1. Introduction
Acrylamide (Figure 1) is a high-production-volume chemical used in a variety of industrial applications (International Agency for Research on Cancer, 1994; Cosmetic Ingredient Review Expert Panel, 2005). Acrylamide is also found in baked and fried starchy foods (e.g., French fries, potato chips, breads, and cereals; Rosén and Hellenäs, 2002; Tareke et al., 2002), due to Maillard reactions involving reducing sugars, such as glucose, and asparagine, a major amino acid present in potatoes and cereals (Mottram et al., 2002; Stadler et al., 2002). Additional sources of acrylamide include coffee (Dybing et al., 2005) and cigarettes (Bergmark, 1997; International Agency for Research on Cancer, 2004). As a consequence of its ubiquitous occurrence, there has been considerable interest in the carcinogenicity of acrylamide, which has led to a number of bioassays being conducted (Bull et al., 1984a; Bull et al., 1984b; Johnson et al., 1986; Robinson et al., 1986; Friedman et al., 1995; Ølstørn et al., 2007; Von Tungeln et al., 2012; National Toxicology Program, 2012; Beland et al., 2013; Maronpot et al., 2015).
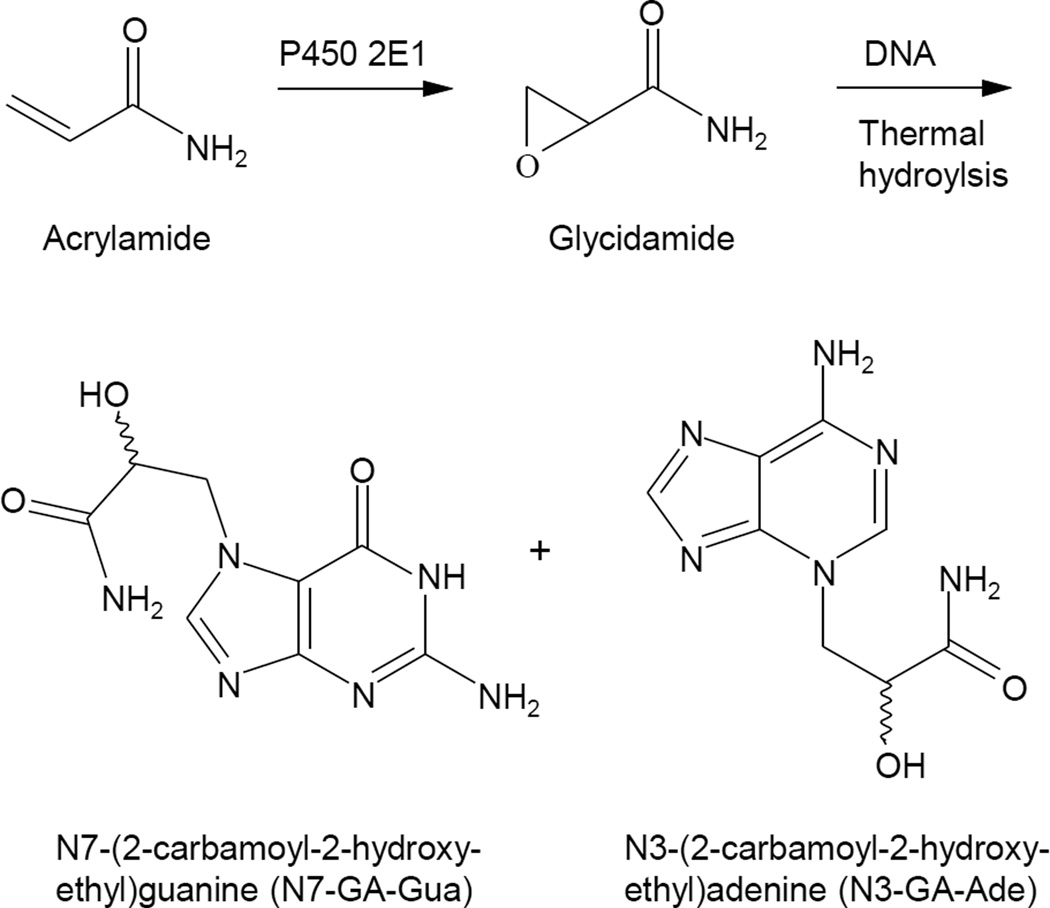
Figure 1.
Structures of acrylamide and glycidamide, and the DNA adducts resulting from the reaction of glycidamide with DNA. N7-GA-Gua and N3-GA-Ade are released from the DNA upon thermal hydrolysis.
Upon ingestion, acrylamide undergoes oxidation by cytochrome P450 2E1 to the epoxide glycidamide (Sumner et al., 1999; Ghanayem et al., 2005a; Figure 1). Although acrylamide will react with DNA, this occurs slowly (Solomon et al., 1985); in contrast, the metabolite glycidamide is considerably more reactive. Several glycidamide-DNA adducts have been characterized (Segerbäck et al., 1995; Solomon, 1999; Gamboa da Costa et al., 2003; Backman et al., 2004; Backman and Kronberg, 2007; Kotova et al., 2011), and two of these, N7-(2-carbamoyl-2-hydroxyethyl)guanine (N7-GA-Gua) and N3-(2-carbamoyl-2-hydroxyethyl)adenine (N3-GA-Ade) (Figure 1), have been detected in mice and rats treated with acrylamide (Segerbäck et al.,1995; Gamboa da Costa et al., 2003; Twaddle et al., 2004; Doerge et al., 2005a; Doerge et al., 2005b; Doerge et al., 2005c; Ghanayem et al., 2005a; Manière et al., 2005; Tareke et al., 2006; Von Tungeln et al., 2009; Zeiger et al., 2009). These data suggest that glycidamide, due to its electrophilic properties, may be responsible for the multi-organ carcinogenic activity of acrylamide. This postulate is supported by a number of observations. Compared to wild-type mice, mice lacking cytochrome P450 2E1 and administered acrylamide have decreased levels of male germ cell mutagenicity, micronuclei, and glycidamide-derived DNA adducts (Ghanayem et al., 2005a; Ghanayem et al., 2005b; Ghanayem et al., 2005c). Transgenic Big Blue mice and rats treated with equimolar doses of acrylamide and glycidamide have mutant frequencies and mutation spectra in the cII transgene consistent with the metabolic conversion of acrylamide to glycidamide (Manjanatha et al., 2006; Wang et al., 2010; Manjantha et al., 2015). Neonatal B6C3F1/Tk+/− mice treated on postnatal days 1, 8, and 15 with glycidamide have an increased mutant frequency at the hypoxanthine-guanine phosphoribosyltransferase gene in splenic T-lymphocytes. This does not occur in mice treated with equimolar doses of acrylamide, which was attributed to the limited capacity of neonatal mice to metabolize acrylamide to glycidamide (Von Tungeln et al., 2009).
The carcinogenicity of glycidamide has been assessed in two studies (Ølstørn et al., 2007; Von Tungeln et al., 2012). In the Ølstørn et al. (2007) experiment, C57BL/6J Min/+ mice, a strain susceptible to intestinal neoplasia, and their wild-type littermates were administered subcutaneous injections of 10 or 50 mg glycidamide per kg body weight (bw) at one and two weeks after birth. In both strains, there was a dose-related induction of tumors of the small intestine, with the increase being significant at 50 mg glycidamide per kg bw. There was no increase in intestinal lesions when acrylamide was given in the same manner at 10 or 50 mg per kg bw. In the Von Tungeln et al. (2012) study, male B6C3F1 mice injected intraperitoneally with 0.70 mmol glycidamide per kg bw on postnatal days 1, 8, and 15 had a significant increase in hepatocellular tumors that was associated with A → G and A → T mutations at codon 61 of the H-ras oncogene. As with the Ølstørn et al. (2007) experiment, mice administered an equimolar amount of acrylamide did not have an increased tumor incidence due to the limited capacity of newborn mice to convert acrylamide to glycidamide.
In a previous study, we assessed the carcinogenicity of acrylamide in B6C3F1 mice and F344/N rats in order to provide data for use in quantitative risk assessments (National Toxicology Program, 2012; Beland et al., 2013). In that experiment, we hypothesized that acrylamide was activated to an ultimate carcinogen through metabolism to glycidamide. To provide additional data for use in quantitative risk assessments and to test this hypothesis, we have now examined the carcinogenicity of glycidamide in B6C3F1 mice and F344/N rats treated chronically with glycidamide in the drinking water for two years.
2. Materials and methods
2.1 Chemicals
Glycidamide (CAS 5694-00–8) was purchased from Toronto Research Chemicals, New York, Ontario, Canada. The purity (> 98%) and identity of the glycidamide were confirmed by gas chromatography coupled with electron impact mass spectrometry, nuclear magnetic resonance spectroscopy, and gas chromatography using flame ionization detection.
2.2 Dose selection
The selection of doses for the two-year glycidamide drinking water study was based upon the effects observed in three-month drinking water studies, conducted simultaneously, with glycidamide (this study) and acrylamide (National Toxicology Program, 2012; Beland et al., 2013). The administration of either 3.52 mM glycidamide or 3.52 mM acrylamide in the drinking water resulted in hind limb paresis and decreased body weight. Decreases in body weight were also observed with 1.41 mM acrylamide. In addition, rats administered 1.41 mM acrylamide displayed hind limb paresis. Since one of the goals of this study was to compare acrylamide with glycidamide, a high dose of 0.70 mM glycidamide was selected for the chronic two-year drinking water study, with the remaining doses being 0.0, 0.0875, 0.175, and 0.35 mM glycidamide. These doses were identical to those used in the two-year chronic bioassay with acrylamide (National Toxicology Program, 2012; Beland et al., 2013).
2.3 Study design
The Institutional Animal Care and Use Committee at the National Center for Toxicological Research (NCTR) reviewed and approved the protocol for these bioassays.
Male and female F344/N Nctr rats and B6C3F1/Nctr (C57BL/6N x C3H/HeN MTV−) mice were obtained from the NCTR breeding colony. Treatment was initiated when the rats were four to five weeks of age and the mice were five to six weeks of age. The rats were housed two of the same sex per cage in polycarbonate cages with hardwood chip bedding. The mice were housed four of the same sex per cage in polycarbonate cages with hardwood chip bedding and micro-isolator tops. Irradiated Purina 5LG6 meal, which was selected for its low acrylamide content (<10 ppb), and Millipore-filtered tap water were provided ad libitum. The stability of glycidamide in the drinking water was assessed for 21 days and determined to be 104 ± 8% (mean ± s.d.; n = 6). Drinking water bottles were replaced weekly. The glycidamide concentrations of the drinking water solutions were determined bi-monthly during the course of the study and found to be acceptable (± 10%, except for the 0.0875 mM dose, where ± 20% was considered to be acceptable). The animal rooms were maintained on a 12-hour light-dark cycle. Environmental controls were set to maintain the temperature at 22 ± 4°C, with a relative humidity of 40 – 70%.
Each dose group consisted of 48 animals per sex per species. Animal inspections were conducted twice daily; body weights, food consumption, and water consumption were measured weekly.
2.4 Necropsy and histopathology
Complete necropsies were performed on all animals, including those that died or became moribund prior to the scheduled terminal sacrifice. Tissues were examined grossly, removed, and preserved in 10% neutral buffered formalin, except the eyes and testes, which were placed in Davidson’s fixative. The tissues were trimmed, processed, and embedded in Formula R® infiltrating medium (Leica Micro Biosystems Division, Richmond, IL), sectioned at approximately 5 µm, and stained with hematoxylin and eosin for microscopic evaluation. In a few cases, special staining procedures were applied to selected lesions to aid in characterizing the pathology changes.
A quality assessment pathologist evaluated slides of all proliferative lesions and target organs. Histopathology slides underpinning the diagnoses made by the study pathologist and the quality assessment pathologist were reviewed by a Pathology Working Group (PWG). Final diagnoses of reviewed lesions represented a consensus between the study pathologist, the quality assessment pathologist, and the PWG.
Acrylamide is neurotoxic in experimental animals (reviewed in National Toxicology Program, 2012), and the possibility exists that glycidamide is likewise. In view of this possibility, an additional pathology quality assessment review was conducted on sections of brain, spinal cord, and peripheral nerve by pathologists from an independent laboratory who have specialized expertise in neuropathology. During this review, all changes in the nervous system were documented, regardless of their severity. Based upon these very stringent criteria, additional lesions were detected. In light of these findings, an additional PWG, consisting of six experienced pathologists, was convened to evaluate the results. This subsequent PWG used criteria similar to those used in the neuropathology quality assessment and concurred that all of the lesions, regardless of their severity, be added to the pathology results.
2.5 Statistical analyses
The SAS Proc Life test procedure was used to obtain Kaplan-Meier (Kaplan and Meier, 1958) estimates of survival times. SAS Proc Phreg was used to conduct Cox proportional hazards regression analyses (Cox, 1972) for comparing dosed to control group hazard rates and testing for linear trends between the hazard and glycidamide dose.
SAS Proc Mixed was used to analyze the effect of glycidamide dose on animal body weight. A sex-stratified, repeated measures, mixed models analysis of variance (ANOVA), with dose and week main effects and a dose by week interaction effect, was fit to the data to obtain least squares estimates of mean body weight for each dose group from weeks 4 to 104 in four-week intervals. Dunnett’s adjusted (Dunnett, 1955) pair-wise comparisons of dosed to control group body weight means were performed to determine if there was a difference between control and individual dosed group means.
Analyses of the effect of glycidamide dose on food and water consumption paralleled those of body weight. The results, however, were determined on a per cage basis, rather than on a per animal basis, and consumption periods were grouped into four-week study periods according to the observation date. The study period was used in lieu of week in the analysis model. Water consumption and body weight data were used to determine glycidamide exposure. Differences in mean food, water, and compound consumption between the previous two-year acrylamide bioassay (National Toxicology Program, 2012; Beland et al., 2013) and the current bioassay were assessed using ANOVA techniques.
Continuity-corrected Poly-3 tests (Bailer and Portier, 1988), modified by Bieler and Williams (1993), were conducted to assess the age-adjusted prevalence of neoplastic and non-neoplastic lesions. P-Values for Poly-3 trend tests were one-sided. Differences in tumor incidences between the previous two-year acrylamide bioassay (National Toxicology Program, 2012; Beland et al., 2013) and the current glycidamide bioassay were also assessed. Logistic regression, using Poly-3 weighted averages, was used to model the dose response of acrylamide and glycidamide and determine if the slopes of these regression lines were equal.
Benchmark doses (BMD) and the lower (BMDL) 95% confidence limits were calculated using Environmental Protection Agency Benchmark Dose software (version 2.1.1; http://www.epa.gov/ncea/bmds). The calculations were conducted using gamma, logistic, log-logistic, log-probit, multistage, probit, and Weibull models to fit the observed neoplastic incidences and the mean doses of glycidamide for the entire two-year study. The BMD10 was defined as the dose that caused a 10% excess risk of the specified adverse effect over that observed in the appropriate control group.
3. Results
Groups of 48 male and 48 female B6C3F1 mice and 48 male and 48 female F344/N rats were administered 0, 0.0875, 0.175, 0.35, and 0.70 mM glycidamide in the drinking water for two years.
3.1 Body weights
3.1.1 B6C3F1 mice
The administration of glycidamide in the drinking water to male and female B6C3F1 mice caused only sporadic statistically significant changes in body weight, with the magnitude of the change never exceeding 5% of the mean control body weight at the same time point (Supplementary Figure S1).
3.1.2 F344/N rats
Glycidamide in the drinking water caused significant dose-related decreasing trends in body weight in male and female F344/N rats (Supplementary Figure S2). Pair-wise comparisons indicated that treatment with 0.70 mM glycidamide resulted in significant decreases in body weight gain beginning at week 8 in male rats. In female rats, treatment with 0.175, 0.35, or 0.70 mM glycidamide resulted in significant decreases in body weight gain beginning at week 4. At the end of the two-year period, male rats administered 0.70 mM glycidamide weighed 82% of the control group; female rats administered 0.70 mM glycidamide weighed 79% of the control group.
3.2 Food consumption
3.2.1 B6C3F1 mice
Glycidamide in the drinking water caused dose-related increasing trends in food consumption beginning at week 84 in male B6C3F1 mice and week 60 in female B6C3F1 mice (Supplementary Figure S3).
3.2.2 F344/N rats
Glycidamide in the drinking water caused only sporadic dose-related trends in food consumption in male and female F344/N rats (Supplementary Figure S4).
3.3 Water consumption
3.3.1 B6C3F1 mice
Glycidamide in the drinking water caused sporadic dose-related increasing trends in water consumption in male B6C3F1 mice (Supplementary Figure S5A). In female B6C3F1 mice, there was a dose-related increasing trend in water consumption beginning at week 76, with water consumption in the 0.70 mM glycidamide group being significantly greater than in the control group beginning at week 80 (Supplementary Figure S5B).
The mean amount of glycidamide consumed by male B6C3F1 mice, calculated at four-week intervals, for the entire two-year experiment was 1.20, 2.65, 5.13, and 9.55 mg glycidamide per kg bw per day for the 0.0875, 0.175, 0.35, and 0.70 mM glycidamide dose groups, respectively. The corresponding values for female B6C3F1 mice were 1.37, 2.89, 5.64, and 12.99 mg glycidamide per kg bw per day.
3.3.2 F344/N rats
Glycidamide in the drinking water did not affect water consumption in either male or female F344/N rats (Supplementary Figure S6). The mean amount of glycidamide consumed by male F344/N rats, calculated at four-week intervals, for the entire two-year experiment was 0.39, 0.79, 1.56, and 3.34 mg glycidamide per kg bw per day for the 0.0875, 0.175, 0.35, and 0.70 mM glycidamide dose groups, respectively. The corresponding values for female rats were 0.54, 1.08, 2.23, and 4.65 mg glycidamide per kg bw per day.
3.4 Survival
3.4.1 B6C3F1 mice
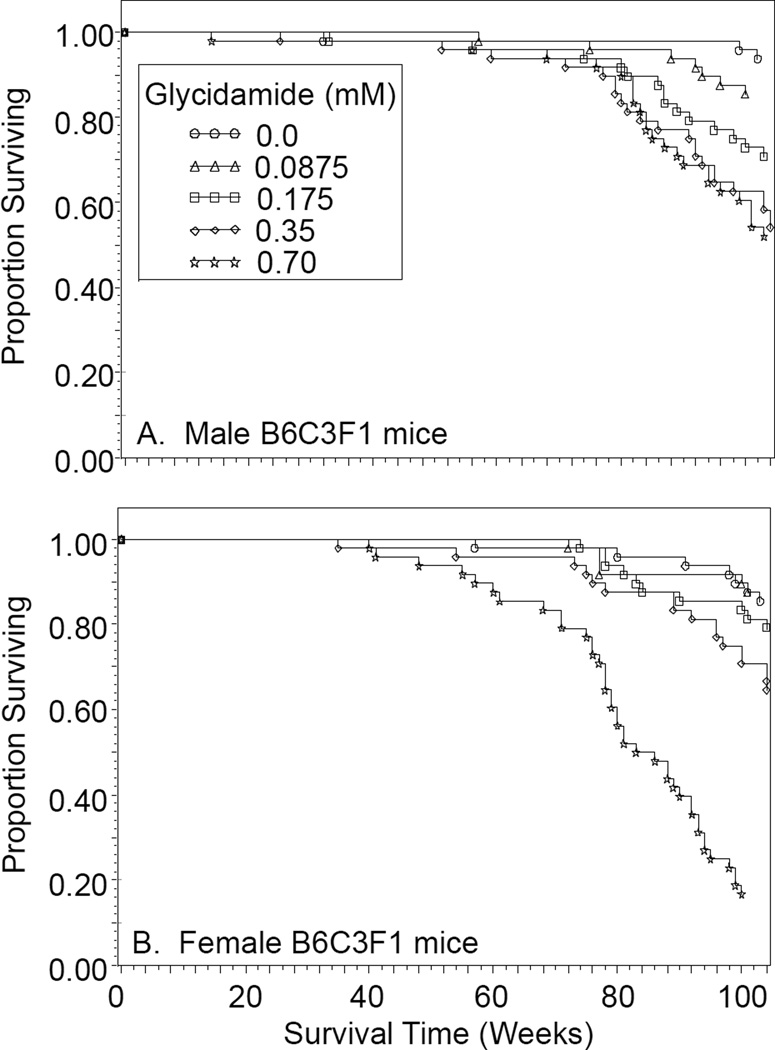
Glycidamide in the drinking water caused a dose-related decreasing trend in survival in male and female B6C3F1 mice (Figure 2). Compared to control mice, male B6C3F1 mice administered 0.175, 0.35, and 0.70 mM glycidamide and female B6C3F1 mice administered 0.35 and 0.70 mM glycidamide had decreased survival.
Figure 2.
Survival of male (A) and female (B) B6C3F1 mice administered 0, 0.0875, 0.175, 0.35, or 0.70 mM glycidamide in the drinking water for two years as a function of the number of weeks on treatment.
3.4.2 F344/N rats
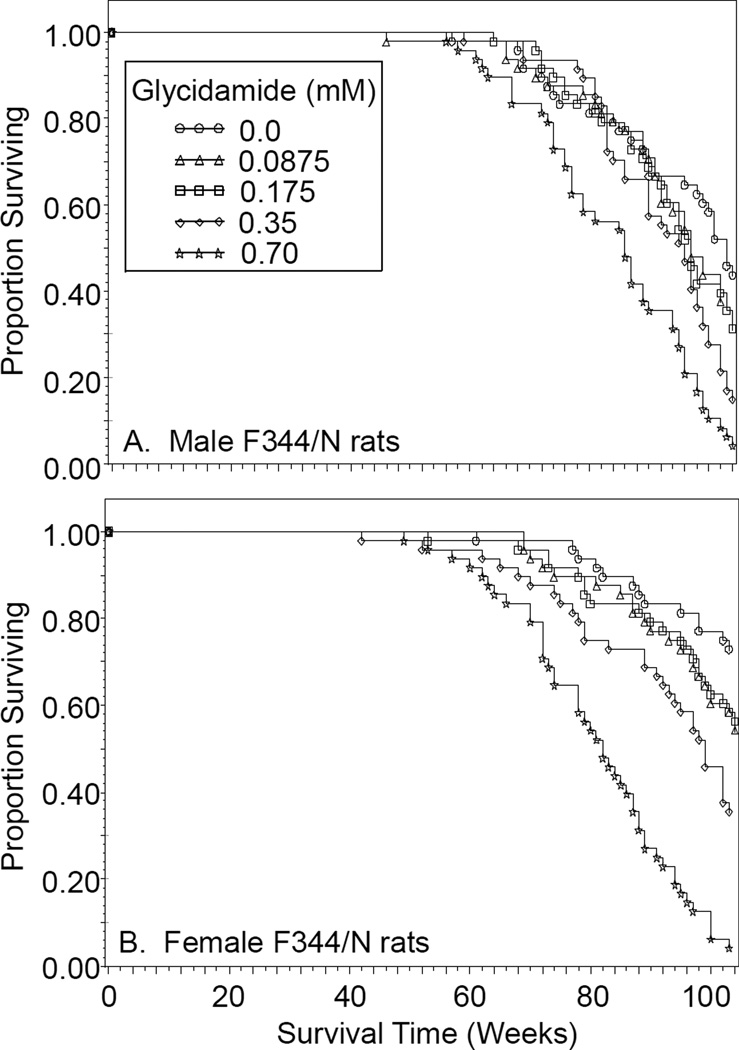
Glycidamide in the drinking water caused a dose-related decreasing trend in survival in male and female F344/N rats (Figure 3). Compared to control rats, both sexes administered 0.35 and 0.70 mM glycidamide had decreased survival.
Figure 3.
Survival of male (A) and female (B) F344/N rats administered 0, 0.0875, 0.175, 0.35, or 0.70 mM glycidamide in the drinking water for two years as a function of the number of weeks on treatment.
3.5 Neoplasms
3.5.1 B6C3F1 mice
Male and female B6C3F1 mice administered glycidamide in the drinking water had dose-related increases in Harderian gland adenoma, with the incidences being significant in both sexes at all doses of glycidamide (Table 1). Harderian gland adenocarcinoma was also observed in one male mouse administered 0.70 mM glycidamide and one female mouse administered 0.175 mM glycidamide.
Table 1.
Incidence of neoplasms in male and female B6C3F1 mice administered 0, 0.0875, 0.175, 0.35, or 0.70 mM glycidamide in the drinking water for two yearsa.
| Neoplasm | Sex | Glycidamide (mM) | ||||
|---|---|---|---|---|---|---|
| 0 | 0.0875 | 0.175 | 0.35 | 0.70 | ||
| Harderian gland adenoma |
Male | 3/47 (6%)*** | 17/47 (36%)*** | 23/47 (49%)*** | 32/46 (70%)*** | 42/47 (89%)*** |
| Female | 2/45 (4%)*** | 19/47 (40%)*** | 20/47 (43%)*** | 24/46 (52%)*** | 40/46 (87%)*** | |
| Lung alveolar/bronchiolar adenoma |
Male | 0/47 (0%)*** | 7/46 (15%)** | 7/47 (15%)** | 13/47 (28%)*** | 17/47 (36%)*** |
| Female | 3/46 (7%)** | 5/48 (10%) | 3/47 (6%) | 7/47 (15%) | 9/44 (20%)** | |
| Stomach (forestomach) squamous cell papilloma |
Male | 0/47 (0%)*** | 2/45 (4%) | 3/48 (6%) | 2/45 (4%) | 10/41 (24%)*** |
| Female | 1/45 (2%)*** | 1/45 (2%) | 1/47 (2%) | 5/45 (11%) | 9/44 (20%)*** | |
| Skin squamous cell papilloma |
Male | 0/47 (0%)*** | 1/48 (2%) | 2/47 (4%) | 1/47 (2%) | 8/46 (17%)*** |
| Skin fibrosarcoma | Female | 0/45 (0%)*** | 1/48 (2%) | 2/47 (4%) | 2/47 (4%) | 9/45 (20%)*** |
| Skin fibrosarcoma or sarcoma |
Female | 0/45 (0%)*** | 1/48 (2%) | 3/47 (6%) | 5/47 (11%)** | 12/45 (27%)*** |
| Mammary gland adenoacanthoma |
Female | 0/45 (0%)*** | 0/48 (0%) | 0/47 (0%) | 1/47 (2%) | 8/45 (18%)*** |
| Mammary gland adenocarcinoma |
Female | 1/45 (2%)*** | 1/48 (2%) | 2/47 (4%) | 9/47 (19%)** | 11/45 (24%)*** |
| Mammary gland adenoacanthoma or adenocarcinoma |
Female | 1/45 (2%)*** | 1/48 (2%) | 2/47 (4%) | 9/47 (19%)** | 18/45 (40%)*** |
| Ovary benign granulosa cell tumor |
Female | 0/45 (0%)* | 0/47 (0%) | 0/47 (0%) | 1/46 (2%) | 2/44 (5%) |
| Ovary malignant granulosa cell tumor |
Female | 0/45 (0%)* | 0/47 (0%) | 0/47 (0%) | 2/46 (4%) | 1/44 (2%) |
| Ovary benign or malignant granulosa cell tumor |
Female | 0/45 (0%)*** | 0/47 (0%) | 0/47 (0%) | 3/46 (7%) | 3/44 (7%) |
The data are reported as the number of animals with a neoplasm per number of animals examined microscopically and (in parentheses) the % incidence. Statistical analyses for dose-related trends and differences in incidence were conducted by survival-adjusted Poly-3 tests.
An asterisk (*) associated with the 0 mM glycidamide incidence indicates a significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) dose-related trend with respect to glycidamide. An asterisk (*) associated with a specific treatment indicates a significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) difference compared to the 0 mM glycidamide incidence.
Dose-related increases in lung alveolar/bronchiolar adenoma occurred in both sexes of B6C3F1 mice, with the incidence being significant at all doses of glycidamide in male mice and at 0.70 mM glycidamide in female mice (Table 1). Low incidences of alveolar/bronchiolar carcinoma (<5%) were also observed in both sexes.
Forestomach neoplasms occurred in both sexes of B6C3F1 mice administered glycidamide, with the incidence being significant at 0.70 mM glycidamide (Table 1). Squamous cell carcinoma of the forestomach was also observed in two male mice administered 0.70 mM glycidamide.
Male B6C3F1 mice exposed to glycidamide had a dose-related increase in squamous cell papilloma of the skin, with the incidence being significant at 0.70 mM glycidamide (Table 1). Squamous cell carcinoma of the skin also occurred in two male mice administered 0.70 mM glycidamide. Female B6C3F1 mice had dose-related increases in malignant mesenchymal skin tumors (fibrosarcoma or combined fibrosarcoma or sarcoma; Table 1). The incidence of fibrosarcoma was significant at 0.70 mM glycidamide and the incidence of combined fibrosarcoma or sarcoma was significant at 0.35 and 0.70 mM glycidamide.
Female B6C3F1 mice administered glycidamide had dose-related increasing trends in adenoacanthoma, adenocarcinoma, and combined adenoacanthoma or adenocarcinoma of the mammary gland (Table 1). The incidence of adenoacanthoma was increased significantly in the 0.70 mM glycidamide dose group, and the incidence of adenocarcinoma and combined adenoacanthoma or adenocarcinoma was increased significantly in the 0.35 and 0.70 mM glycidamide dose groups. Female B6C3F1 mice also had dose-related increasing trends in benign, malignant, and combined benign and malignant granulosa cell tumors of the ovary (Table 1).
3.5.2 F344/N rats
In both sexes of F344/N rats administered glycidamide in the drinking water, there was a dose-related increasing trend in the incidence of thyroid gland follicular cell adenoma, follicular cell carcinoma, and combined follicular cell adenoma or carcinoma (Table 2). In male F344/N rats, the incidence of follicular cell adenoma, follicular cell carcinoma, and combined follicular cell adenoma or carcinoma was significant at 0.70 mM glycidamide. In female F344/N rats, the incidence of follicular cell adenoma and follicular cell carcinoma was significant at 0.70 mM glycidamide, while the incidence of combined follicular cell adenoma or carcinoma was significant at 0.175, 0.35, and 0.70 mM glycidamide.
Table 2.
Incidence of neoplasms in male and female F344/N rats administered 0, 0.0875, 0.175, 0.35, or 0.70 mM glycidamide in the drinking water for two yearsa.
| Neoplasm | Sex | Glycidamide (mM) | ||||
|---|---|---|---|---|---|---|
| 0 | 0.0875 | 0.175 | 0.35 | 0.70 | ||
| Thyroid gland follicular cell adenoma |
Male | 2/47 (4%)*** | 1/42 (2%) | 3/48 (6%) | 3/47 (6%) | 8/46 (17%)** |
| Female | 0/48 (0%)* | 3/48 (6%) | 3/46 (7%) | 1/46 (2%) | 5/47 (11%)** | |
| Thyroid gland follicular cell carcinoma |
Male | 0/47 (0%)** | 2/42 (5%) | 3/48 (6%) | 1/47 (2%) | 5/46 (11%)** |
| Female | 0/48 (0%)** | 0/48 (0%) | 2/46 (4%) | 3/46 (7%) | 3/47 (6%)* | |
| Thyroid gland follicular cell adenoma or carcinoma |
Male | 2/47 (4%)*** | 3/42 (7%) | 6/48 (13%) | 4/47 (9%) | 13/46 (28%)*** |
| Female | 0/48 (0%)*** | 3/48 (6%) | 5/46 (11%)* | 4/46 (9%)* | 8/47 (17%)*** | |
| Oral mucosa squamous cell papilloma |
Male | 1/48 (2%)* | 1/48 (2%) | 0/48 (0%) | 2/47 (4%) | 3/48 (6%) |
| Female | 1/48 (2%)* | 1/48 (2%) | 2/48 (4%) | 0/48 (0%) | 4/48 (8%) | |
| Oral mucosa squamous cell carcinoma |
Male | 1/48 (2%) | 0/48 (0%) | 1/48 (2%) | 1/47 (2%) | 0/48 (0%) |
| Female | 0/48 (0%)* | 0/48 (0%) | 0/48 (0%) | 1/48 (2%) | 2/48 (4%) | |
| Tongue squamous cell papilloma |
Male | 0/48 (0%)** | 1/48 (2%) | 0/48 (0%) | 1/47 (2%) | 4/48 (8%)* |
| Female | 0/48 (0%) | 1/48 (2%) | 0/48 (0%) | 1/48 (2%) | 0/48 (0%) | |
| Tongue squamous cell carcinoma |
Male | 0/48 (0%) | 0/48 (0%) | 1/48 (2%) | 0/47 (0%) | 0/48 (0%) |
| Female | 0/48 (0%) | 0/48 (0%) | 0/48 (0%) | 0/48 (0%) | 1/48 (2%) | |
| Oral mucosa or tongue squamous cell papilloma or carcinoma |
Male | 2/48 (4%)** | 2/48 (4%) | 2/48 (4%) | 3/47 (6%) | 7/48 (15%)* |
| Female | 1/48 (2%)*** | 2/48 (4%) | 2/48 (4%) | 2/48 (4%) | 7/48 (15%)** | |
| Mononuclear cell leukemia |
Male | 21/48 (44%)** | 26/48 (54%) | 27/48 (56%) | 27/47 (57%) | 31/48 (65%)** |
| Female | 14/48 (29%)*** | 11/48 (23%) | 21/48 (44%) | 19/48 (40%) | 27/48 (56%)*** | |
| Epididymis malignant mesothelioma |
Male | 0/48 (0%)*** | 1/45 (2%) | 3/48 (6%) | 10/47 (21%)*** | 17/47 (36%)*** |
| Testes malignant mesothelioma |
Male | 0/48 (0%)*** | 1/47 (2%) | 3/48 (6%) | 6/47 (13%)** | 13/48 (27%)*** |
| Epididymis or testes malignant mesothelioma |
Male | 0/48 (0%)*** | 1/48 (2%) | 3/48 (6%) | 10/47 (21%)*** | 17/48 (35%)*** |
| Heart malignant Schwannoma |
Male | 2/48 (4%)** | 3/48 (6%) | 3/48 (6%) | 7/47 (15%) | 8/48 (17%)* |
| Mammary gland fibroadenoma |
Female | 16/48 (33%)*** | 26/48 (54%)* | 35/48 (73%)*** | 33/48 (69%)*** | 36/48 (75%)*** |
| Clitoral gland adenoma | Female | 6/48 (13%) | 3/48 (6%) | 6/48 (13%) | 3/48 (6%) | 5/47 (11%) |
| Clitoral gland carcinoma | Female | 4/48 (8%)*** | 6/48 (13%) | 7/48 (15%) | 11/48 (23%)* | 14/47 (30%)*** |
| Clitoral gland squamous cell papilloma |
Female | 0/48 (0%)** | 0/48 (0%) | 0/48 (0%) | 1/48 (2%) | 2/47 (4%) |
| Clitoral gland squamous cell carcinoma |
Female | 2/48 (4%) | 0/48 (0%) | 0/48 (0%) | 0/48 (0%) | 0/47 (0%) |
| Clitoral gland adenoma, carcinoma, or squamous cell papilloma or carcinoma |
Female | 11/48 (23%)*** | 9/48 (19%) | 13/48 (27%) | 14/48 (29%) | 20/47 (43%)*** |
| Stomach (forestomach) squamous cell papilloma |
Female | 0/48 (0%)* | 1/48 (2%) | 0/48 (0%) | 0/47 (0%) | 3/46 (7%)* |
The data are reported as the number of animals with a neoplasm per number of animals examined microscopically and (in parentheses) the % incidence. Statistical analyses for dose-related trends and differences in incidence were conducted by survival-adjusted Poly-3 tests.
An asterisk (*) associated with the 0 mM glycidamide incidence indicates a significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) dose-related trend with respect to glycidamide. An asterisk (*) associated with a specific treatment indicates a significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) difference compared to the 0 mM glycidamide incidence.
The administration of glycidamide was associated with a dose-related increase in squamous cell papilloma of the oral mucosa and tongue and combined squamous cell papilloma or carcinoma of the oral mucosa or tongue in male F344/N rats (Table 2). The incidence of squamous cell papilloma of the tongue and combined squamous cell papilloma or carcinoma of the oral mucosa or tongue was significant at 0.70 mM glycidamide. In female F344/N rats, there was a dose-related increase in squamous cell papilloma of the oral mucosa, squamous cell carcinoma of the oral mucosa and combined squamous cell papilloma or carcinoma of the oral mucosa or tongue, with the incidence of combined squamous cell papilloma or carcinoma of the oral mucosa or tongue being significant at 0.70 mM glycidamide. Both sexes of F344/N rats also had dose related increases in the incidence of mononuclear cell leukemia, with the increase in incidence being significant at 0.70 mM glycidamide (Table 2).
Exposure to glycidamide in the drinking water was associated with development of malignant mesothelioma on membranes surrounding the epididymis and on testicular tunicae in male F344/N rats (Table 2). The incidence of malignant mesothelioma was significant in the epididymis, testes, and combined testes or epididymis at 0.35 and 0.70 mM glycidamide. Glycidamide in the drinking water also resulted in a dose-related increase in malignant Schwannoma in the heart of male F344/N rats, with the incidence being significant at 0.70 mM glycidamide (Table 2).
Female F344/N rats exposed to glycidamide in the drinking water had an increased occurrence of fibroadenomas in the mammary gland, with the incidence being significantly increased at all doses (Table 2). Female F344/N rats also had dose-related increases in clitoral gland carcinoma and forestomach squamous cell papilloma. The incidence of clitoral gland carcinoma was significant at 0.35 and 0.70 mM glycidamide, while the occurrence of forestomach squamous cell papilloma was significant at 0.70 mM glycidamide (Table 2).
3.6 Non-neoplastic lesions
3.6.1 B6C3F1 mice
The drinking water administration of glycidamide to B6C3F1 mice resulted in a dose-related increase in cataracts and corneal inflammation in both sexes (Table 3). The incidence of cataracts was increased in the 0.175, 0.35, and 0.70 mM glycidamide dose groups, while the incidence of corneal inflammation was increased at 0.70 mM glycidamide. Glycidamide administration resulted in a dose-related increasing trend in epithelium hyperplasia of the forestomach in both sexes of B6C3F1 mice, with the incidence being significant at 0.70 mM glycidamide in male mice and 0.35 mM glycidamide in female mice. Both sexes of B6C3F1 mice also had increasing dose-related trends in hematopoietic cell proliferation of the spleen, with the incidence being significant at 0.35 and 0.70 mM glycidamide.
Table 3.
Incidence of non-neoplastic lesions in male and female B6C3F1 mice administered 0, 0.0875, 0.175, 0.35, or 0.70 mM glycidamide in the drinking water for two yearsa.
| Non-neoplastic lesion | Sex | Glycidamide (mM) | ||||
|---|---|---|---|---|---|---|
| 0 | 0.0875 | 0.175 | 0.35 | 0.70 | ||
| Eye cataracts | Male | 1/47 (2%)*** | 3/45 (7%) | 7/46 (15%)* | 8/44 (18%)** | 17/42 (40%)*** |
| Female | 1/45 (2%)*** | 2/44 (5%) | 8/47 (17%)* | 8/44 (18%)** | 9/43 (21%)*** | |
| Eye corneal inflammation |
Male | 0/47 (0%)*** | 0/45 (0%) | 2/46 (4%) | 0/44 (0%) | 8/42 (19%)*** |
| Female | 0/45 (0%)** | 2/44 (5%) | 1/47 (2%) | 3/44 (7%) | 5/43 (12%)** | |
| Stomach (forestomach) epithelium hyperplasia |
Male | 5/47 (11%)*** | 2/45 (4%) | 5/48 (10%) | 5/45 (11%) | 12/41 (29%)** |
| Female | 4/45 (9%)* | 4/45 (9%) | 10/47 (21%) | 11/45 (24%)* | 5/44 (11%) | |
| Spleen hematopoietic cell proliferation |
Male | 6/47 (13%)*** | 6/47 (13%) | 12/47 (26%) | 14/46 (30%)* | 17/44 (39%)*** |
| Female | 6/46 (13%)*** | 10/47 (21%) | 11/47 (23%) | 14/47 (30%)* | 29/45 (64%)*** | |
| Lung alveolar epithelium hyperplasia |
Male | 0/47 (0%)** | 1/46 (2%) | 4/47 (9%)* | 3/47 (6%) | 6/47 (13%)** |
| Liver angiectasis | Female | 0/47 (0%)*** | 0/48 (0%) | 1/47 (2%) | 0/46 (0%) | 5/43 (12%)** |
| Liver necrosis | Female | 0/47 (0%)*** | 0/48 (0%) | 0/47 (0%) | 0/46 (0%) | 5/43 (12%)** |
| Spinal cord (cervical) axonal degeneration |
Female | 4/45 (9%)** | 9/44 (20%) | 10/47 (21%) | 9/45 (20%) | 10/43 (23%)** |
The data are reported as the number of animals with a non-neoplastic lesion per number of animals examined microscopically and (in parentheses) the % incidence. Statistical analyses for dose-related trends and differences in incidence were conducted by survival-adjusted Poly-3 tests.
An asterisk (*) associated with the 0 mM glycidamide incidence indicates a significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) dose-related trend with respect to glycidamide. An asterisk (*) associated with a specific treatment indicates a significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) difference compared to the 0 mM glycidamide incidence.
Other treatment-related non-neoplastic lesions in male B6C3F1 mice included alveolar epithelium hyperplasia of the lung (Table 3). Additional non-neoplastic lesions in female B6C3F1 mice included angiectasis and necrosis of the liver and axonal degeneration of the spinal cord.
3.6.2 F344/N rats
Drinking water administration of glycidamide to F334/N rats resulted in a dose-related increase in brain gliosis in both sexes (Table 4). In male rats, the incidence of gliosis was increased in the 0.70 mM glycidamide dose group, while in female rats the incidence was increased at 0.175, 0.35, and 0.70 mM glycidamide. Male F344/N rats also had glycidamide-related increases in hepatocyte degeneration and necrosis in the liver. Additional non-neoplastic lesions associated with glycidamide exposure in female F344/N rats included axonal degeneration of the spinal cord and uterine endometrial hyperplasia.
Table 4.
Incidence of non-neoplastic lesions in male and female F344/N rats administered 0, 0.0875, 0.175, 0.35, or 0.70 mM glycidamide in the drinking water for two yearsa.
| Non-neoplastic lesion | Sex | Glycidamide (mM) | ||||
|---|---|---|---|---|---|---|
| 0 | 0.0875 | 0.175 | 0.35 | 0.70 | ||
| Brain gliosis | Male | 0/48 (0%)** | 1/48 (2%) | 0/48 (0%) | 0/47 (0%) | 4/48 (8%)* |
| Female | 0/48 (0%)** | 0/48 (0%) | 4/48 (8%)* | 4/48 (8%)* | 4/48 (8%)* | |
| Liver hepatocyte degeneration |
Male | 2/47 (4%)** | 6/47 (13%) | 6/48 (13%) | 10/47 (21%)** | 8/47 (17%)** |
| Liver necrosis | Male | 1/47 (2%)* | 5/47 (11%) | 2/48 (4%) | 7/47 (15%)* | 5/47 (11%)* |
| Spinal cord (lumbar) axonal degeneration |
Female | 5/48 (10%)* | 6/48 (13%) | 5/47 (11%) | 6/48 (13%) | 9/48 (19%)* |
| Uterus endometrium cystic hyperplasia |
Female | 11/48 (23%)*** | 17/48 (35%) | 14/48 (29%) | 14/48 (29%) | 23/48 (48%)*** |
The data are reported as the number of animals with a non-neoplastic lesion per number of animals examined microscopically and (in parentheses) the % incidence. Statistical analyses for dose-related trends and differences in incidence were conducted by survival-adjusted Poly-3 tests.
An asterisk (*) associated with the 0 mM glycidamide incidence indicates a significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) dose-related trend with respect to glycidamide. An asterisk (*) associated with a specific treatment indicates a significant (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001) difference compared to the 0 mM glycidamide incidence.
4. Discussion
Acrylamide is a contaminant in baked and fried starchy foods, roasted coffee, and cigarette smoke as a result of Maillard reactions involving asparagine and reducing sugars. Previously we reported that acrylamide is a multi-organ carcinogen in male and female B6C3F1 mice and F344/N rats (National Toxicology Program, 2012; Beland et al., 2013), and we hypothesized that acrylamide was activated to an ultimate carcinogen through cytochrome P450-catalzyed oxidation to the epoxide glycidamide. We have now examined this hypothesis by comparing the carcinogenic effects of glycidamide administered at equimolar doses to the same strains of rodents.
4.1 B6C3F1 mice
The administration of glycidamide in the drinking water to B6C3F1 mice resulted in only sporadic changes in body weight (Supplementary Figure S1). The doses of glycidamide selected for the current bioassay (0, 0.0875, 0.175, 0.35, and 0.70 mM in the drinking water) were identical to those used in our previous bioassay with acrylamide, and there were only sporadic differences in body weights when comparisons were made between mice administered glycidamide and mice given acrylamide. The survival of the B6C3F1 mice was affected by glycidamide, with significant decreases in survival being observed at the three highest dose levels in male mice and the two highest dose levels in female mice (Figure 2). Similar trends existed with B6C3F1 mice given acrylamide. In addition to having comparable body weights and survival, B6C3F1 mice exposed to glycidamide or acrylamide typically consumed similar (±10%) amounts of each of the compounds (on a µmol per kg body weight basis). These results indicate that any differences in the incidence of neoplasms between mice given glycidamide and those administered acrylamide are not a consequence of differences in body weights, survival, or amount of test compound consumed.
The most sensitive site for tumor induction in B6C3F1 mice administered glycidamide in the drinking water was the Harderian gland (Table 1), and even at the lowest dose of glycidamide (0.0875 mM), the incidence of Harderian gland adenoma exceeded the range observed in control male and female B6C3F1 mice in experiments conducted at the NCTR. As we reported previously, the Harderian gland was also the most sensitive site for tumor induction in the B6C3F1 mice administered acrylamide in the drinking water (National Toxicology Program, 2012, Beland et al., 2013), and a comparison of the bioassays (Supplementary Table S1) indicates that the tumor incidences were similar. These data, plus the fact that other low-molecular-weight carcinogens thought to be metabolized to electrophilic epoxides also target the Harderian gland in B6C3F1 mice (Bucher et al., 1990; Melnick and Sills, 2001; Melnick, 2002; Beland et al., 2005a), strongly support the concept that the carcinogenic activity of acrylamide in the Harderian gland of B6C3F1 mice is due to its metabolism to glycidamide.
In both sexes of B6C3F1 mice, there were significant dose-dependent increases in alveolar/bronchiolar adenoma of the lung (Table 1), and the incidences in the 0.35 and 0.70 mM glycidamide dose groups exceeded the range observed in control B6C3F1 mice in experiments conducted at the NCTR. As with Harderian gland adenoma, the incidence of alveolar/bronchiolar adenoma in B6C3F1 mice administered glycidamide did not differ significantly from the incidence in mice given acrylamide (Supplementary Table S1). Furthermore, Big Blue mice administered equimolar quantities of acrylamide or glycidamide had similar increases in the mutant frequencies at the cII transgene in their lungs (Manjanatha et al., 2015). These results, coupled with the observation that glycidamide and acrylamide give very similar DNA adduct profiles in the lungs of B6C3F1 mice and other strains of mice (Gamboa da Costa et al., 2003; Doerge et al., 2005c; Von Tungeln et al., 2009) are consistent with the premise that the lung neoplasms induced in B6C3F1 mice are due to acrylamide being metabolized to glycidamide.
In addition to Harderian gland and lung adenoma, the drinking water exposure to glycidamide resulted in significant dose-related increases in forestomach and skin neoplasms in both sexes of B6C3F1 mice and mammary gland neoplasms in female B6C3F1 mice. Forestomach neoplasms were also observed in male B6C3F1 mice administered acrylamide in the drinking water (National Toxicology Program, 2012; Beland et al., 2013), and the incidence of these tumors did not differ significantly between the two compounds (Supplementary Table S1). Likewise, skin and mammary gland neoplasms occurred in female B6C3F1 mice treated with acrylamide, and as was the case with forestomach neoplasms in male mice, the incidence of these neoplasms did not differ significantly between female mice given glycidamide and those treated with acrylamide (Supplementary Table S1). Other low-molecular-weight epoxides (e.g., glycidol; Irwin et al., 1996) or alkenes that are thought to be metabolized to electrophilic epoxides (e.g., 1,3-butadiene, chloroprene, and urethane; Melnick and Sills, 2001; Melnick, 2002; Beland et al., 2005a) also induce mammary gland neoplasms in female B6C3F1 mice.
Male B6C3F1 mice treated neonatally with glycidamide developed a high incidence of combined hepatocellular adenoma or carcinoma (Von Tungeln et al., 2012). In other studies, high levels of N7-GA-Gua and N3-GA-Ade have been detected in liver DNA from mice treated as adults with glycidamide (Gamboa da Costa et al., 2003; Doerge et al., 2005a; Doerge et al., 2005c; Tareke et al., 2006) and an increase in mutant frequency has been observed in the cII transgene in the livers of adult Big Blue mice dosed orally with glycidamide for four weeks (Manjanatha et al., 2006). These data suggested that glycidamide had the potential to be hepatocarcinogenic in the current bioassays. Liver necrosis was observed in female B6C3F1 mice (Table 3) administered glycidamide; nonetheless, an increased incidence of hepatocellular tumors was not observed. B6C3F1 mice treated with acrylamide also did not have increased incidences of liver neoplasms (National Toxicology Program, 2012; Beland et al., 2013). While unexpected, there is precedent for the induction of liver tumors in mice treated as newborns but not as adults. The administration of benzo[a]pyrene to neonatal B6C3F1 mice results in an increased incidence of liver tumors (Wiseman et al., 1987; Flammang et al, 1997); however, although substantial levels of benzo[a]pyrene-derived DNA adducts were detected in the livers of adult B6C3F1 mice fed diets containing benzo[a]pyrene (Beland et al., 2005b), this did not lead to an increased hepatic tumor incidence in a two-year chronic bioassay (Culp et al., 1998). Likewise, treating newborn B6C3F1 mice with aflatoxin B1 resulted in the induction of liver tumors (Vessolinovitch et al., 1972), whereas liver tumors were not induced in various strains of adult mice fed diets containing aflatoxin B1 (Wogan, 1969).
4.2 F344/N rats
The administration of glycidamide in the drinking water to F344/N rats resulted in significant dose-related decreases in body weight, with the effect being most pronounced in the 0.70 mM glycidamide dose group (Supplementary Figure S2). F344/N rats administered acrylamide in the drinking water for two years also had significant dose-related decreases in body weight (National Toxicology Program, 2012; Beland et al., 2013), and a comparison of body weights between these two bioassays indicated that the mean body weights for all dose groups through the entire two year treatment period were typically within 5% of one another.
The survival of the F344/N rats also was affected by glycidamide, with significant decreases in survival being observed at the 0.35 and 0.70 mM doses (Figure 3). Similar trends existed with F344/N rats given acrylamide (National Toxicology Program, 2012; Beland et al., 2013). Furthermore, F344/N rats exposed to glycidamide or acrylamide typically consumed similar (±10%) amounts of each of the compounds (on a µmol per kg body weight basis), thus permitting a direct comparison between the neoplasms arising as a result of the treatments.
Glycidamide induced follicular cell adenoma or carcinoma of the thyroid gland in both male and female F344/N rats. In male rats, the incidence of combined follicular cell adenoma or carcinoma was significantly increased at 0.70 mM glycidamide and in female rats the incidence was increased significantly at 0.175, 0.35, and 0.70 mM glycidamide (Table 2). In both sexes, the incidence in each of the glycidamide dose groups exceeded the historical range observed in control F344/N rats at the NCTR. Follicular cell carcinoma also was detected in all the glycidamide dose groups of F344/N rats, with the exception of female rats administered 0.0875 mM glycidamide. Follicular cell carcinoma of the thyroid gland has not been observed in either male or female control F344/N rats in bioassays conducted at the NCTR. Follicular cell adenoma or carcinoma has been reported in F344 and Wistar Han rats given acrylamide (Johnson et al., 1986; Friedman et al., 1995; National Toxicology Program, 2012; Beland et al., 2013; Maronpot et al., 2015). The incidence of combined follicular cell adenoma or carcinoma of the thyroid gland induced by acrylamide in the bioassay conducted at NCTR did not differ statistically from that induced by glycidamide (Supplementary Table S1). In addition to having similar incidences of follicular cell neoplasms, F344 rats treated with equimolar levels of acrylamide or glycidamide have similar levels of N7-GA-Gua in their thyroid gland DNA (Doerge et al., 2005c). Furthermore, Big Blue rats administered equimolar quantities of acrylamide or glycidamide have increased mutant frequencies at the cII transgene of their thyroid glands (Mei et al., 2010). The totality of these data is consistent with the conversion of acrylamide to glycidamide being an important step in the induction of follicular cell tumors in the thyroid gland of F344/N rats.
The most sensitive site for tumor induction in female F344/N rats administered glycidamide in the drinking water was the mammary gland, where there was a significant increase in fibroadenoma at all dose levels of glycidamide (Table 2). Furthermore, the incidence of mammary gland fibroadenoma in each of the glycidamide dose groups exceeded the range observed in control female F344/N rats at the NCTR. The mammary gland was also the most sensitive site for tumor induction in female F344/N Nctr rats administered acrylamide in the drinking water (National Toxicology Program, 2012; Beland et al., 2013) and the fibroadenoma incidences observed with glycidamide were very similar to those occurring with acrylamide (Supplementary Table S1).
In addition to having similar incidences of mammary gland fibroadenomas, female F344 rats administered a single intraperitoneal injection of 0.7 mmol acrylamide or glycidamide per kg body weight form high levels of N7-GA-Gua in their mammary gland DNA (Doerge et al., 2005c), which supports the concept that the fibroadenomas result from a genotoxic mechanism as a consequence of the metabolic conversion of acrylamide to glycidamide. This interpretation conflicts with the fact that Big Blue rats treated with 0.12 mmol glycidamide or acrylamide per kg body weight per day for two months did not have an increased mutant frequency in the cII transgene in the mammary gland (Mei et al., 2010); however, this may be a consequence of the high spontaneous mutant frequencies that occur with transgenic mutation assays in general. Other low-molecular-weight epoxides, such as glycidol, and alkenes that are thought to be metabolized to epoxides, such as 1,3-butadiene and chloroprene, also induce mammary gland tumors in female F344 rats (Irwin et al., 1996; Melnick and Sills, 2001; Melnick, 2002). In addition, an increased incidence in mammary gland fibroadenoma has recently been reported in female Wistar Han rats administered acrylamide for two years (Maronpot et al., 2015).
Female F344/N rats treated with glycidamide in the drinking water also had increased incidences of clitoral gland carcinoma and oral cavity neoplasms (primarily squamous cell papilloma of the oral mucosa or tongue; Table 2) that, in each of the glycidamide dose groups, exceeded the NCTR historical range for control female F344/N rats. These neoplasms were also observed in female F344/N rats administered acrylamide in the drinking water, with incidences very similar to those recorded in the glycidamide bioassay (Supplementary Table S1). Oral cavity neoplasms (primarily squamous cell papilloma) also occurred in male F344/N rats given glycidamide (Table 2). The incidence in the 0.70 mM glycidamide group was significant and the incidence in all dose groups, including the control group, exceeded the historical control range for male F344/N rats at the NCTR. These neoplasms were also observed in male F344/N rats administered acrylamide, but the incidences were not significant (National Toxicology Program, 2012).
Female F344/N rats treated with 0.70 mM glycidamide had a low, but statistically significant, occurrence of forestomach papilloma (Table 2), the incidence of which exceeded the NCTR historical control range. Mononuclear cell leukemia was also observed in all dose groups of male and female F344/N rats, with the incidence in the 0.70 mM glycidamide group (Table 2) exceeding the NCTR historical control range for female F344/N rats.
In addition to thyroid gland neoplasms, oral cavity neoplasms, and leukemia, malignant mesothelioma of the epididymis or testes was observed in male F344/N rats administered glycidamide (Table 2). The incidence of these neoplasms was significant at 0.35 and 0.70 mM glycidamide and exceeded the NCTR historical control range. Malignant mesothelioma of the epididymis or testes has been reported in F344 rats given acrylamide (Johnson et al., 1986; Friedman et al., 1995; National Toxicology Program, 2012; Beland et al, 2013) and the incidence of these neoplasms was similar to that induced by acrylamide at NCTR except at the 0.70 mM dose, where the incidence was greater with glycidamide (Supplementary Table S1). The higher tumor incidence in rats treated with 0.70 mM glycidamide compared to 0.70 mM acrylamide may reflect the saturation of enzymatic oxidation that occurs at high doses of acrylamide in rats (Bergmark et al., 1991). Higher levels of N7-GA-Gua have also been detected in testicular DNA from rats administered glycidamide as compared to acrylamide (Doerge et al., 2005c), which again may be a consequence of metabolic saturation occurring with acrylamide.
The concordance in the incidence in malignant mesothelioma of the epididymis or testes that occurred at the lower doses of glycidamide and acrylamide supports the concept that the testicular tumors are a result of acrylamide being converted to glycidamide. Although these data suggest a genotoxic mechanism for the induction of testicular tumors, Big Blue rats treated with either acrylamide or glycidamide did not show an increased mutant frequency in the testes (Mei et al., 2010); however this may be a consequence of the fact that the entire testicular tissue rather than the target tunica vaginalis was assessed.
As an alternative to a genotoxic mechanism, malignant mesothelioma of the tunica vaginalis has been proposed to be a consequence of Leydig cell tumor proliferation resulting from carcinogen-induced decreases in testosterone and increases in luteinizing hormone (Shipp et al., 2006; Maronpot et al., 2009). Maronpot et al. (2015) recently reported that acrylamide did not induce malignant mesothelioma in male Wistar Han rats, a strain with a very low incidence of spontaneous Leydig cell tumors compared to that observed in F344/N rats. F344 rats administered relatively high doses of acrylamide (≥ 10 mg acrylamide per kg bw) do have decreased serum levels of testosterone and increased serum levels of luteinizing hormone; nonetheless, Leydig cell proliferation was not evident (Camacho et al., 2012).
Male F344/N rats administered glycidamide developed malignant Schwannoma of the heart (Table 2), with the incidence in all dose groups, including the control group, exceeding the historical control range at the NCTR. This neoplasm also occurred in male F344/N rats treated with acrylamide, at an incidence similar to that found in the current bioassay with glycidamide (Supplementary Table S1). An increased incidence of adenoma or carcinoma of the pancreatic islets was observed in male F344/N rats treated with acrylamide (National Toxicology Program, 2012; Beland et al., 2013). This did not occur with glycidamide.
During the histopathological examinations, special attention was given to the brain and spinal cord tumors of glial cell origin that have been reported in F344 rats administered acrylamide (Johnson et al., 1986). These tumors were not observed in two subsequent bioassays with acrylamide in F344 rats (Friedman et al., 1995; National Toxicology Program, 2012; Beland et al., 2013) nor were they observed in the current bioassay with glycidamide. A treatment-related non-neoplastic brain lesion was gliosis, which was detected in both male and female F344/N rats (Table 4). A non-neoplastic lesion reported in F344 and Wistar Han rats during previous two-year bioassays with acrylamide was peripheral nerve degeneration (Johnson et al., 1986; Friedman et al., 1995; National Toxicology Program, 2012; Beland et al., 2013; Maronpot et al., 2015). A dose-related prevalence of this lesion was not observed in F344/N rats in the current study with glycidamide, although it should be noted that a high incidence of the lesion was detected in all dose groups, including the controls.
4.3 Benchmark dose comparisons
At the initiation of this study we hypothesized that acrylamide is carcinogenic due to its metabolic conversion to glycidamide. To evaluate this hypothesis, benchmark dose modeling was conducted to estimate the doses of glycidamide that would give a 10% excess risk for specific neoplasms (BMD10); these doses were then compared to BMD10 values previously determined for acrylamide (Beland et al., 2013). In B6C3F1 mice, the most sensitive site for tumor induction by glycidamide was the Harderian gland, with a BMD10 for Harderian gland adenoma of 5.24 to 5.91 µmol glycidamide per kg body weight per day in male mice and 4.55 µmol glycidamide per kg body weight per day in female mice (Supplementary Table S2). In F344/N rats, the most sensitive site for tumor induction was the mammary gland in female rats (BMD10 of 2.39 µmol glycidamide per kg body weight per day for fibroadenoma) and the epididymis or testes in male rats (BMD10 of 11.23 to 17.94 µmol glycidamide per kg body weight per day for malignant mesothelioma) (Supplementary Table S2).
A comparison of these data with those previously reported for acrylamide (Beland et al., 2013) indicates that both chemicals have similar potencies in the target tissues (Table 5). For example, the BMD10 for Harderian gland adenoma in male B6C3F1 mice was 5.51 to 5.91 µmol per kg body weight per day for glycidamide as compared to 5.14 to 5.39 µmol per kg body weight per day for acrylamide. Likewise, in female B6C3F1 mice the BMD10 for Harderian gland adenoma was 4.55 µmol per kg body weight per day for glycidamide as compared to 6.65 µmol per kg body weight per day for acrylamide. In female F344/N rats, the BMD10 for mammary gland fibroadenoma was 2.39 µmol per kg body weight per day for glycidamide and 7.74 µmol per kg body weight per day for acrylamide, and in male F344/N rats, the BMD10 for malignant mesothelioma of the epididymis or testes was 11.23 to 17.94 µmol per kg body weight per day for glycidamide and 29.90 to 30.66 µmol per kg body weight per day for acrylamide.
Table 5.
Comparison of BMD10 for selected neoplasms in male and female B6C3F1 mice and F344/N rats administered 0, 0.0875, 0.175, 0.35, or 0.70 mM glycidamide or acrylamide in the drinking water for two years.
| Species | Neoplasm | Sex | Model | Glycidamide (BMD10, µmol/kg body weight/day)a |
Acrylamide (BMD10, µmol/kg body weight/day)b |
|---|---|---|---|---|---|
| B6C3F1 mice |
Harderian gland, adenoma |
Male | Log-Logistic | 5.51 | 5.14 |
| Log-Probit | 5.91 | 5.39 | |||
| Female | Log-Logistic | 4.55 | 6.65 | ||
| B6C3F1 mice |
Lung, alveolar/ bronchiolar adenoma |
Male | Log-Logistic | 17.16 | 29.59 |
| Female | Gamma | 99.36 | 27.69 | ||
| Logistic | 112.72 | 56.06 | |||
| Log-Logistic | 99.08 | 27.15 | |||
| Log-Probit | 100.00 | 26.89 | |||
| Multistage | 98.98 | 27.69 | |||
| Probit | 110.13 | 51.97 | |||
| Weibull | 99.33 | 27.69 | |||
| B6C3F1 mice |
Stomach (forestomach), squamous cell papilloma |
Male | Gamma | 52.55 | 63.98 |
| Logistic | 76.50 | 105.74 | |||
| Log-Logistic | 51.96 | 62.30 | |||
| Log-Probit | 49.72 | 60.51 | |||
| Multistage | 55.10 | 63.98 | |||
| Probit | 73.15 | 101.39 | |||
| Weibull | 52.55 | 63.98 | |||
| B6C3F1 mice |
Mammary gland, adenoacanthoma |
Female | Gamma | 117.96 | 140.56 |
| Logistic | 129.97 | 146.10 | |||
| Log-Logistic | 119.46 | 142.80 | |||
| Log-Probit | 115.08 | 172.55 | |||
| Multistage | 116.96 | 140.56 | |||
| Probit | 125.67 | 145.74 | |||
| Weibull | 120.56 | 140.56 | |||
| B6C3F1 mice |
Mammary gland, adenoacanthoma or adenocarcinoma |
Female | Gamma | 53.46 | 31.22 |
| Log-Logistic | 52.72 | 28.31 | |||
| Log-Probit | 51.98 | 24.23 | |||
| Multistage | 55.63 | 31.22 | |||
| Weibull | 53.41 | 31.22 | |||
| F344/N rats |
Thyroid gland, follicular cell carcinoma |
Male | Gamma | 40.32 | 28.57 |
| Logistic | 42.24 | 35.95 | |||
| Log-Logistic | 39.76 | 27.87 | |||
| Log-Probit | 70.34 | 26.72 | |||
| Multistage | 40.32 | 28.57 | |||
| Probit | 42.65 | 35.00 | |||
| Weibull | 40.32 | 28.57 | |||
| F344/N rats |
Thyroid gland, follicular cell adenoma or carcinoma |
Male | Gamma | 19.33 | 20.37 |
| Logistic | 22.62 | 28.19 | |||
| Log-Logistic | 20.77 | 19.47 | |||
| Multistage | 20.85 | 20.37 | |||
| Probit | 21.65 | 27.14 | |||
| Weibull | 20.50 | 20.37 | |||
| Female | Gamma | 27.00 | 54.15 | ||
| Logistic | 42.74 | 60.24 | |||
| Log-Logistic | 24.05 | 54.80 | |||
| Multistage | 27.00 | 54.15 | |||
| Probit | 41.31 | 59.36 | |||
| Weibull | 27.00 | 54.15 | |||
| F344/N rats |
Heart, malignant Schwannoma |
Male | Gamma | 25.06 | 34.13 |
| Logistic | 31.09 | 37.68 | |||
| Log-Logistic | 24.40 | 33.91 | |||
| Log-Probit | 23.23 | 34.76 | |||
| Multistage | 25.06 | 34.13 | |||
| Probit | 30.17 | 37.23 | |||
| Weibull | 25.06 | 34.13 | |||
| F344/N rats |
Epididymis or testes, malignant mesothelioma |
Male | Gamma | 11.71 | 29.90 |
| Multistage | 11.59 | 30.66 | |||
| Probit | 17.94 | 30.36 | |||
| Weibull | 11.69 | 30.06 | |||
| F344/N rats |
Mammary gland, fibroadenoma |
Female | Log-Logistic | 2.39 | 7.74 |
| F344/N rats |
Oral mucosa or tongue, squamous cell papilloma or carcinoma |
Female | Gamma | 50.94 | 49.49 |
| Logistic | 48.94 | 58.01 | |||
| Log-Logistic | 51.03 | 48.42 | |||
| Log-Probit | 50.82 | 61.93 | |||
| Multistage | 49.97 | 49.49 | |||
| Probit | 48.73 | 57.54 | |||
| Weibull | 51.09 | 49.49 |
The BMD10 for glycidamide in µmol/kg body weight/day are from the data presented in Supplementary Table S2.
The BMD10 for acrylamide, in µmol/kg body weight/day, were calculated from the data presented in Beland et al. (2013).
5. Conclusions
Male and female B6C3F1 mice and F344/N rats were exposed to glycidamide in the drinking water for two years. In male and female B6C3F1 mice, there were significant dose-related increases in tumors of the Harderian gland, lung, forestomach, and skin. Female B6C3F1 mice also had a significantly increased incidence of tumors of the mammary gland and ovary. In male and female F344/N rats, there were significant increases in thyroid gland and oral cavity neoplasms, and mononuclear cell leukemia. Male F344/N rats also had significant dose-related increases in tumors of the epididymis/testes and heart, while female F344/N rats demonstrated significant increases in tumors of the mammary gland, clitoral gland, and forestomach.
In B6C3F1 mice, the most sensitive site for tumor induction by glycidamide was the Harderian gland, with a BMD10 of 5.51 to 5.91 µmol glycidamide per kg body weight per day in male mice and 4.55 µmol glycidamide per kg body weight per day in female mice. In F344/N rats, the most sensitive site for tumor induction was the mammary gland in female rats (BMD10 of 2.39 µmol glycidamide per kg body weight per day) and the epididymis/testes in male rats (BMD10 of 11.23 to 17.94 µmol glycidamide per kg body weight per day). Similar values were obtained in B6C3F1 mice and F344/N rats administered acrylamide in the drinking water for two years. These data indicate that, under the conditions of these bioassays, acrylamide is efficiently metabolized to glycidamide in both sexes of both species and that the carcinogenic activity of acrylamide is due to its metabolic conversion into glycidamide.
Supplementary Material
Highlights.
Glycidamide is an electrophilic metabolite of the food contaminant acrylamide.
The carcinogenicity of glycidamide was assessed in a two-year bioassay.
The tumors observed from glycidamide correspond to those found with acrylamide.
The carcinogenicity of acrylamide is due to metabolic conversion to glycidamide.
Acknowledgments
We thank L. Patrice McDaniel for serving as the study coordinator, and James Carson, Andy Matson, and Michelle Vanlandingham for providing animal diets and care. This work was supported by an Interagency Agreement between the National Institute of Environmental Health Sciences, National Toxicology Program, and the U.S. Food and Drug Administration, National Center for Toxicological Research (NCTR/FDA IAG #224-12-0003; NIH/NTP IAG #AES12013) and formed the basis for NTP Technical Report 588. Financial support was also provided by a research grant from Fundação para a Ciência e a Tecnologia, Portugal (RECI/QEQ-MED/0330/2012; UID/QUI/00100/2013). The opinions expressed in this paper do not necessarily represent those of the U.S. Food and Drug Administration.
Abbreviations
- ANOVA
analysis of variance
- BMD
benchmark dose
- BMDL
lower limit of benchmark dose
- N3-GA-Ade
N3-(2-carbamoyl-2-hydroxyethyl)adenine
- N7-GA-Gua
N7-(2-carbamoyl-2-hydroxyethyl)guanine
- NCTR
National Center for Toxicological Research
- PWG
Pathology Working Group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backman J, Sjöholm R, Kronberg L. Characterization of the adducts formed in the reactions of glycidamide with thymidine and cytidine. Chem. Res. Toxicol. 2004;17:1652–1658. doi: 10.1021/tx049823i. [DOI] [PubMed] [Google Scholar]
- Backman J, Kronberg L. Reaction of glycidamide with 2′-deoxyadenosine and 2′-deoxyguanosine-mechanism for the amide hydrolysis. Nucleosides Nucleotides Nucleic Acids. 2007;26:129–148. doi: 10.1080/15257770601112697. [DOI] [PubMed] [Google Scholar]
- Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induced mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–431. [PubMed] [Google Scholar]
- Beland FA, Benson RW, Mellick PW, Kovatch RM, Roberts DW, Fang J-L, Doerge DR. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem. Toxicol. 2005a;43:1–19. doi: 10.1016/j.fct.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Beland FA, Churchwell MI, Von Tungeln LS, Chen S, Fu PP, Culp SJ, Schoket B, Győrffy E, Minárovits J, Poirier MC, Bowman ED, Weston A, Doerge DR. High-performance liquid chromatography electrospray ionization tandem mass spectrometry for the detection and quantitation of benzo[a]pyrene-DNA adducts. Chem. Res. Toxicol. 2005b;18:1306–1315. doi: 10.1021/tx050068y. [DOI] [PubMed] [Google Scholar]
- Beland FA, Mellick PW, Olson GR, Mendoza MCB, Marques MM, Doerge DR. Carcinogenicity of acrylamide in B6C3F1 mice and F344/N rats from a 2-year drinking water exposure. Food Chem. Toxicol. 2013;51:149–159. doi: 10.1016/j.fct.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Bergmark E, Calleman CJ, Costa LG. Formation of hemoglobin adducts of acrylamide and its epoxide metabolite glycidamide in the rat. Toxicol. Appl. Pharmacol. 1991;111:352–363. doi: 10.1016/0041-008x(91)90036-e. [DOI] [PubMed] [Google Scholar]
- Bergmark E. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chem. Res. Toxicol. 1997;10:78–84. doi: 10.1021/tx960113p. [DOI] [PubMed] [Google Scholar]
- Bieler GS, Williams RL. Ratio estimates, the delta method, and quantal response tests for increased carcinogenicity. Biometrics. 1993;49:793–801. [PubMed] [Google Scholar]
- Bucher JR, Huff J, Haseman JK, Eustis SL, Peters A, Toft JD. Neurotoxicity and carcinogenicity of N-methylolacrylamide in F344 rats and B6C3F1 mice. J. Toxicol. Environ. Health. 1990;31:161–177. doi: 10.1080/15287399009531446. [DOI] [PubMed] [Google Scholar]
- Bull RJ, Robinson M, Laurie RD, Stoner GD, Greisiger E, Meier JR, Stober J. Carcinogenic effects of acrylamide in Sencar and A/J mice. Cancer Res. 1984a;44:107–111. [PubMed] [Google Scholar]
- Bull RJ, Robinson M, Stober JA. Carcinogenic activity of acrylamide in the skin and lung of Swiss-ICR mice. Cancer Lett. 1984b;24:209–212. doi: 10.1016/0304-3835(84)90138-1. [DOI] [PubMed] [Google Scholar]
- Camacho L, Latendresse JR, Muskhelishvili L, Patton R, Bowyer JF, Thomas M, Doerge DR. Effects of acrylamide exposure on serum hormones, gene expression, cell proliferation, and histopathology in male reproductive tissues of Fischer 344 rats. Toxicol. Lett. 2012;211:135–143. doi: 10.1016/j.toxlet.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmetic Ingredient Review Expert Panel. Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int. J. Toxicol. 2005;24(Suppl. 2):21–50. doi: 10.1080/10915810590953842. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J. Royal Stat. Soc. B. 1972;34:187–220. [Google Scholar]
- Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis. 1998;19:117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Young JF, McDaniel LP, Twaddle NC, Churchwell MI. Toxicokinetics of acrylamide and glycidamide in B6C3F1 mice. Toxicol. Appl. Pharmacol. 2005a;202:258–267. doi: 10.1016/j.taap.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Young JF, McDaniel LP, Twaddle NC, Churchwell MI. Toxicokinetics of acrylamide and glycidamide in Fischer 344 rats. Toxicol. Appl. Pharmacol. 2005b;208:199–209. doi: 10.1016/j.taap.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Gamboa da Costa G, McDaniel LP, Churchwell MI, Twaddle NC, Beland FA. DNA adducts derived from administration of acrylamide and glycidamide to mice and rats. Mutat. Res. 2005c;580:131–141. doi: 10.1016/j.mrgentox.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J. Amer. Stat. Assoc. 1955;50:1096–1121. [Google Scholar]
- Dybing E, Farmer PB, Andersen M, Fennell TR, Lalljie SPD, Müller DJG, Olin S, Petersen BJ, Schlatter J, Scholz G, Scimeca JA, Slimani N, Törnqvist M, Tuijtelaars S, Verger P. Human exposure and internal dose assessments of acrylamide in food. Food Chem. Toxicol. 2005;43:365–410. doi: 10.1016/j.fct.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Flammang TJ, Von Tungeln LS, Kadlubar FF, Fu PP. Neonatal mouse assay for tumorigenicity: alternative to the chronic rodent bioassay. Regul. Toxicol. Pharmacol. 1997;26:230–240. doi: 10.1006/rtph.1997.1125. [DOI] [PubMed] [Google Scholar]
- Friedman MA, Dulak LH, Stedham MA. A lifetime oncogenicity study in rats with acrylamide. Fundam. Appl. Toxicol. 1995;27:95–105. doi: 10.1093/toxsci/27.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa da Costa G, Churchwell MI, Hamilton LP, Von Tungeln LS, Beland FA, Marques MM, Doerge DR. DNA adduct formation from acrylamide via conversion to glycidamide in adult and neonatal mice. Chem. Res. Toxicol. 2003;16:1328–1337. doi: 10.1021/tx034108e. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, McDaniel LP, Churchwell MI, Twaddle NC, Snyder R, Fennell TR, Doerge DR. Role of CYP2E1 in the epoxidation of acrylamide to glycidamide and formation of DNA and hemoglobin adducts. Toxicol. Sci. 2005a;88:311–318. doi: 10.1093/toxsci/kfi307. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, Witt KL, El-Hadri L, Hoffler U, Kissling GE, Shelby MD, Bishop JB. Comparison of germ cell mutagenicity in male CYP2E1-null and wild-type mice treated with acrylamide: evidence supporting a glycidamide-mediated effect. Biol. Reprod. 2005b;72:157–163. doi: 10.1095/biolreprod.104.033308. [DOI] [PubMed] [Google Scholar]
- Ghanayem BI, Witt KL, Kissling GE, Tice RR, Recio L. Absence of acrylamide-induced genotoxicity in CYP2E1-null mice: evidence consistent with a glycidamide-mediated effect. Mutation Res. 2005c;578:284–297. doi: 10.1016/j.mrfmmm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Some Industrial Chemicals. Vol. 60. Lyon: International Agency for Research on Cancer; 1994. Acrylamide. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; pp. 389–433. [Google Scholar]
- International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking. Vol. 83. Lyon: International Agency for Research on Cancer; 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; pp. 1–1418. [PMC free article] [PubMed] [Google Scholar]
- Irwin RD, Eustis SL, Stefanski S, Haseman JK. Carcinogenicity of glycidol in F344 rats and B6C3F1 mice. J. Appl. Toxicol. 1996;16:201–209. doi: 10.1002/(SICI)1099-1263(199605)16:3<201::AID-JAT333>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Gorzinski SJ, Bodner KM, Campbell RA, Wolf CH, Friedman MA, Mast RW. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol. Appl. Pharmacol. 1986;85:154–168. doi: 10.1016/0041-008x(86)90109-2. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. Amer. Stat. Assoc. 1958;53:457–481. [Google Scholar]
- Kotova N, Jurén T, Myöhänen K, Cornelius M, Abramsson-Zetterberg L, Backman J, Menzel U, Rydberg P, Kronberg L, Vähäkangas K, Segerbäck D. ³²P-HPLC analysis of N1-(2-carboxy-2-hydroxyethyl)deoxyadenosine: a DNA adduct of the acrylamide-derived epoxide glycidamide. Toxicol. Lett. 2011;207:18–24. doi: 10.1016/j.toxlet.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Manière I, Godard T, Doerge DR, Churchwell MI, Guffroy M, Laurentie M, Poul J-M. DNA damage and DNA adduct formation in rat tissues following oral administration of acrylamide. Mutat. Res. 2005;580:119–129. doi: 10.1016/j.mrgentox.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Manjanatha MG, Aidoo A, Shelton SD, Bishop ME, McDaniel LP, Lyn-Cook LE, Doerge DR. Genotoxicity of acrylamide and its metabolite glycidamide administered in drinking water to male and female Big Blue mice. Environ. Mol. Mutagen. 2006;47:6–17. doi: 10.1002/em.20157. [DOI] [PubMed] [Google Scholar]
- Manjanatha MG, Guo L-W, Shelton SD, Doerge DR. Acrylamide-induced carcinogenicity in mouse lung involves mutagenicity: cII gene mutations in the lung of Big Blue mice exposed to acrylamide and glycidamide for up to 4 weeks. Environ. Mol. Mutagen. 2015;56:446–456. doi: 10.1002/em.21939. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Zeiger E, McConnell EE, Kolenda-Roberts H, Wall H, Friedman MA. Induction of tunica vaginalis mesotheliomas in rats by xenobiotics. Crit. Rev. Toxicol. 2009;39:512–537. doi: 10.1080/10408440902969430. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Thoolen RJMM, Hansen B. Two-year carcinogenicity study of acrylamide in Wistar Han rats with in utero exposure. Exp. Toxicol. Pathol. 2015;67:189–195. doi: 10.1016/j.etp.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Mei N, McDaniel LP, Dobrovolsky VN, Guo X, Shaddock JG, Mittelstaedt RA, Azuma M, Shelton SD, McGarrity LJ, Doerge DR, Heflich RH. The genotoxicity of acrylamide and glycidamide in Big Blue rats. Toxicol. Sci. 2010;115:412–421. doi: 10.1093/toxsci/kfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick RL, Sills RC. Comparative carcinogenicity of 1,3-butadiene, isoprene, and chloroprene in rats and mice. Chem.-Biol. Interact. 2001;135–136:27–42. doi: 10.1016/s0009-2797(01)00213-7. [DOI] [PubMed] [Google Scholar]
- Melnick RL. Carcinogenicity and mechanistic insights on the behavior of epoxides and epoxide-forming chemicals. Ann. N.Y. Acad. Sci. 2002;982:177–189. doi: 10.1111/j.1749-6632.2002.tb04932.x. [DOI] [PubMed] [Google Scholar]
- Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. Technical Report Series No. 575. NIH Publication No. 12-5917. Park, NC: National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services, Research Triangle; 2012. Technical Report on the Toxicology and Carcinogenesis Studies of Acrylamide (CAS No. 79-06-1) in F344/N Rats and B6C3F1 Mice (Feed and Drinking Water Studies) pp. 1–233. [Google Scholar]
- Ølstørn HBA, Paulsen JE, Alexander J. Effects of perinatal exposure to acrylamide and glycidamide on intestinal tumorigenesis in Min/+ mice and their wild-type litter mates. Anticancer Res. 2007;27:3855–3864. [PubMed] [Google Scholar]
- Robinson M, Bull RJ, Knutsen GL, Shields RP, Stober J. A combined carcinogen bioassay utilizing both the lung adenoma and skin papilloma protocols. Environ. Health Perspect. 1986;68:141–145. doi: 10.1289/ehp.8668141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén J, Hellenäs K-E. Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry. Analyst. 2002;127:880–882. doi: 10.1039/b204938d. [DOI] [PubMed] [Google Scholar]
- Segerbäck D, Calleman CJ, Schroeder JL, Costa LG, Faustman EM. Formation of N-7-(2-carbamoyl-2-hydroxyethyl)guanine in DNA of the mouse and the rat following intraperitoneal administration of [14C]acrylamide. Carcinogenesis. 1995;16:1161–1165. doi: 10.1093/carcin/16.5.1161. [DOI] [PubMed] [Google Scholar]
- Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C. Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit. Rev. Toxicol. 2006;36:481–608. doi: 10.1080/10408440600851377. [DOI] [PubMed] [Google Scholar]
- Solomon JJ, Fedyk J, Mukai F, Segal A. Direct alkylation of 2’-deoxynucleosides and DNA following in vitro reaction with acrylamide. Cancer Res. 1985;45:3465–3470. [PubMed] [Google Scholar]
- Solomon JJ. Cyclic adducts and intermediates induced by simple epoxides. In: Singer B, Bartsch H, editors. Exocyclic DNA Adducts in Mutagenesis and Carcinogenesis. IARC Scientific Publications No. 150. Lyon: International Agency for Research on Cancer; 1999. pp. 123–135. [PubMed] [Google Scholar]
- Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert M-C, Riediker S. Acrylamide from Maillard reaction products. Nature. 2002;419:449–450. doi: 10.1038/419449a. [DOI] [PubMed] [Google Scholar]
- Sumner SCJ, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem. Res. Toxicol. 1999;12:1110–1116. doi: 10.1021/tx990040k. [DOI] [PubMed] [Google Scholar]
- Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002;50:4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- Tareke E, Twaddle NC, McDaniel LP, Churchwell MI, Young JF, Doerge DR. Relationships between biomarkers of exposure and toxicokinetics in Fischer 344 rats and B6C3F1 mice administered single doses of acrylamide and glycidamide and multiple doses of acrylamide. Toxicol. Appl. Pharmacol. 2006;217:63–75. doi: 10.1016/j.taap.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Twaddle NC, McDaniel LP, Gamboa da Costa G, Churchwell MI, Beland FA, Doerge DR. Determination of acrylamide and glycidamide serum toxicokinetics in B6C3F1 mice using LC-ES/MS/MS. Cancer Lett. 2004;207:9–17. doi: 10.1016/j.canlet.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch SD, Mihailovich N, Wogan GN, Lombard LS, Rao KVN. Aflatoxin B1, a hepatocarcinogen in the infant mouse. Cancer Res. 1972;32:2289–2291. [PubMed] [Google Scholar]
- Von Tungeln LS, Churchwell MI, Doerge DR, Shaddock JG, McGarrity LJ, Heflich RH, Gamboa da Costa G, Marques MM, Beland FA. DNA adduct formation and induction of micronuclei and mutations in B6C3F1/Tk mice treated neonatally with acrylamide or glycidamide. Int. J. Cancer. 2009;124:2006–2015. doi: 10.1002/ijc.24165. [DOI] [PubMed] [Google Scholar]
- Von Tungeln LS, Doerge DR, Gamboa da Costa G, Marques MM, Witt WM, Koturbash I, Pogribny IP, Beland FA. Tumorigenicity of acrylamide and its metabolite glycidamide in the neonatal mouse bioassay. Int. J. Cancer. 2012;131:2008–2015. doi: 10.1002/ijc.27493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R-S, McDaniel LP, Manjanatha MG, Shelton SD, Doerge DR, Mei N. Mutagenicity of acrylamide and glycidamide in the testes of Big Blue mice. Toxicol. Sci. 2010;117:72–80. doi: 10.1093/toxsci/kfq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman RW, Miller EC, Miller JA, Liem A. Structure-activity studies of the hepatocarcinogenicities of alkenylbenzene derivatives related to estragole and safrole on administration to preweanling male C57BL/6J x C3H/HeJ F1 mice. Cancer Res. 1987;47:2275–2283. [PubMed] [Google Scholar]
- Wogan GN. Naturally occurring carcinogens in foods. Prog. Exp. Tumor Res. 1969;11:134–162. doi: 10.1159/000391392. [DOI] [PubMed] [Google Scholar]
- Zeiger E, Recio L, Fennell TR, Haseman JK, Snyder RW, Friedman M. Investigation of the low-dose response in the in vivo induction of micronuclei and adducts by acrylamide. Toxicol. Sci. 2009;107:247–257. doi: 10.1093/toxsci/kfn214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.