a phoP mutant (Fig. 2A). phoS expression was induced by polymyxin and also by protamine. As expected, phoS expression was no longer induced by protamine in a phoP background (Fig. 2B). Expression of dltB and phoS was induced by a common inducer, polymyxin, but the regulators responsible for this induction have not yet been identified.
FIG 2.
Regulation of dltB and phoS. The dltB-uidA (A) and phoS-lacZ (B) fusions of strains A5394 and A5749, respectively, were assayed in the presence of protamine or polymyxin in a phoP background. Activities shown are the mean values ± standard deviations (SDs) from at least four separate experiments and are expressed in micromoles of o-nitrophenol or p-nitrophenol produced per minute and per milligram of bacterial dry weight.
arcA activates genes involved in resistance to AMPs.
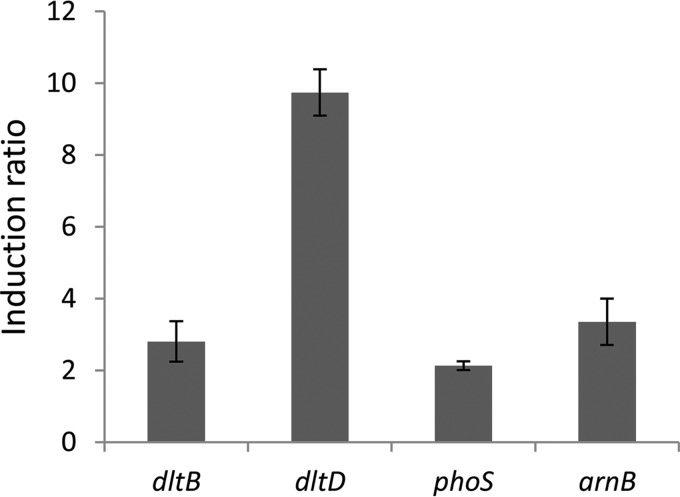
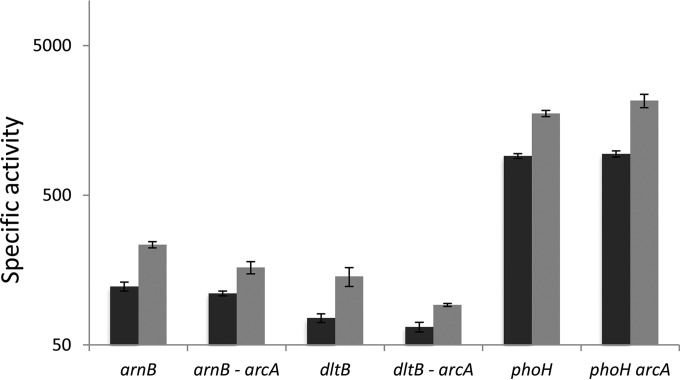
To identify genes controlling dlt gene expression, we introduced a D. dadantii gene library into strain A5403 containing a dltB-lacZ transcriptional fusion. Transformants were selected on a medium containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and darker- or lighter-blue clones were used to identify any mutants that overexpressed or underexpressed the fusion. Among about 20,000 transformants, two dark-blue colonies were found. Analysis of their plasmid content found that they contained the arcA gene. In Escherichia coli, ArcA is the response regulator of the two-component regulatory system ArcA-ArcB that regulates genes in response to changes in the respiratory and fermentative state of the cell (24, 25). A plasmid bearing arcA was introduced into strains containing reporter-gene fusions with dltB, dltD, phoS, and arnB. The presence of this plasmid induced the expression of all these genes from 2-fold (phoS) to 10-fold (dltD), indicating that it can act a general activator of genes involved in resistance to AMPs (Fig. 3). Since all these genes are induced by polymyxin, we supposed that this compound might be detected by the sensor interacting with ArcA, ArcB. Surprisingly, arcB is a pseudogene in D. dadantii 3937, which is not the case in the other D. dadantii strains sequenced. Overexpression of a response regulator can activate the target genes even in the absence of a sensor protein because of nonspecific phosphorylation of the regulator. However, there might be cross talk between ArcA and a sensor responding to polymyxin. Thus, we tested whether polymyxin induced arnB and dltB in an arcA background. The basal level of expression of the fusion was unchanged. Induction by polymyxin still occurred, albeit at a lower level, probably because the double mutants were very sensitive to the drug, which inhibits their growth (Fig. 4). Thus, induction by polymyxin does not seem to occur through ArcA.
FIG 3.
Activation of genes involved in AMP resistance by arcA. Plasmid pGEM-T or pGEM-T-arcA was introduced into strains containing a dltB-uidA, dltD-uidA, phoS-lacZ, or arnB-uidA transcriptional fusion. The ratios of expression in strains with pGEM-T-arcA versus those in strains with pGEM-T are shown. Values represent the means ± SDs from at least four separate experiments.
FIG 4.
arcA is not involved in induction by polymyxin. Fusions in the indicated genes in the WT or arcA background were assayed in the absence (dark bars) or presence (gray bars) of 5 μg/ml polymyxin. Values represent the means ± SDs from at least four separate experiments. All results comparing gene expression in the presence or absence of polymyxin for each strain were statistically different by the Wilcoxon test (P < 0.05).
To confirm the independence between the regulation by arcA and the induction by polymyxin, we looked for a gene controlled by arcA which would not be induced by polymyxin. A study of the targets of ArcA in E. coli revealed that phoH possesses 4 ArcA binding sites and that its expression is strongly modulated by this regulator (26). PhoH has an ATP-binding site but its function in the cell is unknown (27). It was never identified among genes involved in AMP resistance in previous studies. We constructed the D. dadantii phoH-uidA reporter strain A5632. When we introduced a plasmid bearing arcA in this strain, we observed that phoH was neither induced nor repressed by ArcA, indicating that its regulation is different from that in E. coli. Surprisingly, phoH expression was induced 2-fold by polymyxin, even in an arcA background (Fig. 4). This result confirms that regulation by arcA and polymyxin are independent. The addition of protamine did not modify phoH expression, which indicates that it is not regulated by PhoP (data not shown).
dltB and phoS mutations modify LPSs.
While trying to find a function for the dlt operon, we analyzed the presence of the outer-membrane protein KdgM by Western blotting with dlt mutants. We noticed that the lanes containing WT strain extracts presented a background smear that was absent from the lanes containing dltB mutant samples (Fig. 5). KdgM is an outer-membrane protein involved in the import of oligogalacturonates (28). The KdgM antibody was prepared with the protein extracted from the outer-membrane fraction of an overproducing D. dadantii strain (22). Thus, the protein might have been contaminated with LPS. The high immunogenicity of LPS can result in a serum containing antibodies against KdgM, LPS, and more specifically, the O-antigen. To check this hypothesis, we tested highly purified D. dadantii LPSs with this antibody and obtained a strong signal (Fig. 5, lane 9). We then tested for the presence of the O-antigen in other mutants with the KdgM antibody. No signal was detected in the gmd mutant, which lacks the O-antigen (29), or with the phoS mutant. The dltD, phoH, and arnB mutants reacted the same as the wild-type strain did. The arcA mutant behaved like the dltB mutant, i.e., no O-chain was detected, in agreement with its role as an activator of dltB and phoS expression. Thus, the dltB, phoS, and arcA mutants lack the O-chain of LPS. An analysis by SDS-PAGE of the membrane protein content of the dltB, phoS and arcA mutants did not enable us to detect any difference in the membrane composition (data not shown).
FIG 5.
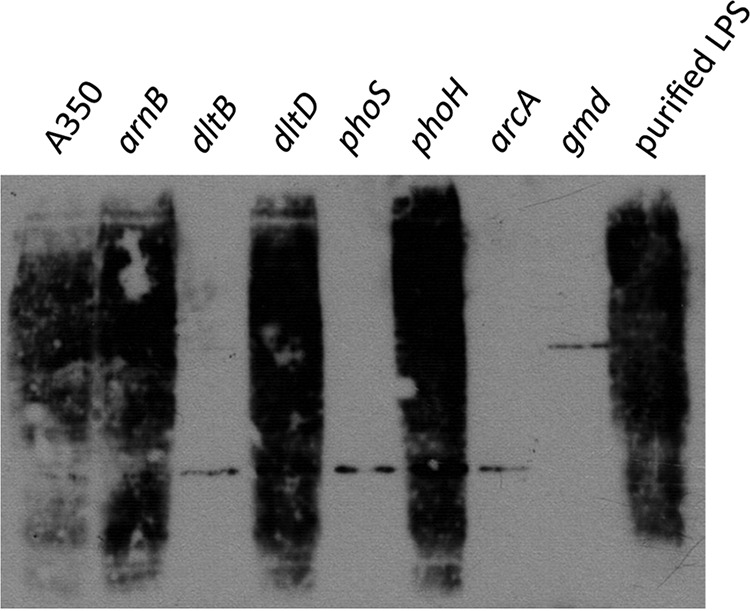
Analysis of the LPS of various mutants. Whole cells from different strains (lane 1, A350; lane 2, A5256; lane 3, A5394; lane 4, A5823; lane 5, A5749; lane 6, A5632; lane 7, A5537; lane 8, A3092) or purified LPS from strain A5256 was separated by SDS-PAGE and blotted onto a PVDF membrane. LPS was revealed with anti-KdgM antibodies.
dltB, phoS, arcA, and phoH are involved in resistance to AMPs.
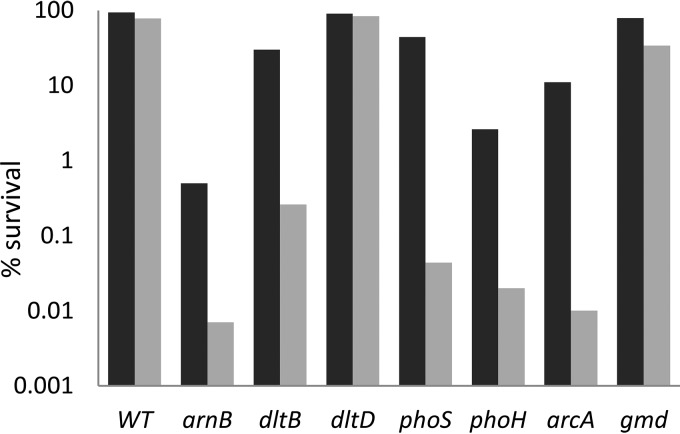
To investigate whether all the genes identified in this study are involved in resistance to AMPs, we performed a survival test involving a 1-h exposure to 1 or 10 μg/ml polymyxin. The arnB mutant, which cannot perform 4-NAra addition on LPS, was the most affected with a more than 104-fold reduction in viability at the highest dose (Fig. 6). The dltB and the phoS mutants were also affected but to a lesser degree. To determine if the absence of the LPS O-chain observed in these mutants was the reason for their sensitivity to polymyxin, we tested a gmd mutant that cannot synthesize the O-antigen (29). This mutant was only weakly sensitive to polymyxin, indicating that a loss of the O-antigen is not the cause of susceptibility to polymyxin in the dltB and phoS mutants. The arcA mutant was among the most strongly affected, which is not surprising since it regulates several genes involved in the resistance to AMPs, including the arnB gene. Finally, the phoH mutant was also more sensitive to the drug than the wild-type strain. A role of phoH in resistance to AMPs has not been described previously. However, induction of the phoH gene by polymyxin and increased sensitivity of the mutant to this drug suggest that its function might be related to AMP resistance in D. dadantii.
FIG 6.
Survival of various mutants against polymyxin. Wild-type and various mutants in genes involved in resistance to AMPs (arnB, dltB, dltD, phoS, phoH, arcA, and gmd) were incubated in the presence of 1 μg/ml (dark bars) or 10 μg/ml (gray bars) polymyxin for 1 h. Samples were diluted and plated onto LB agar plates to assess bacterial viability. Survival values are relative to the original inocula. This experiment was repeated three times. The trends found in the three independent experiments were the same, but variations prevented statistical analysis. Results of a representative experiment are shown.
dltB and phoS are required for fitness of D. dadantii in chicory leaves.
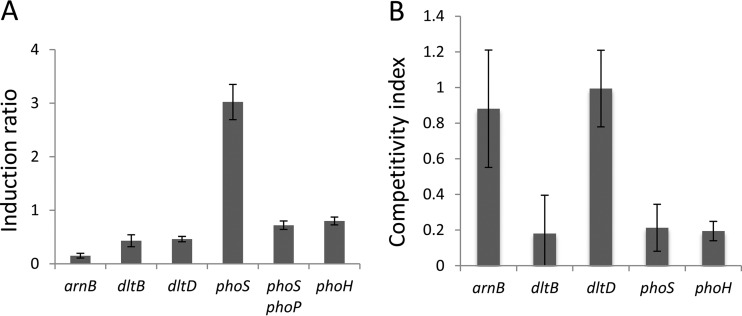
Since the dlt operon is found in all the sequenced Dickeya and Pectobacterium strains, we thought that the presence of dltB and phoS might be related to the plant pathogen lifestyle of these bacteria. First, we examined the induction of the genes involved in AMP resistance when bacteria were grown in minimal medium in the presence of chicory chips to mimic plant infection. While expression of most of the genes tested was unaffected (phoH) or repressed (arnB, dltB, and dltD) under this condition, that of phoS was induced 3-fold in a phoP-dependent manner (Fig. 7A).
FIG 7.
Competitivity of various mutants in plant infection. (A) Induction of fusions in genes involved in resistance to AMP in the presence of plant chips. Bacteria were grown in M63 medium with glycerol in the absence or presence of chicory chips. Values are the ratios of the activity of the fusion under induced conditions versus those under noninduced conditions. Values are the means ± SDs from at least four separate experiments. (B) Competitivity indexes of various mutants in chicory leaf infections. The wild-type and mutant strains were inoculated in a 1/1 ratio in a chicory leaf. After 24 h, rotten tissue was collected, homogenized, and diluted, and the numbers of bacteria were counted. The competitivity index is the ratio (number of mutant bacteria/number of WT bacteria) in the rotten tissue/(number of mutant bacteria/number of WT bacteria) in the inoculum. Values are the averages ± standard deviations of data from at least five infected leaves.
To determine the fitness of the mutants in plants, we coinfected chicory leaves with a 1:1 mixture of the WT and mutant strains. After 24 h, the ratio of the two strains in the rotten tissue was determined to calculate a competitivity index. To verify that the mutants had no growth defect, controls were grown in M63 medium containing glycerol. For all strains, the competitivity index was around 1 after 10 generations. The multiplication of dltD and arnB mutants was not significantly affected in the plant. In contrast, the dltB, phoS, and phoH mutants were counterselected, indicating that the products of these genes are important for the survival of the bacteria during plant infection (Fig. 7B).
DISCUSSION
While the role of the dlt operon in modifying TA in Gram-positive bacteria is well documented (8), the function of these genes in the small number of Gram-negative bacteria in which they have been detected is still unclear. The D. dadantii dlt operon encodes five proteins that present sequence or structural homology with their Gram-positive counterparts. DltA is a protein that might activate and ligate an amino acid to the carrier protein DltC. The activated amino acid, which is alanine in Gram-positive bacteria, has not been conclusively determined in Gram-negative bacteria. It has been suggested that it might also be alanine in B. pertussis (14). However, in V. cholerae, a group of three alm genes confers resistance to AMPs by adding glycine to LPS. The initial steps of this process require the homologues DltA and AlmE and the homologues DltC and AlmF, which activate glycine (4). Thus, a similar mechanism can be used to incorporate various amino acids. The role of DltB is unclear. It is a member of the membrane-bound O-acyl transferase (MBOAT) family of proteins that transfer organic acids onto the hydroxyl groups of membrane-embedded components. Sequence homology between the proteins in Gram-positive bacteria and D. dadantii suggests that this multimembrane-spanning protein might have the same function in binding the amino acid to its substrate. The third V. cholerae protein required to add glycine to LPS, AlmG, is also an inner-membrane protein, but it has no homology with DltB, suggesting that there is a different target or mechanism for modifying its substrate (4). A mutant with deletion of the four B. pertussis dra (dlt) genes shows an increased sensitivity to AMP, but a specific role of the individual genes has not been tested (14). The function of DltD is unknown, even in Gram-positive bacteria. A D. dadantii dltB mutant is more sensitive to polymyxin than the wild-type strain, but in contrast, a dltD mutant is as resistant as the wild-type strain. The absence of this gene does not seem to prevent the modification that confers resistance to AMPs. However, it might have a subtle role that was not detected by our test. Mutations that we constructed in dltD are polar and should have inactivated the downstream genes of the same operon. Since the dltD mutant does not have the dltB polymyxin-sensitivity phenotype, an additional promoter is probably present in front of dltB, allowing expression of dltBAC independent of that of dltD. In V. cholerae, only three genes are required to add glycine to LPS, and no homologue of DltD has been detected. The complete dlt operon of Gram-positive bacteria may have been transferred to Gram-negative bacteria, but dltBAC alone might be sufficient to confer AMP resistance in these organisms. Attempts to identify the substrate of the B. pertussis dra genes indicate the presence of unidentified outer-membrane proteins (14). Nevertheless, we favor a hypothesis where the modified substrate is LPS, since polymyxin binds to the lipid A region of LPS (30). The dltB and phoS mutants have no O-antigen chain. This absence is not the cause of their sensitivity to AMP, since a gmd mutant is not sensitive to polymyxin. However, for an unknown reason, these two phenotypes are linked and point to LPS as a target of the dlt genes.
Several induction circuits control the genes involved in AMP resistance in D. dadantii. Induction by protamine occurs through PhoP-PhoQ. arnB, dltB, and phoS expression was induced in the presence of polymyxin, although the regulator involved in this control mechanism has not been characterized. In an attempt to discover this regulator, we identified arcA as an activator of these genes. The ArcA-ArcB two-component system has been characterized in E. coli. It controls gene expression in response to changes in the respiratory and fermentative states of the cell (26). However, it may regulate genes that have a function other than in redox metabolism, since Liu et al. (31) showed that 9% of the E. coli genes are differentially expressed in an arcA mutant. This regulation might not be direct. Park et al. (26) found that only 85 of the 229 regulons controlled by arcA that they identified possess ArcA binding sites. In E. coli, the sensor ArcB detects the oxidation state of quinones. arcB is a pseudogene in D. dadantii, and the redox state of the cell cannot be a signal for genes regulated by arcA. arcA-regulated genes are not induced or repressed by anaerobiosis (data not shown). Cross talk between noncognate pairs of sensors and regulators has been demonstrated. ArcA could be phosphorylated by another sensor, and thus respond to a different signal than anaerobiosis (32). The identity of the sensor and whether the regulation of genes involved in AMP resistance by arcA is direct remain to be established.
The role of PhoH in the resistance to AMPs was found fortuitously during this study. While looking for an arcA-regulated gene, we discovered that phoH expression is induced by polymyxin, that a phoH mutant has an increased sensitivity to this AMP, and that it was outcompeted by the WT strain in a coinoculation experiment in chicory leaves. The phoH gene was first described in E. coli as a phosphate starvation-induced gene, but its role in phosphate metabolism has not been explained (33). Homologues of PhoH are present in many organisms, and they can be classified into three groups. The first group, which is present in most bacteria, is proposed to be functionally linked to phospholipid metabolism and RNA modification. Proteins of the second group, which are present in aerobes, are members of fatty acid beta-oxidation regulons. The third group is specific to enterobacteria. PhoH homologues have been found to be auxiliary metabolic genes of phages with a high prevalence in marine phage genomes, and phoH was used to examine phage diversity in the marine environment (34, 35). PhoH shows ATP-binding activity and may be an ATPase. Moreover, when fused to a PilT N-terminal domain in PhoH2 proteins, it possesses ATPase and RNA helicase activity (33, 36). Thus, PhoH proteins seem to be very versatile, and their ATPase activity may have been recruited to provide energy in various processes. In D. dadantii for example, it might be the modification of a component of the cell envelope that confers resistance to AMPs.
In addition to modifications found in many bacteria, such as the addition of 4-aminoarabinose or phosphoethanolamine, other modifications seem to exist more sporadically in bacteria. The presence of GlcN has been described on phosphate groups of Bordetella bronchiseptica lipid A (37) and on B. pertussis (38), along with most of the species of this genus. It has not been described for other genera. This substitution of the Bordetella lipid A phosphate groups with GlcN was shown to confer Bordetella resistance to AMPs (39).
Screens to find genes involved in resistance to cationic AMPs or polymyxin identified 10 genes in Yersinia pestis and 41 loci in Pseudomonas aeruginosa, respectively (40, 41). Although some of these genes encode functions related to membrane biogenesis or changes that can modify resistance to AMPs, the modes of action of others have not been determined. Moreover, some of these genes do not confer a general resistance to AMPs but to only one of them (40). phoS is found only in a few bacteria and seems to have been acquired recently through lateral transfer by D. dadantii. Its absence confers sensitivity to polymyxin, and its expression is regulated by PhoP. Its induction by the plant and the reduced fitness of the mutant in plants suggest that its product may be required for resistance to plant AMPs. PhoS localization in the outer membrane indicates that it can modify LPSs, since lipid A modification systems are usually located in the extracytoplasmic compartment of bacteria (4).
Coinoculation experiments in chicory leaves identified three genes required for the fitness of D. dadantii, phoS, dltB, and phoH. arnB, which is important for the survival of the bacteria in insects (13), seems to play a minor role in plants. The presence in D. dadantii of dlt genes, rarely found in Gram-negative bacteria but present in all soft-rot enterobacteria, and of phoS may be due to the phytopathogenic lifestyle of the bacteria. These genes may protect the bacteria against plant AMPs. A role for some genes in the protection against one class of AMPs has already been described, but it has never been related to the host of the bacterium (30, 40).
ACKNOWLEDGMENTS
Stephanie Le Moullec (IGM, Université de Paris-Sud) is acknowledged for technical assistance for LPS extraction, and Valerie James is acknowledged for correcting the manuscript.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Yeaman MR, Yount NY. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Gunn JS. 2008. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol 16:284–290. doi: 10.1016/j.tim.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Needham BD, Trent MS. 2013. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat Rev Microbiol 11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. 2012. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in Gram-positive and Gram-negative bacteria. Proc Natl Acad Sci U S A 109:8722–8727. doi: 10.1073/pnas.1201313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, Fischer W. 1995. Incorporation of d-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J Biol Chem 270:15598–15606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- 6.Heaton MP, Neuhaus FC. 1994. Role of the d-alanyl carrier protein in the biosynthesis of d-alanyl-lipoteichoic acid. J Bacteriol 176:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debabov DV, Heaton MP, Zhang Q, Stewart KD, Lambalot RH, Neuhaus FC. 1996. The d-alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC. J Bacteriol 178:3869–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichmann NT, Cassona CP, Grundling A. 2013. Revised mechanism of d-alanine incorporation into cell wall polymers in Gram-positive bacteria. Microbiology 159:1868–1877. doi: 10.1099/mic.0.069898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debabov DV, Kiriukhin MY, Neuhaus FC. 2000. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J Bacteriol 182:2855–2864. doi: 10.1128/JB.182.10.2855-2864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charkowski A, Blanco C, Condemine G, Expert D, Franza T, Hayes C, Hugouvieux-Cotte-Pattat N, Lopez Solanilla E, Low D, Moleleki L, Pirhonen M, Pitman A, Perna N, Reverchon S, Rodriguez Palenzuela P, San Francisco M, Toth I, Tsuyumu S, van der Walls J, van der Wolf J, Van Gijsegem F, Yang CH, Yedidia I. 2012. The role of secretion systems and small molecules in soft-rot Enterobacteriaceae pathogenicity. Annu Rev Phytopathol 50:425–449. doi: 10.1146/annurev-phyto-081211-173013. [DOI] [PubMed] [Google Scholar]
- 11.Grenier AM, Duport G, Pages S, Condemine G, Rahbe Y. 2006. The phytopathogen Dickeya dadantii (Erwinia chrysanthemi 3937) is a pathogen of the pea aphid. Appl Environ Microbiol 72:1956–1965. doi: 10.1128/AEM.72.3.1956-1965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costechareyre D, Balmand S, Condemine G, Rahbe Y. 2012. Dickeya dadantii, a plant pathogenic bacterium producing Cyt-Like entomotoxins causes septicemia in the pea aphid Acyrthosiphon pisum. PLoS One 7:e30702. doi: 10.1371/journal.pone.0030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costechareyre D, Chich JF, Strub JM, Rahbe Y, Condemine G. 2013. Transcriptome of Dickeya dadantii infecting Acyrthosiphon pisum reveals a strong defense against antimicrobial peptides. PLoS One 8:e54118. doi: 10.1371/journal.pone.0054118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taneja NK, Ganguly T, Bakaletz LO, Nelson KJ, Dubey P, Poole LB, Deora R. 2013. d-Alanine modification of a protease-susceptible outer membrane component by the Bordetella pertussis dra locus promotes resistance to antimicrobial peptides and polymorphonuclear leukocyte-mediated killing. J Bacteriol 195:5102–5111. doi: 10.1128/JB.00510-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abi Khattar Z, Rejasse A, Destoumieux-Garzon D, Escoubas JM, Sanchis V, Lereclus D, Givaudan A, Kallassy M, Nielsen-Leroux C, Gaudriault S. 2009. The dlt operon of Bacillus cereus is required for resistance to cationic antimicrobial peptides and for virulence in insects. J Bacteriol 191:7063–7073. doi: 10.1128/JB.00892-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Résibois A, Colet M, Faelen M, Schoonejans E, Toussaint A. 1984. phiEC2, a new generalized transducing phage of Erwinia chrysanthemi. Virology 137:102–112. doi: 10.1016/0042-6822(84)90013-8. [DOI] [PubMed] [Google Scholar]
- 17.Roeder DL, Collmer A. 1985. Marker-exchange mutagenesis of a pectate lyase isozyme gene in Erwinia chrysanthemi. J Bacteriol 164:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardonnet N, Blanco C. 1991. Improved vectors for transcriptional signal screening in corynebacteria. FEMS Microbiol Lett 68:97–102. [DOI] [PubMed] [Google Scholar]
- 19.Caroff M. June 2004. Novel method for isolating endotoxins. French patent WO 2004062690 A1.
- 20.Basheer SM, Bouchez V, Novikov A, Augusto LA, Guiso N, Caroff M. 2016. Structure activity characterization of Bordetella petrii lipid A, from environment to human isolates. Biochimie 120:87–95. doi: 10.1016/j.biochi.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 21.El Hamidi A, Tirsoaga A, Novikov A, Hussein A, Caroff M. 2005. Microextraction of bacterial lipid A: easy and rapid method for mass spectrometric characterization. J Lipid Res 46:1773–1778. doi: 10.1194/jlr.D500014-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Blot N, Berrier C, Hugouvieux-Cotte-Pattat N, Ghazi A, Condemine G. 2002. The oligogalacturonate-specific porin KdgM of Erwinia chrysanthemi belongs to a new porin family. J Biol Chem 277:7936–7944. doi: 10.1074/jbc.M109193200. [DOI] [PubMed] [Google Scholar]
- 23.Rio-Alvarez I, Rodriguez-Herva JJ, Cuartas-Lanza R, Toth I, Pritchard L, Rodriguez-Palenzuela P, Lopez-Solanilla E. 2012. Genome-wide analysis of the response of Dickeya dadantii 3937 to plant antimicrobial peptides. Mol Plant Microbe Interact 25:523–533. doi: 10.1094/MPMI-09-11-0247. [DOI] [PubMed] [Google Scholar]
- 24.Georgellis D, Kwon O, Lin EC. 2001. Quinones as the redox signal for the arc two-component system of bacteria. Science 292:2314–2316. doi: 10.1126/science.1059361. [DOI] [PubMed] [Google Scholar]
- 25.Rolfe MD, Ter Beek A, Graham AI, Trotter EW, Asif HM, Sanguinetti G, de Mattos JT, Poole RK, Green J. 2011. Transcript profiling and inference of Escherichia coli K-12 ArcA activity across the range of physiologically relevant oxygen concentrations. J Biol Chem 286:10147–10154. doi: 10.1074/jbc.M110.211144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park DM, Akhtar MS, Ansari AZ, Landick R, Kiley PJ. 2013. The bacterial response regulator ArcA uses a diverse binding site architecture to regulate carbon oxidation globally. PLoS Genet 9:e1003839. doi: 10.1371/journal.pgen.1003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazakov AE, Vassieva O, Gelfand MS, Osterman A, Overbeek R. 2003. Bioinformatics classification and functional analysis of PhoH homologs. In Silico Biol 3:3–15. [PubMed] [Google Scholar]
- 28.Hutter CA, Lehner R, Wirth C, Condemine G, Peneff C, Schirmer T. 2014. Structure of the oligogalacturonate-specific KdgM porin. Acta Crystallogr D Biol Crystallogr 70:1770–1778. doi: 10.1107/S1399004714007147. [DOI] [PubMed] [Google Scholar]
- 29.Touze T, Goude R, Georgeault S, Blanco C, Bonnassie S. 2004. Erwinia chrysanthemi O antigen is required for betaine osmoprotection in high-salt media. J Bacteriol 186:5547–5550. doi: 10.1128/JB.186.16.5547-5550.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández L, Alvarez-Ortega C, Wiegand I, Olivares J, Kocincova D, Lam JS, Martinez JL, Hancock RE. 2013. Characterization of the polymyxin B resistome of Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:110–119. doi: 10.1128/AAC.01583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, De Wulf P. 2004. Probing the ArcA-P modulon of Escherichia coli by whole genome transcriptional analysis and sequence recognition profiling. J Biol Chem 279:12588–12597. doi: 10.1074/jbc.M313454200. [DOI] [PubMed] [Google Scholar]
- 32.Groban ES, Clarke EJ, Salis HM, Miller SM, Voigt CA. 2009. Kinetic buffering of cross talk between bacterial two-component sensors. J Mol Biol 390:380–393. doi: 10.1016/j.jmb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SK, Makino K, Amemura M, Shinagawa H, Nakata A. 1993. Molecular analysis of the phoH gene, belonging to the phosphate regulon in Escherichia coli. J Bacteriol 175:1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldsmith DB, Crosti G, Dwivedi B, McDaniel LD, Varsani A, Suttle CA, Weinbauer MG, Sandaa RA, Breitbart M. 2011. Development of phoH as a novel signature gene for assessing marine phage diversity. Appl Environ Microbiol 77:7730–7739. doi: 10.1128/AEM.05531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldsmith DB, Parsons RJ, Beyene D, Salamon P, Breitbart M. 2015. Deep sequencing of the viral phoH gene reveals temporal variation, depth-specific composition, and persistent dominance of the same viral phoH genes in the Sargasso Sea. PeerJ 3:e997. doi: 10.7717/peerj.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews ES, Arcus VL. 2015. The mycobacterial PhoH2 proteins are type II toxin antitoxins coupled to RNA helicase domains. Tuberculosis (Edinb) 95:385–394. doi: 10.1016/j.tube.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Marr N, Tirsoaga A, Blanot D, Fernandez R, Caroff M. 2008. Glucosamine found as a substituent of both phosphate groups in Bordetella lipid A backbones: role of a BvgAS-activated ArnT ortholog. J Bacteriol 190:4281–4290. doi: 10.1128/JB.01875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marr N, Hajjar AM, Shah NR, Novikov A, Yam CS, Caroff M, Fernandez RC. 2010. Substitution of the Bordetella pertussis lipid A phosphate groups with glucosamine is required for robust NF-kappaB activation and release of proinflammatory cytokines in cells expressing human but not murine Toll-like receptor 4-MD-2-CD14. Infect Immun 78:2060–2069. doi: 10.1128/IAI.01346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah NR, Hancock RE, Fernandez RC. 2014. Bordetella pertussis lipid A glucosamine modification confers resistance to cationic antimicrobial peptides and increases resistance to outer membrane perturbation. Antimicrob Agents Chemother 58:4931–4934. doi: 10.1128/AAC.02590-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J, Nair MK, Galvan EM, Liu SL, Schifferli DM. 2011. Tn5AraOut mutagenesis for the identification of Yersinia pestis genes involved in resistance towards cationic antimicrobial peptides. Microb Pathog 51:121–132. doi: 10.1016/j.micpath.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutu AD, Sgambati N, Strasbourger P, Brannon MK, Jacobs MA, Haugen E, Kaul RK, Johansen HK, Hoiby N, Moskowitz SM. 2013. Polymyxin resistance of Pseudomonas aeruginosa phoQ mutants is dependent on additional two-component regulatory systems. Antimicrob Agents Chemother 57:2204–2215. doi: 10.1128/AAC.02353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hugouvieux-Cotte-Pattat N, Robert-Baudouy J. 1985. Lactose metabolism in Erwinia chrysanthemi. J Bacteriol 162:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]