Summary
Background
Dehydration due to diarrhoea is a leading cause of child death worldwide, yet no clinical tools for assessing dehydration have been validated in resource-limited settings. The Dehydration: Assessing Kids Accurately (DHAKA) score was derived for assessing dehydration in children with diarrhoea in a low-income country setting. In this study, we aimed to externally validate the DHAKA score in a new population of children and compare its accuracy and reliability to the current Integrated Management of Childhood Illness (IMCI) algorithm.
Methods
DHAKA was a prospective cohort study done in children younger than 60 months presenting to the International Centre for Diarrhoeal Disease Research, Bangladesh, with acute diarrhoea (defined by WHO as three or more loose stools per day for less than 14 days). Local nurses assessed children and classified their dehydration status using both the DHAKA score and the IMCI algorithm. Serial weights were obtained and dehydration status was established by percentage weight change with rehydration. We did regression analyses to validate the DHAKA score and compared the accuracy and reliability of the DHAKA score and IMCI algorithm with receiver operator characteristic (ROC) curves and the weighted κ statistic. This study was registered with ClinicalTrials.gov, number NCT02007733.
Findings
Between March 22, 2015, and May 15, 2015, 496 patients were included in our primary analyses. On the basis of our criterion standard, 242 (49%) of 496 children had no dehydration, 184 (37%) of 496 had some dehydration, and 70 (14%) of 496 had severe dehydration. In multivariable regression analyses, each 1-point increase in the DHAKA score predicted an increase of 0·6% in the percentage dehydration of the child and increased the odds of both some and severe dehydration by a factor of 1·4. Both the accuracy and reliability of the DHAKA score were significantly greater than those of the IMCI algorithm.
Interpretation
The DHAKA score is the first clinical tool for assessing dehydration in children with acute diarrhoea to be externally validated in a low-income country. Further validation studies in a diverse range of settings and paediatric populations are warranted.
Funding
National Institutes of Health Fogarty International Center.
Introduction
Despite tremendous progress over the past several decades, diarrhoeal disease remains a leading cause of death in children worldwide. Annually, children younger than 60 months have an estimated 1·7 billion diarrhoeal episodes, leading to 124 million outpatient visits, 9 million hospital admissions, and 520 000 deaths.1–4 Accurate and rapid assessment of dehydration status is crucial to prevent morbidity and mortality in children with diarrhoeal disease. Global health authorities recommend classifying such children into one of three categories on the basis of their initial presentation: severe dehydration, some dehydration, or no dehydration.5–8 For children with some dehydration, oral rehydration therapy is highly cost-effective and associated with shorter hospital stays and fewer adverse events than treatment with intravenous fluid, particularly in resource-limited settings.7,9,10
However, the tools available to assess dehydration status in children with diarrhoeal disease are inadequate. Neither clinician gestalt nor any individual clinical sign, symptom, laboratory, or imaging test has been found to have adequate sensitivity, specificity, and reliability for detecting dehydration in children.11–13 The WHO Integrated Management of Childhood Illness (IMCI) guidelines recommend using a combination of clinical signs to classify children as having no, some, or severe dehydration (figure 1).14 However, the IMCI algorithm was developed based largely on expert opinion, and studies15–17 have not found it to be an accurate predictor of dehydration in children. Other dehydration scales have been derived in high-resource settings for use by physician providers but have not been validated for use by nurses and other non-physician providers in resource-limited settings.18–21
Figure 1. IMCI algorithm.
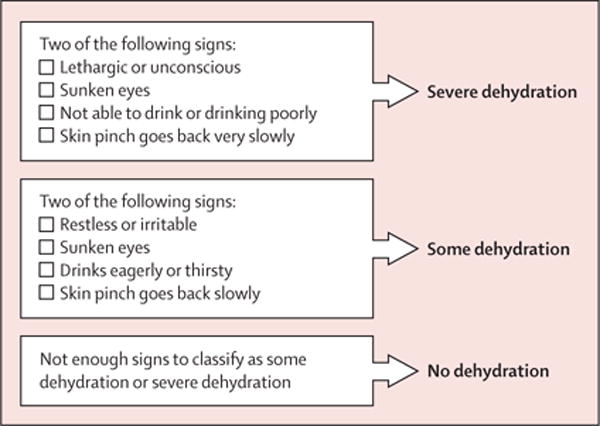
IMCI=Integrated Management of Childhood Illness.
In response, the Dehydration: Assessing Kids Accurately (DHAKA) study22 empirically derived and internally validated a new clinical score for assessing dehydration in children with acute diarrhoea in a low-income country (figure 2). In this study, we sought to externally validate the DHAKA score and compare its accuracy and reliability to the IMCI algorithm.
Figure 2. DHAKA score.
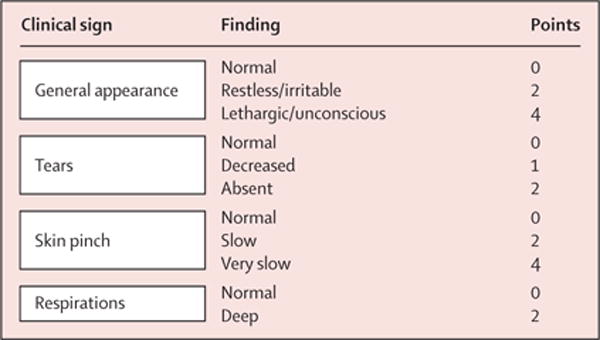
A score of 4 or more was deemed severe dehydration, a score of 2–3 as some dehydration, and a score of 0–1 as no dehydration. DHAKA=Dehydration: Assessing Kids Accurately.
Methods
Study design and participants
The DHAKA validation study was a prospective cohort study that included all eligible children presenting to the International Centre for Diarrhoeal Disease Research, Bangladesh. Enrolment took place in the International Centre for Diarrhoeal Disease Research, Bangladesh, rehydration (short stay) unit. The International Centre for Diarrhoeal Disease Research, Bangladesh, provides free clinical services to the population of Dhaka, Bangladesh, and the surrounding rural and suburban districts, with a catchment area of more than 17 million people.23
All children younger than 60 months presenting to the rehydration unit with acute diarrhoea, defined by WHO as three or more loose stools per day for less than 14 days, were eligible for enrolment.5 Research staff randomly selected children younger than 60 months for screening 24 h a day and established whether each child met any of the four predefined exclusion criteria, which were fewer than three loose stools per day, diarrhoea for more than 14 days, a diagnosis other than gastroenteritis as established by the treating physician, and previous enrolment in the DHAKA validation study. Random selection was accomplished by pulling coloured marbles from a black pouch. A blue marble was for inclusion, a white marble for exclusion.
For children who did not meet any exclusion criteria, research staff explained the risks and benefits of the study and obtained informed consent from the parent or guardian of the child in the local language, Bengali. Ethical approval was obtained from the International Centre for Diarrhoeal Disease Research, Bangladesh, the Ethical Review Committee, and the Lifespan Institutional Review Board.
Procedures
Local general practice nurses with 4–6 years of clinical experience collected all data for this study. Beforehand, they received 5 days of training in all study procedures. This included didactic, video, and practical instruction in how to appropriately apply both the DHAKA score and the IMCI algorithm in children (appendix).
After obtaining informed consent, children were immediately undressed and weighed to the nearest tenth of a kilogram with an electronic scale. Study staff recorded the volume of fluid received before baseline weight measurement. Participants were then assessed clinically by a study nurse, who classified their dehydration status first with the DHAKA score and second with the IMCI algorithm. Participants were also assessed clinically by a second study nurse when available, masked to the exam done by the first nurse. Study nurses collected baseline historical and demographic data for each child from their parent or guardian including age, sex, home district, days of diarrhoea, diarrhoeal episodes in the past 24 h, and type of diarrhoea (bloody, watery, rice water). Nutritional status was assessed for each patient on arrival by measuring mid-upper arm circumference with a standard measuring tape.
Patients were rehydrated according to International Centre for Diarrhoeal Disease Research, Bangladesh, protocols. Patients were weighed every 8 h, on the same scale and without clothing, to establish their post-hydration stable weight. Children who did not achieve a stable weight before discharge were telephoned daily and asked to return for a post-illness weight check once their diarrhoea had resolved completely.
Outcomes
We averaged each child’s two highest consecutive weight measurements that differed by less than 2% to establish their stable weight, as described previously.18 Generally, children with dehydration rapidly gain weight as they are rehydrated until they achieve their pre-illness weight, or stable weight, after which they stop gaining weight as their kidneys diurese excess fluid. For each participant with a valid stable weight, we calculated the percentage weight change with rehydration, our criterion standard for percent dehydration, using the formula:
For children who did not achieve a stable weight before discharge, their post-illness weight was used instead of their stable weight in the formula above to calculate the percentage dehydration. We then categorised children as having severe (>9%) dehydration, some (3–9%) dehydration, or no (<3%) dehydration. We also did a sensitivity analysis, eliminating children who lost more than 3% of their weight during their admission, suggesting either over-hydration before arrival or inadequate hydration with ongoing diarrhoea during their admission.
Standard guidelines from the scientific literature, including the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis guidelines, were used to validate the DHAKA score.24 We used both linear regression and ordinal logistic regression to establish whether the DHAKA score predicted the percentage dehydration and dehydration category of the child on arrival, controlling for age, sex, district type, and nutritional status.
Statistical analysis
As methods have yet to be developed for calculating the sample size needed for the validation of a diagnostic model, we based our sample size calculations on the test characteristics of the DHAKA score. Assuming a sensitivity of about 85% for the assessment of severe dehydration on the basis of the performance of the DHAKA score in its derivation population, with a power (type II error) of 80%, an α (type I error) of 0·05, and a 10% prevalence of severe dehydration, we estimated a sample size of at least 490 patients to ensure 95% CI around our test characteristics of less than 10%.22,25 To allow for up to 10% loss to follow-up, we planned to enrol at least 540 patients in our study.
Baseline demographic and historical data were summarised for all children. The proportion of children with undernutrition was calculated with a mid-upper arm circumference <115 mm for severe acute mal nutrition and mid-upper arm circumference of 116–125 mm for moderate acute malnutrition.26 Data for children included and excluded from primary analyses was compared with the χ2 test or equality of medians testing as appropriate.
To directly compare the accuracy of the DHAKA score and the IMCI algorithm, we calculated the area under the receiver operator characteristic (ROC) curves (AUC), or c-statistic, for each model against the true dehydration category of the child. We first calculated pairwise AUC comparisons (none vs some, some vs severe, and none vs severe) and then computed the dichotomised ordinal c-index using the weighted average of the pairwise AUC values to create a single measure of accuracy for each model.27 The ordinal c-index is interpreted like the traditional AUC for testing the discrimination of a diagnostic model for a binary outcome: a value of 0·5 is no better than chance, whereas a value of 1 represents a perfect model. We used bootstrapping (selection with replacement) with 1000 replicates in order to calculate a p value for the comparison of the ordinal c-index for the DHAKA score and IMCI algorithm. We also compared the test characteristics, including sensitivity, specificity, and positive and negative likelihood ratios of the DHAKA score and the IMCI algorithm for the diagnosis of some (any) dehydration and severe dehydration.
We assessed the inter-rater reliability of the DHAKA score using the interclass correlation coefficient by testing agreement of individual scores between the initial exam and repeat exam for the subset of children that had repeat exams. We also compared the reliability of the DHAKA score and the IMCI algorithm for the assessment of dehydration category using Cohen’s κ (weighted) and bootstrapping with 1000 replicates. We used the standard p value cutoff of 0·05 for all analyses. Primary analyses were done with R software, version 3.0.2. This study was registered with ClinicalTrials.gov, number NCT02007733.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 22, 2015, and May 15, 2015, 858 children younger than 60 months were randomly selected on arrival, of which 546 were enrolled in this study and 496 were included in our primary analyses (figure 3). The median age of included children was 16 months (range 2–60). Overall, 209 (42%) of 496 included children were younger than 12 months and 105 (21%) of 496 had moderate-to-severe malnutrition. Children excluded from analyses because of loss to follow-up were older, but no significant differences in other baseline characteristics were noted (table 1).
Figure 3. Flowchart for DHAKA validation study.
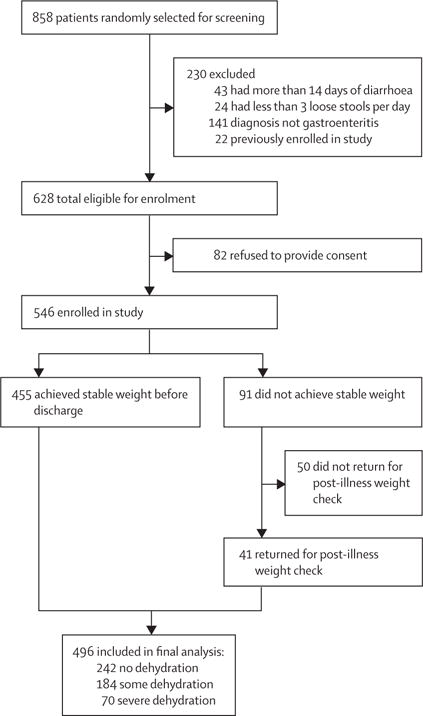
DHAKA=Dehydration: Assessing Kids Accurately.
Table 1.
Baseline characteristics
| Included in final analysis (n=496) | Excluded, no final weight (n=50) | p value | |
|---|---|---|---|
| Age (months) | 16 (9–30) | 28 (15–42) | 0·001 |
|
| |||
| Sex | 0·263 | ||
| Female | 217 (44%) | 26 (52%) | ·· |
| Male | 279 (56%) | 24 (48%) | ·· |
|
| |||
| Home district | 0·348 | ||
| Urban (Dhaka) | 356 (72%) | 39 (78%) | ·· |
| Rural or suburban | 140 (28%) | 11 (22%) | ·· |
|
| |||
| Nutritional status (MUAC) | 0·517 | ||
| No acute malnutrition | 391 (79%) | 40 (80%) | ·· |
| Moderate acute malnutrition | 77 (15%) | 9 (18%) | ·· |
| Severe acute malnutrition | 28 (6%) | 1 (2%) | ·· |
|
| |||
| Days of diarrhoea before arrival | 2 (2–3) | 2 (1–3) | 0·144 |
|
| |||
| Loose stools in past 24 h | 10 (8–18) | 12 (7–15) | 0·451 |
|
| |||
| Diarrhoea type | 0·227 | ||
| Watery | 368 (74%) | 32 (64%) | ·· |
| Rice water | 125 (25%) | 18 (36%) | ·· |
| Bloody | 3 (1%) | 0 | ·· |
Data are median (IQR) or n (%). MUAC=mid-upper arm circumference.
The median percentage weight change with rehydration was 3% (IQR 1–7) in our study population. On the basis of our criterion standard, 242 (49%) of 496 children had no dehydration, 184 (37%) of 496 had some dehydration, and 70 (14%) of 496 had severe dehydration. Median time from admission to stable or return weight was 14 h (IQR 11–22). Although 32% of patients received some fluid before hospital admission weight was measured, the median amount of fluid received in these patients was only 8 mL (IQR 4–12).
The median time from initial weight to assessment of the DHAKA score in enrolled children was 2 min (IQR 2–3). After controlling for potential confounders, the DHAKA score remained an independent predictor of the percentage dehydration in children on arrival, with each 1-point increase in the DHAKA score predicting an increase of 0·6% in the child’s percentage dehydration (table 2). Similarly, after controlling for potential confounders, the DHAKA score remained an independent predictor of dehydration category (table 2), with each 1-point increase in the DHAKA score increasing the odds of dehydration by 1·4. The median percentage dehydration and the probability of each dehydration category by DHAKA score are shown (figures 4, 5).
Table 2.
DHAKA score linear and ordinal logistic regression analysis
| Percentage dehydration measured with linear regression
|
Dehydration category measured with ordinal logistic regression
|
|||
|---|---|---|---|---|
| Coefficient | p value | Odds ratio | p value | |
| DHAKA score | 0·006 (0·005 to 0·007) | <0·0001 | 1·422 (1·340 to 1·507) | <0·0001 |
|
| ||||
| Age (months) | 0·000 (−0·001 to 0·000) | 0·057 | 0·989 (0·975 to 1·004) | 0·159 |
|
| ||||
| Sex | −0·001 (−0·008 to 0·005) | 0·721 | 0·837 (0·572 to 1·224) | 0·358 |
|
| ||||
| Nutrition status | −0·002 (−0·008 to 0·004) | 0·481 | 1·019 (0·717 to 1·448) | 0·914 |
|
| ||||
| Urban location | 0·007 (−0·001 to 0·014) | 0·080 | 1·126 (0·731 to 1·735) | 0·591 |
|
| ||||
| Constant | 0·011 (0·003 to 0·019) | 0·009 | ·· | ·· |
DHAKA=Dehydration: Assessing Kids Accurately.
Figure 4. Median percentage dehydration by DHAKA score.
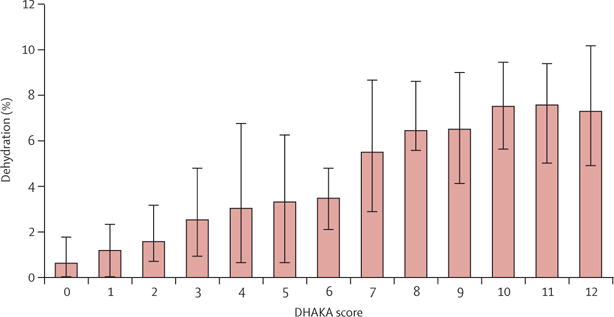
Error bars show interquartile range. DHAKA=Dehydration: Assessing Kids Accurately.
Figure 5. Predicted probability of each dehydration category by DHAKA score.
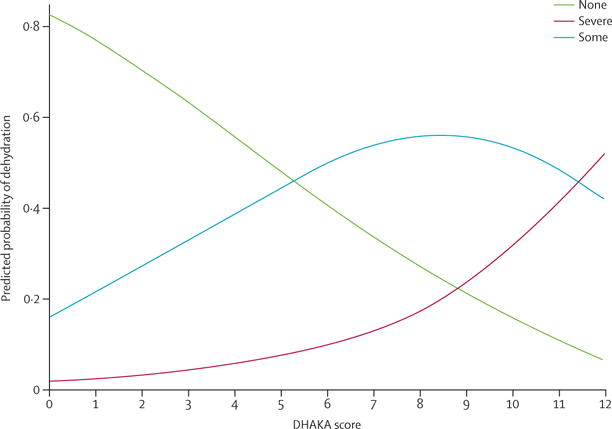
DHAKA=Dehydration: Assessing Kids Accurately.
The calculated ordinal c-index for the DHAKA score was 0·82 (95% CI 0·78–0·85), whereas the calculated ordinal c-index for the IMCI algorithm was 0·76 (0·73–0·79). Among 1000 bootstrap simulations, the accuracy of the DHAKA score was significantly better than that of the IMCI algorithm (p<0·0001). The test characteristics of the IMCI algorithm and the DHAKA score at a series of different cutoff values for the diagnosis of some (any) dehydration and severe dehydration, including the cutoff value of 2 for some dehydration and 4 for severe dehydration that was recommended in the original derivation study for the DHAKA score, are shown in table 3. The test characteristics of the individual clinical components of the DHAKA score and IMCI algorithm for detecting some and severe dehydration are shown in table 4.
Table 3.
Test characteristics for the current IMCI algorithm and the DHAKA score by cutoffs
| Sensitivity | Specificity | LR positive (95% CI) | LR negative (95% CI) | |
|---|---|---|---|---|
| Some dehydration | ||||
|
| ||||
| IMCI algorithm | ||||
| ≥2 dehydration signs* | 97% | 26% | 1·3 (1·2−1·4) | 0·11 (0·05−0·23) |
| DHAKA score | ||||
| ≥1 | 97% | 30% | 1·4 (1·3−1·5) | 0·11 (0·05−0·22) |
| ≥2* | 93% | 50% | 1·9 (1·6−2·1) | 0·14 (0·09−0·23) |
| ≥3 | 89% | 63% | 2·4 (2·0−2·9) | 0·18 (0·13−0·26) |
| ≥4 | 78% | 76% | 3·3 (2·6−4·1) | 0·29 (0·23−0·37) |
| ≥5 | 74% | 79% | 3·6 (2·8−4·7) | 0·32 (0·26−0·40) |
| ≥6 | 70% | 84% | 4·3 (3·2−5·8) | 0·36 (0·30−0·44) |
| ≥7 | 69% | 85% | 4·6 (3·4−6·3) | 0·37 (0·31−0·45) |
| ≥8 | 57% | 89% | 5·3 (3·6−7·8) | 0·48 0·41−0·56) |
|
| ||||
| Severe dehydration | ||||
|
| ||||
| IMCI algorithm | ||||
| ≥2 severe signs* | 77% | 67% | 2·3 (1·9−2·8) | 0·34 (0·22−0·53) |
| DHAKA score | ||||
| ≥3 | 94% | 42% | 1·6 (1·5−1·8) | 0·14 (0·05−0·36) |
| ≥4* | 86% | 54% | 1·9 (1·6−2·1) | 0·26 (0·15−0·47) |
| ≥5 | 84% | 58% | 2·0 (1·7−2·3) | 0·27 (0·16−0·47) |
| ≥6 | 83% | 63% | 2·2 (1·9−2·6) | 0·27 (0·16−0·46) |
| ≥7 | 81% | 64% | 2·3 (1·9−2·7) | 0·29 (0·18−0·48) |
| ≥8 | 70% | 71% | 2·4 (2·0−3·0) | 0·42 (0·29−0·60) |
| ≥9 | 69% | 75% | 2·7 (2·2−3·4) | 0·42 (0·30−0·60) |
| ≥10 | 44% | 86% | 3·2 (2·2−4·6) | 0·65 (0·52−0·80) |
Original cutoffs from the IMCI guidelines and the DHAKA derivation study.
LR=likelihood ratio. IMCI=Integrated Management of Childhood Illness. DHAKA=Dehydration: Assessing Kids Accurately.
Table 4.
Test characteristics of individual clinical signs for some dehydration (unshaded) and severe dehydration (shaded)
| Sensitivity | Specificity | LR positive (95% CI) | LR negative (95% CI) | χ2 | Reliability* | |
|---|---|---|---|---|---|---|
| General appearance | 0·95 | |||||
| Restless or irritable (some dehydration) | 0·81 | 0·67 | 2·4 (2·0−2·9) | 0·28 (0·21−0·37) | 117·38 | ·· |
| Lethargic or unconscious (severe dehydration) | 0·73 | 0·69 | 2·3 (1·9−2·9) | 0·39 (0·27−0·58) | 44·66 | ·· |
|
| ||||||
| Skin pinch | 0·85 | |||||
| Slow (some dehydration) | 0·84 | 0·68 | 2·6 (2·2−3·2) | 0·23 (0·17−0·31) | 138·51 | ·· |
| Very slow (severe dehydration) | 0·40 | 0·88 | 3·3 (2·2−4·8) | 0·68 (0·56−0·83) | 34·33 | ·· |
|
| ||||||
| Tears | 0·63 | |||||
| Decreased (some dehydration) | 0·83 | 0·47 | 1·6 (1·4−1·8) | 0·36 (0·27−0·49) | 52·17 | ·· |
| Absent (severe dehydration) | 0·29 | 0·90 | 2·8 (1·8−4·5) | 0·79 (0·68−0·92) | 18·51 | ·· |
|
| ||||||
| Respirations | 0·77 | |||||
| Normal (some dehydration) | 0·67 | 0·81 | 3·5 (2·7−4·6) | 0·41 (0·34−0·49) | 115·76 | ·· |
| Deep (severe dehydration) | 0·36 | 0·92 | 4·2 (2·7−6·6) | 0·70 (0·59−0·84) | 41·43 | ·· |
|
| ||||||
| Eyes | 0·67 | |||||
| Sunken eyes (some dehydration) | 0·95 | 0·26 | 1·3 (1·2−1·4) | 0·20 (0·11–0·35) | 41·78 | ·· |
| Sunken eyes (severe dehydration) | 0·59 | 0·82 | 3·2 (2·4−4·2) | 0·51 (0·38−0·67) | 53·44 | ·· |
|
| ||||||
| Thirst | 0·19 | |||||
| Drinks eagerly and thirsty (some dehydration) | 0·93 | 0·21 | 1·2 (1·1−1·3) | 0·34 (0·20−0·56) | 20·25 | ·· |
| Not able to drink or drinks poorly (severe dehydration) | 0·13 | 0·96 | 3·4 (1·6−7·4) | 0·91 (0·83−0·99) | 10·40 | ·· |
LR=likelihood ratio.
Reliability assessed by Cohen’s κ (weighted).
Repeat clinical exams were available for 416 (84%) of 496 patients. The median time between initial exam and repeat exam was 4 min (IQR 3–5). The interclass correlation coefficient for the DHAKA score was 0·94 (95% CI 0·93–0·95). The weighted κ statistic for the DHAKA score was 0·93, whereas the weighted κ statistic for the IMCI algorithm was 0·80 for the assessment of dehydration category (p<0·0001). Of the 496 children included in our analysis, eight (2%) lost more than 3% of their bodyweight. Removal of these children had no effect on any of the analyses (appendix).
Discussion
Overall, the DHAKA score did well in this validation study, showing good discriminative ability in differentiating between children with severe, some, and no dehydration. The score also had excellent inter-rater reliability, indicating its consistency in practice. Moreover, the DHAKA score was statistically more accurate and reliable than the IMCI algorithm for the assessment of dehydration status in children with acute diarrhoea in a low-income country.
Global health authorities recommend classifying children with acute diarrhoea into one of three categories, with significant differences in management based on the category assigned.5–8 Children classified with no dehydration receive only expectant outpatient management. Children classified with some dehydration are rehydrated with oral rehydration therapy, an inexpensive but logistically intensive process. According to IMCI guidelines, the child should be observed in the health facility for a minimum of 4–8 h, while the mother slowly spoons oral rehydration therapy into the child’s mouth, followed by a repeat dehydration assessment.14 Finally, children with severe dehydration are resuscitated with intravenous fluid resuscitation, which generally requires the child to be transferred to an inpatient facility. Not only is intravenous fluid resuscitation more expensive and human resource intensive than oral rehydration therapy, but it can also lead to longer hospital lengths of stay and more adverse events in resource-limited settings.9,10 At best, inappropriate categorisation of children with diarrhoea will result in overuse of precious health resources, negatively affecting the health-care system as a whole. At worst, it might result in direct harm to the child.
Despite the importance of this initial diagnostic decision, no clinical tools for the assessment of dehydration have previously been derived and validated for use in resource-limited settings, where most diarrhoea morbidity and mortality occurs in children. Worldwide, the most common clinical tool for assessing dehydration status is the IMCI algorithm, but previous studies15–17 comparing it to a physiological gold standard such as percentage weight change with rehydration have found it to be a poor predictor of dehydration, especially in infants under 12 months, with AUCs for the prediction of severe dehydration ranging from 0·58 to 0·72 depending on the study. Although other paediatric dehydration scales have been derived previously, none have been externally validated in resource-limited settings for use by non-physician providers.18–21 Clinical scales found to be accurate in high-resource settings might not perform well in low-resource settings, where children have higher rates of malnutrition and more severe forms of diarrhoea, and where providers might have less training.
Both the IMCI algorithm and the DHAKA score include four clinical signs that can be rapidly and easily assessed by non-physician providers. The primary difference between the DHAKA score and the IMCI algorithm, which likely accounts for its greater accuracy and reliability, is the absence of two particularly subjective clinical signs: thirst and sunken eyes. Differentiating children who are drinking “normally” from those who are drinking “eagerly”, and differentiating those who are refusing to drink because they are not thirsty from those who are refusing to drink because they are irritable, might be difficult in practice, as suggested by the poor reliability of this sign in our study. Additionally, previous research has identified the presence of a statistical interaction between general appearance and sunken eyes, whereby sunken eyes are a strong predictor of dehydration status in children with a normal general appearance but a weak predictor in children with a lethargic general appearance.22 However, the presence of tears might be difficult to assess in very young infants, who might not begin to form tears until the age of 2 months. Diarrhoea is very uncommon in this age group, however, as most children younger than 3 months in low-income countries are exclusively breastfed and therefore not routinely exposed to enteral pathogens.
The DHAKA score can be adapted to different settings more easily than the IMCI algorithm because it allows health policy makers to choose different cutoff values for the three dehydration categories. For instance, although we recommend using a DHAKA score of 4 or above to determine severe dehydration, policy makers wanting a more specific test for the diagnosis of severe dehydration, perhaps because of limitations in the availability of intravenous fluid resuscitation or inpatient beds, could choose to use a higher cutoff value, as shown in table 3. Similarly, although we recommend using a DHAKA score cutoff of 2 or above for some dehydration, policy makers wanting a more sensitive test for some dehydration could use a score of 1 or above. Furthermore, because higher scores correlate directly with the severity of dehydration, clinicians can also use the DHAKA score to better differentiate between patients. For instance, although a patient with a score of 5 and another with a score of 10 might both receive intravenous fluid resuscitation, the clinician knows that the child with a score of 10 is likely to be more dehydrated, and should be prioritised for more urgent management.
Although the derivation and validation phases of the DHAKA study collectively enrolled nearly 1400 children with acute diarrhoea, they were both done in the same low-income country. The DHAKA score might not perform as well when used in other settings, and additional external validation studies are warranted. Additionally, we did not perform subgroup analyses to assess the performance of the DHAKA score in specific populations, such as very young infants, children with undernutrition, or children with hyponatraemia or hypernatraemia. However, the populations enrolled in both the DHAKA derivation and validation studies included a diverse casemix, with children from both urban and rural settings, younger and older children, undernourished and well nourished children, and those with watery diarrhoea (typically viral) and rice-water diarrhoea (typically cholera). Furthermore, because all clinical services are free, the International Centre for Diarrhoeal Disease Research, Bangladesh, treated children from across the socioeconomic spectrum. Finally, about 90% of the children presenting to the rehydration unit at International Centre for Diarrhoeal Disease Research, Bangladesh, arrived directly from home, with only 10% referred from other health facilities, making its casemix more similar to primary health centres than to secondary referral hospitals.
Given that the accuracy of clinical exam findings are clinician dependent, the DHAKA score might not perform as well when used by less skilled providers. To ensure generalisability of our study results, we used local nurses without substantial previous training in paediatrics or dehydration assessment. Further research, however, is necessary to assess the accuracy and reliability of the DHAKA score when done by community health workers and other types of providers. We have made available the specific criteria used by the research nurses to assess each of the clinical signs in this study, including accompanying videos, so they can be easily replicated in future provider trainings (appendix).
Although the best physiological criterion standard for dehydration remains the percentage difference between pre-illness and admission weight, accurate pre-illness weights are rarely available for children in resource-limited settings. Instead, we used the percentage weight change with rehydration as the criterion standard for percent dehydration in our study, which correlates almost perfectly with percentage volume loss and has been used in nearly all previous studies of dehydration in children.11,18,28
Finally, although the DHAKA score was statistically more accurate overall than the IMCI algorithm, whether this difference is clinically meaningful in practice will depend on the setting in which it is used and the exact cutoffs chosen for the DHAKA score. Additionally, this study did not assess which of these tests is easier to use in practice. Future operational research should assess provider preference for each method in real world clinical settings.
The DHAKA score represents the first dehydration assessment tool both empirically derived and externally validated for use in a low-income country. In this study, it was also found to be both more accurate and reliable than the IMCI algorithm. Further validation studies of the DHAKA score in different regions of the world and with different populations of children and providers should be prioritised to determine its generalisability to children with acute diarrhoea worldwide.
Supplementary Material
Research in context.
Evidence before this study
We systematically reviewed the scientific literature to identify studies published up to Feb 1, 2016, addressing the assessment of dehydration in children with diarrhoea. We searched PubMed, Cochrane Libraries, and Google Scholar to identify all published and unpublished trials in English using combinations of the following search terms: dehydration, dehydration scale, dehydration assessment, diarrhoea, gastroenteritis, child, pediatric. Additional studies were identified by hand searching references from included studies. Our literature search identified four previous clinical prediction models for dehydration that have been derived using data from prospective cohorts of children against a valid criterion standard. All four studies were done in high-income or middle-income countries, and none of these previous studies were based on cohorts of children large enough to develop a stable clinical prediction model. Only three previous studies have validated the Integrated Management of Childhood Illness (IMCI) algorithm against a physiological criterion standard, and all three studies found it to be a poor predictor of dehydration in children.
Added value of this study
The Dehydration: Assessing Kids Accurately (DHAKA) score is the first clinical diagnostic model for dehydration in children with diarrhoea derived in a low-income country using local nurses to perform all exams. In this study, we externally validated the DHAKA score in a new population of children and compared it with the IMCI algorithm. The DHAKA score was shown to be significantly more reliable and accurate than the IMCI algorithm (p<0·0001).
Implications of all the available evidence
The DHAKA score is the first dehydration assessment tool empirically derived and externally validated for use in a low-income country. Further validation studies of the DHAKA score in different regions of the world and with different populations of children and providers should be prioritised to establish its generalisability to children with acute diarrhoea worldwide.
Acknowledgments
We thank the National Institutes of Health Fogarty International Center for providing funding for this research (grant number 1K01TW009208-01A1), and the International Centre for Diarrhoeal Disease Research, Bangladesh, for their support of this project. We would also thank Charles Carpenter for his contribution to this Article.
Footnotes
See Online for appendix
Contributors
ACL and NHA designed the research study. SN, BA, PM, NHA, and ACL supervised data collection. JG-B, SRe, SRo, CHS, and ACL cleaned and analysed data. ACL, JG-B, PM, SN, BA, SRe, SRo, CHS, and NHA wrote the manuscript. All authors read and approved the final manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–16. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto C, Black RE. Diarrhoea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health. 2012;12:220. doi: 10.1186/1471-2458-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–72. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. The treatment of diarrhoea: a manual for physicians and other senior health workers. Geneva: World Health Organization; 2005. [Google Scholar]
- 6.King CK, Glass R, Bresee JS, Duggan C, Centers for Disease Control and Prevention Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52:1–16. [PubMed] [Google Scholar]
- 7.National Collaborating Centre for Women’s and Children’s Health (UK) Diarrhoea and vomiting caused by gastroenteritis: diagnosis, assessment and management in children younger than 5 years. London: RCOG Press; 2009. [PubMed] [Google Scholar]
- 8.Farthing M, Salam MA, Lindberg G, et al. Acute diarrhoea in adults and children: a global perspective. J Clin Gastroenterol. 2013;47:12–20. doi: 10.1097/MCG.0b013e31826df662. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca BK, Holdgate A, Craig JC. Enteral vs intravenous rehydration therapy for children with gastroenteritis: a meta-analysis of randomized controlled trials. Arch Pediatr Adolesc Med. 2004;158:483–90. doi: 10.1001/archpedi.158.5.483. [DOI] [PubMed] [Google Scholar]
- 10.Ngabo F, Mvundura M, Gazley L, et al. The economic burden attributable to a child’s inpatient admission for diarrhoeal disease in Rwanda. PLoS One. 2016;11:e0149805. doi: 10.1371/journal.pone.0149805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291:2746–54. doi: 10.1001/jama.291.22.2746. [DOI] [PubMed] [Google Scholar]
- 12.Freedman SB, Vandermeer B, Milne A, Hartling L, Pediatric Emergency Research Canada Gastroenteritis Study Group Diagnosing clinically significant dehydration in children with acute gastroenteritis using noninvasive methods: a meta-analysis. J Pediatr. 2015;166:908–16. doi: 10.1016/j.jpeds.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Modi P, Glavis-Bloom J, Nasrin S, et al. Accuracy of Inferior Vena Cava Ultrasound for Predicting Dehydration in Children with Acute Diarrhoea in Resource-Limited Settings. PLoS One. 2016;11:e0146859. doi: 10.1371/journal.pone.0146859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Handbook: IMCI integrated management of childhood illness. Geneva: World Health Organization; 2005. [Google Scholar]
- 15.Pringle K, Shah SP, Umulisa I, et al. Comparing the accuracy of the three popular clinical dehydration scales in children with diarrhoea. Int J Emerg Med. 2011;4:58. doi: 10.1186/1865-1380-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine AC, Munyaneza RM, Glavis-Bloom J, et al. Prediction of severe disease in children with diarrhoea in a resource-limited setting. PLoS One. 2013;8:e82386. doi: 10.1371/journal.pone.0082386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jauregui J, Nelson D, Choo E, et al. External validation and comparison of three pediatric clinical dehydration scales. PLoS One. 2014;9:e95739. doi: 10.1371/journal.pone.0095739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorelick MH, Shaw KN, Murphy KO. Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics. 1997;99:E6. doi: 10.1542/peds.99.5.e6. [DOI] [PubMed] [Google Scholar]
- 19.Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997;13:179–82. doi: 10.1097/00006565-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Duggan C, Refat M, Hashem M, Wolff M, Fayad I, Santosham M. How valid are clinical signs of dehydration in infants? J Pediatr Gastroenterol Nutr. 1996;22:56–61. doi: 10.1097/00005176-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Parkin PC, Macarthur C, Khambalia A, Goldman RD, Friedman JN. Clinical and Laboratory Assessment of Dehydration Severity in Children With Acute Gastroenteritis. Clin Pediatr (Phila) 2010;49:235–39. doi: 10.1177/0009922809336670. [DOI] [PubMed] [Google Scholar]
- 22.Levine AC, Glavis-Bloom J, Modi P, et al. Empirically derived dehydration scoring and decision tree models for children with diarrhoea: assessment and internal validation in a prospective cohort study in Dhaka, Bangladesh. Glob Health Sci Pract. 2015;3:405–18. doi: 10.9745/GHSP-D-15-00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardhan PK. Annual statistics of Dhaka hospital. Bangladesh: International Center for Diarrhoeal Disease Research; 2012. [Google Scholar]
- 24.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 25.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: Sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–70. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 26.WHO, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children’s Fund. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 27.Van Calster B, Van Belle V, Vergouwe Y, Steyerberg EW. Discrimination ability of prediction models for ordinal outcomes: relationships between existing measures and a new measure. Biom J. 2012;54:674–85. doi: 10.1002/bimj.201200026. [DOI] [PubMed] [Google Scholar]
- 28.Pruvost I, Dubos F, Chazard E, Hue V, Duhamel A, Martinot A. The value of body weight measurement to assess dehydration in children. PLoS One. 2013;8:e55063. doi: 10.1371/journal.pone.0055063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


