Abstract
Background/Aims
Glypican-3 (GPC3) protein is highly expressed in hepatocellular carcinoma (HCC) tissue. It has been suggested as a diagnostic biomarker, but its inconsistent performance means that it requires further assessment. We therefore investigated the diagnostic value of the plasma GPC3 level compared to the alpha-fetoprotein (AFP) level as a diagnostic biomarker of HCC.
Methods
We enrolled 157 consecutive patients with newly diagnosed HCC and 156 patients with liver cirrhosis (LC) as the control group. GPC3 plasma levels were measured using two commercially available enzyme-linked immunosorbent assays (ELISAs, named as Assay 1 and 2), and AFP levels were measured using an enzyme-linked chemiluminescent immunoassay. The diagnostic accuracy was analyzed using the receiver operating characteristics (ROC) curve.
Results
Plasma GPC3 levels in HCC patients were very low (0–3.09 ng/mL) in Assay 1, while only 3 of the 157 patients (1.9%) showed detectable GPC3 levels in Assay 2. The median GPC3 level was not significantly elevated in the HCC group (0.80 ng/mL) compared with the LC group (0.60 ng/mL). The area under the ROC curve (AUC) for GPC3 was 0.559 in Assay 1. In contrast, the median AFP level was significantly higher in HCC (27.72 ng/mL) than in LC (4.74 ng/mL), with an AUC of 0.729.
Conclusion
The plasma level of GPC3 is a poor diagnostic marker for HCC, being far inferior to AFP. The development of a consistent detection system for the blood level of GPC3 is warranted.
Keywords: Alpha-fetoprotein; Biomarkers; Diagnosis; Glypican-3; Hepatocellular carcinoma; Sensitivity, Specificity
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 80-90% of liver cancer, which is the 6th most frequent cancer and the third leading cause of all cancer related deaths [1,2].
While typical diagnostic methods for HCC include imaging methods and histological analysis, in the former it is difficult to detect and differentiate small nodules, whereas the latter is invasive and holds high-risk, especially in patients with cirrhotic backgrounds. Blood alpha-fetoprotein (AFP) levels have been used as a marker for HCC diagnosis and to supplement radiological findings. The Asian Pacific Association for the Study of the Liver guidelines recommend AFP as a tumor marker for HCC with a cutoff value at 200 ng/mL in addition to radiological examinations [3]. However, the current guidelines of the American Association for the Study of Liver Disease and the European Association for the Study of the Liver have excluded AFP based on its limited accuracy [4]. AFP has moderate sensitivity for HCC, but lacks specificity as it is elevated in benign hepatic condition including cirrhosis and hepatitis [5]. Therefore, additional biomarkers that are HCC-specific and -sensitive require investigation.
The heparin sulfate proteoglycan glypican-3 (GPC3) is a member of the glypican family and is bound to the membrane through a glycophosphatidylinositol (GPI) anchor [6]. It is highly expressed in HCC cells and promotes HCC proliferation through the canonical Wnt pathway, and therapeutic methods that target GPC3 are currently being investigated [6-8]. However, GPC3 elevation in the sera of HCC patients is less conclusive. Initial studies reported high sensitivity of GPC3 as a specific biomarker for HCC [5,9]. Nonetheless, subsequent studies have led to inconsistent results, questioning plasma GPC3’s value as a diagnostic biomarker [6]. The aim of this study was to address this concern by assessing and comparing the diagnostic accuracy of GPC3 and AFP plasma level in a South Korean HCC population.
PATIENTS AND METHODS
Patients
The study design was a cross sectional, retrospective, case-control study including 157 HCC patients and a control group of 156 liver cirrhosis (LC) patients at the Seoul National University Bundang Hospital (Seongnam, Republic of Korea) from August 2006 through September 2013. This study was approved by the Seoul National University Bundang Hospital’s Institutional Review Board (IRB, B-1307/210-006). Informed consent was confirmed by the IRB and received from all HCC patients. LC plasma samples were derived from repository samples, and thus informed consent was waived by the IRB.
HCC was diagnosed by histological and imaging findings outlined by American Association for the Study of Liver Disease guidelines and staged by the Barcelona Clinic Liver Cancer (BCLC) system. Patients with double primary cancer in non-liver organs or those who underwent intent-to-cure treatment (resection, liver transplantation, radiofrequency ablation, transarterial chemoembolization, or percutaneous ethanol injection) were excluded from this study. LC was diagnosed by histological examination or by one or more clinical findings of portal hypertension: 1) cirrhotic appearance of the liver with splenomegaly on imaging studies (ultrasonography, computed tomography or magnetic resonance image), 2) thrombocytopenia (platelet < 120,000/mm3), 3) presence of esophagogastric varices on endoscopy, 4) presence of ascites, and 5) presence of portosystemic encephalopathy. LC patients for whom the presence of HCC was not evaluated within six months before enrollment were excluded.
Sample storage and measurement of AFP and GPC3
Blood samples were collected from HCC patients before curative treatment and from LC patients at the time of clinic visit. Plasma aliquots were stored at -70o C until time of measurement. Plasma AFP was measured using an automated enzyme-linked chemiluminescent immunoassay (ELICA). Plasma GPC3 levels were assessed using two different commercially available enzyme-linked immunosorbent assay (ELISA) kits named as Assay 1 and 2 (Assay 1: USCN Life Science Inc., Wuhan, China and Assay 2: R&D Systems, Inc., Minneapolis, MN, USA), which were compared in Table 1. Tests were performed in accordance to manufacturer’s instructions and in duplicates by two experienced researchers (YSC and YJ). In Assay 2, a sample dilution of 1:1 did not yield results within the range studied. Sample dilutions (1:1, 1:10, 1:50, 1:100, 1:500, and 1:1000) showed that 1:100 yielded consistent and peak GPC3 values. This dilution factor was therefore used for all samples.
Table 1.
Descriptions of Assay 1 and Assay 2 used to measure plasma GPC3 levels
| Category | Assay 1 | Assay 2 |
|---|---|---|
| Manufacturer | USCN Life Science Inc | R&D Systems, Inc. |
| Capture antibody | Monoclonal mouse | Monoclonal mouse |
| Detection antibody | Polyclonal rabbit | Polyclonal sheep |
| Plate | Pre-coated with capture antibody | Manual capture antibody coating |
| Incubation temperature | 37°C | Room temperature |
| Biotin binding protein | Avidin | Streptavidin |
| Wash | Aspirate but no wash between adding sample and detecting antibody | Aspirate and wash between adding sample and detecting antibody |
| Detection range | 0.156 ng/mL – 10 ng/mL | 0.312 ng/mL – 20 ng/mL |
| Sample dilution factor | 1:1 | 1:100 |
ELISA, enzyme-linked immunosorbent assay.
Statistical analysis
Pearson’s χ2 (categorical variables), Student’s independent t-test (continuous parametric variables), and Mann-Whitney U test (continuous nonparametric variables) were used to compare HCC and LC groups. Significance was defined as P<0.05. Receiver operating characteristic (ROC) curve and area under the curve (AUC) analyses were performed to compare diagnostic accuracies of GPC3 and AFP. The maximum sum of sensitivity and specificity on the ROC curve determined the GPC3 cut-off level. All statistical analyses were performed via SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Characteristics of HCC patients and control group of cirrhosis patients
The clinical characteristics of the study population (157 HCC and 156 LC) were summarized in Table 2. The HCC group had a higher proportion of males and was older than the LC control group. In both groups, hepatitis B virus (HBV) was the most common etiology (67.5% in HCC and 74.4% in LC). Among the HCC patients, 6.4% and 87.3% had underlying liver diseases of chronic hepatitis and liver cirrhosis, respectively. There were no significant differences in Child-Pugh class between the two groups. BCLC staging was 0 in 13.4%, A in 35.7%, B in 7.0%, C in 39.5% and D in 4.5% of the HCC patients. Major portal vein invasion was observed in 20.4% of the HCC patients.
Table 2.
Clinical characteristics of the study population
| Total (N=313) | LC (N=156) | HCC (N=157) | P-value | |
|---|---|---|---|---|
| Age, year* | 58.8 (11.5) | 56.7 (10.8) | 60.8 (11.8) | 0.002 |
| Gender† | ||||
| Male | 217 (69.3) | 90 (57.7) | 127 (80.9) | <0.001 |
| Female | 96 (30.7) | 66 (42.3) | 30 (19.1) | |
| BMI, kg/m2 * | 23.88 (4.12) | 23.92 (4.6) | 23.84 (3.6) | 0.860 |
| Etiology† | ||||
| Alcohol Consumption | 20 (6.4) | 3 (1.9) | 17 (10.8) | |
| HBV | 222 (70.9) | 116 (74.4) | 106 (67.5) | |
| HCV | 54 (17.3) | 37 (23.7) | 17 (10.8) | |
| Cryptogenic | 14 (4.5) | 0 (0.0) | 14 (8.9) | |
| Others | 3 (0.9) | 0 (0.0) | 3 (1.9) | |
| Child-Pugh class† | ||||
| A | 261 (83.0) | 133 (85.3) | 128 (81.5) | 0.516 |
| B | 45 (14.8) | 19 (12.2) | 26 (16.6) | |
| C | 7 (2.3) | 4 (2.5) | 3 (1.9) | |
| Underlying liver disease† | ||||
| Chronic hepatitis | 10 (3.2) | 0 (0.0) | 10 (6.4) | <0.001 |
| Liver cirrhosis | 293 (93.6) | 156 (100.0) | 137 (87.3) | |
| Tumor characteristics† | ||||
| BCLC stage | ||||
| 0 | - | - | 21 (13.4) | |
| A | - | - | 56 (35.7) | |
| B | - | - | 11 (7.0) | |
| C | - | - | 62 (39.5) | |
| D | - | - | 7 (4.5) | |
| Diffuse type | - | - | 18 (11.5) | |
| With major PVI | - | - | 32 (20.4) |
LC, liver cirrhosis; HCC, hepatocellular carcinoma; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, barcelona clinic liver cancer; PVI, portal vein invasion.
Mean (Standard Deviation).
Number (Percentage).
Plasma levels of GPC3 and AFP in HCC and LC patients
The median and mean levels of GPC3 and AFP were summarized in Table 3.
Table 3.
Plasma GPC3 and AFP levels in HCC and LC patients
| Total (N=313) | LC (N=156) | HCC (N=157) | P-value | |
|---|---|---|---|---|
| AFP level | <0.001 | |||
| Median | 7.44 | 4.74 | 27.72 | |
| Range | 0.72-42000 | 1.08-145.08 | 0.72-42000.00 | |
| Mean | 1960.79 | 9.90 | 3899.26 | |
| SD | 8026.26 | 17.72 | 11011.53 | |
| GPC3 in Assay 1 | 0.255 | |||
| Median | 0.65 | 0.60 | 0.80 | |
| Range | 0-7.40 | 0.07-7.40 | 0-3.09 | |
| Mean | 0.89 | 0.86 | 0.92 | |
| SD | 0.78 | 0.86 | 0.69 | |
| GPC3 in Assay 2 * | ||||
| Median | 14.79 | All Results | 14.79 | |
| Range | 10.59-83.16 | Out of | 10.59-83.16 | |
| Mean | 36.18 | Range | 36.18 | |
| SD | 40.74 | 40.74 |
All values are in ng/mL.
GPC3, Glypican-3; AFP, Alpha-fetoprotein; HCC, Hepatocellular carcinoma; LC, Liver cirrhosis; SD, Standard deviation.
Only three HCC samples had detectable GPC3 values.
In Assay 1, the median GPC3 value for HCC patients was 0.80 ng/mL (range, 0.00 ng/mL to 3.09 ng/mL) for HCC patients, and for LC patients was 0.60 ng/mL (range, 0.07 ng/mL to 7.40 ng/mL). These differences were not significant (P=0.255).
In Assay 2, all but 3 HCC samples were “Out of Range”. None of the LC samples reached concentrations within the range of the assay. Because of the lack of HCC patients with detectable GPC3 levels, AUC analysis was not performed based on this data. Clinical characteristics and GPC3 concentrations based on Assay 1 and 2 of these samples were listed in Table 4.
Table 4.
Comparison of clinical characteristics for three patients with detectable plasma GPC3 levels in both ELISA assays
| Patient | Age | Etiology | Cirrhosis | BCLC stage | Tumor number | Max tumor size (cm) | GPC3 (ng/mL) |
|
|---|---|---|---|---|---|---|---|---|
| Assay 1 | Assay 2 | |||||||
| A | 66 | HBV | Yes | A | 1 | 3.0 | 0.18 | 83.16 |
| B | 62 | HBV | Yes | A | 2 | 1.7 | 1.11 | 14.79 |
| C | 51 | HBV | Yes | C | 1 | 4.6 | 0.78 | 10.59 |
ELISA, enzyme-linked immunosorbent assay; BCLC, barcelona clinic liver cancer; GPC3, glypican-3; HBV, hepatitis B virus.
Median plasma AFP level was 27.72 ng/mL (range, 0.72 ng/mL to 42,000.0 ng/mL) in HCC patients and 4.74 ng/mL in LC patients (range, 1.08 ng/mL to 145.08 ng/mL), which showed significantly elevated levels in HCC patients compared to LC patients (P<0.001).
In addition, when comparing LC GPC3 and AFP median values to just that of early HCC patients (BCLC 0~B, n=88), again only AFP was significantly elevated in the latter (GPC3 0.65 ng/mL P=0.916, AFP=11.94 ng/mL P<0.001; data not shown).
Diagnostic Accuracy of GPC3 and AFP
Using the ROC curves (Fig. 1), AUCs were calculated to assess the diagnostic accuracy of plasma AFP and GPC3 for HCC. Based on maximum sum of sensitivity and specificity, a GPC3 cut-off of 0.61 ng/mL was determined. At this cut-off, GPC3 had a sensitivity of 60% and a specificity of 52%. At a cut-off of 20 ng/mL, AFP had a sensitivity of a 55% and specificity 91%. The AUC was 0.559 and 0.729 for GPC3 and AFP respectively, indicating AFP as a stronger diagnostic tool. Combining the two markers only provided a marginal change; GPC3 and AFP (at cut-offs of 0.61 ng/mL and 20 ng/mL respectively) had an AUC of 0.744, sensitivity of 55%, and specificity of 91%. Furthermore, when comparing the LC group to the early HCC subgroup, at the same cut-offs, AFP (AUC=0.665) was a superior diagnostic biomarker compared to GPC3 (AUC=0.527).
Figure 1.
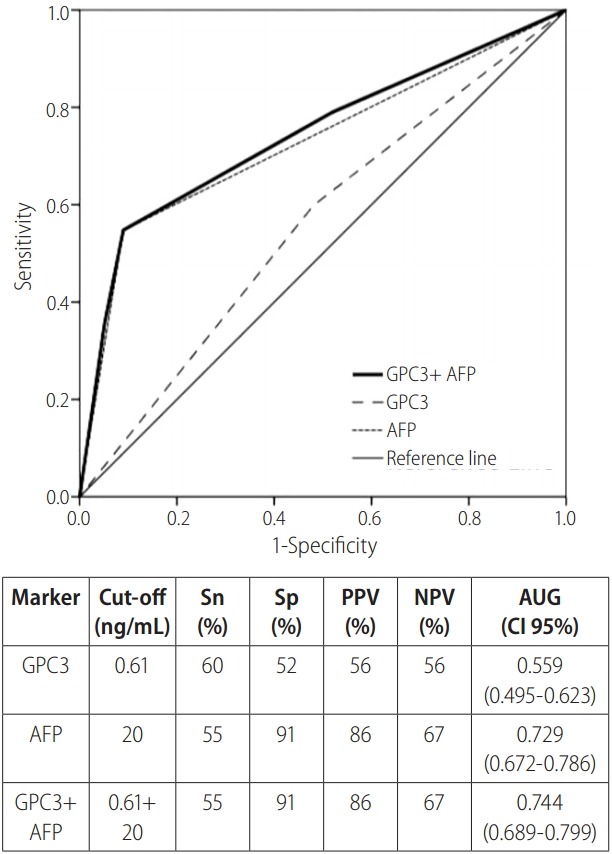
ROC curves of plasma GPC3 and AFP levels for HCC diagnosis. ROC curve of GPC3 (cut off level of 0.61 ng/mL), AFP (cut off level of 20 ng/mL), combined GPC3 and AFP, and reference for hepatocellular carcinoma diagnosis displayed with data summary. ROC, receiver operating characteristic; GPC3, glypican-3; AFP, alpha-fetoprotein; Sn, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CI, confidence interval.
DISCUSSION
We set out to determine whether GPC3 could be useful as a diagnostic biomarker for HCC in comparison to the traditional AFP in an HBV endemic, South Korean background. While AFP levels were significantly elevated in HCC patients compared to LC patients, plasma GPC3 levels were either very low or undetectable. Our study reported comparable sensitivity, but much lower specificity and AUC values for GPC3 (52% and 0.559) compared to other studies (84% and 0.762) [10]. The ROC determined cut-off value for GPC3 of 0.61 ng/mL was lower than most reported values, though it has been reported to range from 3.9 pg/mL to 300 ng/mL [10]. Meanwhile, AFP sensitivity, specificity, and AUC were comparable to reported results [10]. Overall, the plasma level of GPC3 was a poor diagnostic biomarker for HCC compared to AFP.
Though we used 2 commercially available GPC3 assays, GPC3 values detected by the two assays showed discrepancies. Assay 1 resulted in lower GPC3 values in HCC patients than those reported by previous studies, although some studies have reported similarly low values [11-14]. In Assay 2, only 1.9% of the HCC patients had detectable values in this study. The technical differences between the two assays included sample dilution factor and washing method. These differences may have contributed to discrepancies between the two assays.
Since first reported to be present in the plasma of 53% HCC patients but undetectable in healthy liver controls, GPC3 has been studied as a biomarker for HCC to replace or complement AFP [5]. However, subsequent studies have yielded inconsistent results as summarized in Table 5, showing inter- and intra-ELISA variances. Differences in capture and detection antibodies could explain inter-method differences [5,9,11-20]. While Capurro et al. developed antibodies targeting the COOH-terminus for their ELISA, Hippo et al. used antibodies targeting the NH2-terminus [5,9]. Though Capurro et al. and Hippo et al. reported similarly elevated rates of GPC3 in HCC patients (53% and 51%, respectively), their scales were completely different (0-2924 ng/mL vs 0-~60 ng/mL) [5,9].
Table 5.
Summary of reported studies measuring blood GPC3 levels in HCC patients using ELISA
| Author (year) | Country | HCC cases | GPC3 mean | GPC3 median | GPC3 range | ELISA kit |
|---|---|---|---|---|---|---|
| Beale et al. (2008) [13] | UK | 50 | 161.41 ng/mL | 56.57 ng/mL | BioMosaics limited (Burlington, VT, USA) | |
| Yasuda et al. (2010) [11] | Japan | 200 | 924.8 pg/mL | |||
| Lee et al. (2014) [15] | South Korea | 120 | 75.8 ng/mL | 21.7-482.5 ng/mL | Cusabio (Wuhan, China) | |
| Wang et al (2013) [14] | China | 84 | 4.55 ng/mL | |||
| Badr et al. (2014) [16] | Egypt | 30 | 551.47 µg/mL | USCN Life Science Inc. (Wuhan, China) | ||
| El Gawad et al. (2014) [17] | Egypt | 40 | 7.7 ng/mL | |||
| El-Shenawy et al. (2012) [18] | Egypt | 85 | 1,646.3 ng/mL | |||
| Ozkan et al. (2011) [12] | Turkey | 75 | 3.9 pg/mL | EIAab Science Co. (Wuhan China) | ||
| Capurro et al. (2003) [5] | Canada | 34 | 0-2924 ng/mL | Non-Commercial | ||
| Chen et al. (2013) [19] | China | 155 | 99.94 ng/mL | 15.11 ng/mL | 0–2400.00 ng/mL | |
| Hippo et al. (2004) [9] | Japan | 69 | 4.84 ng/mL | |||
| Tangkijvanich et al. (2010)* [20] | Thailand | 100 | 46.3 ng/mL | 0-7826.6 ng/mL |
Reported to use same procedure as (5).
GPC3, glypican-3; HCC, hepatocellular carcinoma; ELISA, enzyme-linked immunosorbent assay.
The nature of plasma GPC3 may impact ELISA utility. Plasma GPC3 can be produced when an extracellular lipase (Notum) cleaves the GPI anchor of membrane bound GPC3 or when secreted, possibly by endogenous Notum or GPI-phospholipase D, by the cell [21]. Various species of GPC3 may also be found, as Hippo et al. was able to detect a 50-kDa fragment produced by a cleavage site in the COOH-terminal in addition to the commonly detected glycanated GPC3 [22]. Furthermore, plasma GPC3 competitively binds with several growth factors, which could interfere with antibody binding [23]. Given these possibilities, it is clear that a uniform understanding of plasma GPC3 has not been reached. There may be ways to circumvent these proposed obstacles. Comparison of cell lysate and supernatant could help differentiate membrane bound and secreted GPC3; an antibody that targets the NH2 terminal may detect both the 50kDa and glycanated forms of GPC3; and a dilution of the plasma could help prevent interference of antibody binding [22]. Nonetheless, for plasma GPC3 to be a universal biomarker, the biochemistry behind plasma GPC3 concerning its origin as well as structure(s) must be elucidated.
Finally, heterogeneity in HCC composition, ethnicity, etiology, and clinical manifestation may lead to variability [8,24]. Moreover, meta-studies point out methodological issues such as differences in inclusion and exclusion criteria or use of reference standard tests [6,10].
The limitations of this study were that it was a single center, cross-sectional design without external validation samples, no assessment of concurrent GPC3 tissue expression, and usage of plasma samples. It also included only 2 commercially available ELISA.
In conclusion, plasma GPC3 level measured by currently available 2 ELISA kits clearly failed to be a useful diagnostic biomarker for HCC compared to AFP. Persistent inconsistency in GPC3 values questions its utility. Future studies regarding the development of more reliable detection systems perhaps including blood GPC3 levels are warranted.
Abbreviations
- AFP
alpha-fetorprotien
- BCLC
Barcelona Clinic Liver Cancer
- ELICA
enzymelinked chemiluminescent immunoassay
- ELISA
enzyme-linked immunosorbent assay
- GPC3
glypican-3
- GPI
glycophosphatidylinositol
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- LC
liver cirrhosis
- ROC
receiver operating characteristic
Funding Support
This study was supported by an intramural grant (No.02-2014-024) from the Seoul National University Bundang Hospital. We are grateful to Hyein Mann of Seoul National University Bundang Hospital for her devotion to sample collection and storage. We are also grateful to Professor Emeritus J. Patrick Barron Ph.D. of Tokyo Medical University for his pro bono editing of this manuscript.
Conflicts of Interest: The authors have no conflicts to disclose.
REFERENCES
- 1.Feng M, Ho M. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett. 2014;588:377–382. doi: 10.1016/j.febslet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu AX, Gold PJ, El-Khoueiry AB, Abrams TA, Morikawa H, Ohishi N, et al. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2013;19:920–928. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 3.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song do S, Bae SH. Changes of guidelines diagnosing hepatocellular carcinoma during the last ten-year period. Clin Mol Hepatol. 2012;18:258–267. doi: 10.3350/cmh.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, et al. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 6.Liu XF, Hu ZD, Liu XC, Cao Y, Ding CM, Hu CJ. Diagnostic accuracy of serum glypican-3 for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Biochem. 2014;47:196–200. doi: 10.1016/j.clinbiochem.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280:2471–2476. doi: 10.1111/febs.12126. [DOI] [PubMed] [Google Scholar]
- 9.Hippo Y, Watanabe K, Watanabe A, Midorikawa Y, Yamamoto S, Ihara S, et al. Identification of soluble NH2-terminal fragment of glypican-3 as a serological marker for early-stage hepatocellular carcinoma. Cancer Res. 2004;64:2418–2423. doi: 10.1158/0008-5472.can-03-2191. [DOI] [PubMed] [Google Scholar]
- 10.Jia X, Liu J, Gao Y, Huang Y, Du Z. Diagnosis accuracy of serum glypican-3 in patients with hepatocellular carcinoma: a systematic review with meta-analysis. Arch Med Res. 2014;45:580–588. doi: 10.1016/j.arcmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda E, Kumada T, Toyoda H, Kaneoka Y, Maeda A, Okuda S, et al. Evaluation for clinical utility of GPC3, measured by a commercially available ELISA kit with Glypican-3 (GPC3) antibody, as a serological and histological marker for hepatocellular carcinoma. Hepatol Res. 2010;40:477–485. doi: 10.1111/j.1872-034X.2010.00624.x. [DOI] [PubMed] [Google Scholar]
- 12.Ozkan H, Erdal H, Kocak E, Tutkak H, Karaeren Z, Yakut M, et al. Diagnostic and prognostic role of serum glypican 3 in patients with hepatocellular carcinoma. J Clin Lab Anal. 2011;25:350–353. doi: 10.1002/jcla.20484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beale G, Chattopadhyay D, Gray J, Stewart S, Hudson M, Day C, et al. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer. 2008;8:200. doi: 10.1186/1471-2407-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Yang H, Xu H, Lu X, Sang X, Zhong S, et al. Golgi protein 73, not Glypican-3, may be a tumor marker complementary to alpha-Fetoprotein for hepatocellular carcinoma diagnosis. J Gastroenterol Hepatol. 2014;29:597–602. doi: 10.1111/jgh.12461. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, Yeon JE, Suh SJ, Lee SJ, Yoon EL, Kang K, et al. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma. Gut Liver. 2014;8:177–185. doi: 10.5009/gnl.2014.8.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badr EAE, Korah TE, Ghani AA, El-Sayed S, Badr S. Role of serum glypican-3 in the diagnosis and differentiation of small hepatocellular carcinoma from hepatitis-C virus cirrhosis. Alexandria Med J. 2014;50:221–226. [Google Scholar]
- 17.Abd El Gawad IA, Mossallam GI, Radwan NH, Elzawahry HM, Elhifnawy NM. Comparing Prothrombin induced by vitamin K absence-II (PIVKA-II) with the oncofetal proteins Glypican-3, Alpha feto protein and Carcinoembryonic antigen in diagnosing hepatocellular carcinoma among Egyptian patients. J Egypt Natl Canc Inst. 2014;26:79–85. doi: 10.1016/j.jnci.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 18.El-Shenawy S, El Sabawi M, Sheble N, Abd El-Raof M, Allam M, Fath Allah S. Diagnostic role of serum Glypican-3 as a tumor marker for hepatocellular carcinoma. Nat Sci. 2012;10:32–38. [Google Scholar]
- 19.Chen M, Li G, Yan J, Lu X, Cui J, Ni Z, et al. Reevaluation of glypican-3 as a serological marker for hepatocellular carcinoma. Clin Chim Acta. 2013;423:105–111. doi: 10.1016/j.cca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Tangkijvanich P, Chanmee T, Komtong S, Mahachai V, Wisedopas N, Pothacharoen P, et al. Diagnostic role of serum glypican-3 in differentiating hepatocellular carcinoma from non-malignant chronic liver disease and other liver cancers. J Gastroenterol Hepatol. 2010;25:129–137. doi: 10.1111/j.1440-1746.2009.05988.x. [DOI] [PubMed] [Google Scholar]
- 21.Traister A, Shi W, Filmus J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem J. 2008;410:503–511. doi: 10.1042/BJ20070511. [DOI] [PubMed] [Google Scholar]
- 22.Capurro M, Filmus J. Glypican-3 as a serum marker for hepatocellular carcinoma. Cancer Res. 2005;65:372. [PubMed] [Google Scholar]
- 23.Zittermann SI, Capurro MI, Shi W, Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer. 2010;126:1291–1301. doi: 10.1002/ijc.24941. [DOI] [PubMed] [Google Scholar]
- 24.Bedossa P, Paradis V. Hepatocellular Carcinoma. Practical Hepatic Pathology: A Diagnostic Approach. In: Saxena R, editor. Hepatocellular Carcinoma, Vol I. Philadelphia: Elsevier Inc; 2011. pp. 489–501. [Google Scholar]


