Abstract
Background. Despite the low and decreasing prevalence of tuberculosis (TB) in the United States, there remain certain high-risk groups with high incidence rates. The targeted screening and treatment of latent TB infection (LTBI) among these high-risk groups are needed to achieve TB elimination; however, by most accounts, LTBI treatment completion rates remain low.
Methods. We retrospectively studied all patients accepting treatment for LTBI at the Fulton County Health Department TB clinic over 2 years. Medical chart abstraction was performed to collect information on sociodemographics, medical, and LTBI treatment history. Treatment completion was defined as finishing ≥88% of the prescribed regimen. Logistic regression analysis was performed to identify predictors of treatment completion.
Results. Among 547 adults offered LTBI treatment, 424 (78%) accepted treatment and 298 of 424 (70%) completed treatment. The median age was 42 years, most patients were black (77%), and close to one third did not have stable housing. No significant difference in completion rates was found between the 3 regimens of 9 months isoniazid (65%), 4 months rifampin (71%), and 3 months of weekly rifapentine and isoniazid (79%). In multivariate analysis, having stable housing increased the odds of finishing treatment, whereas tobacco use and an adverse event decreased the odds.
Conclusion. Utilizing comprehensive case management, we demonstrated high rates of LTBI treatment completion, including among those receiving a 3-month regimen. Completion rates were higher among persons with stable housing, and this finding highlights the need to develop strategies that will improve adherence among homeless persons.
Keywords: adherence, latent tuberculosis, treatment, 3HP
There has been significant progress in the fight against tuberculosis (TB) in the United States over the last few decades. In 2014, there were a total of 9412 TB cases reported in the United States for an incidence rate of 3.0 cases per 100 000 population [1]. This declining number of TB cases has led to a record low incidence in the United States and makes it one of lowest burdened TB countries in the world [2]. However, this low rate of TB obscures the fact that there remain subpopulations with high rates of TB including the homeless, foreign born, and persons with immunosuppressive conditions [3–5]. Finding ways to optimize the identification and treatment completion rates of latent TB infection (LTBI) among such high-risk groups is needed for the United States to achieve the goal of TB elimination set by the World Health Organization's Stop TB Program [2].
Although the number of active TB cases in the United States continues to decline, the estimated prevalence of LTBI unexpectedly increased from 1999–2000 to 2011–2012, by 4.3% to 4.7%, respectively, with rates in foreign-born persons remaining high at 20.5% [6]. This estimated 13 million person reservoir of Mycobacterium tuberculosis remains a huge obstacle to eliminating TB, especially considering that approximately 80% of active TB cases in the United States are thought to be a result of reactivation of LTBI [7]. A targeted screening approach for LTBI among persons with the highest risk of progressing to active TB and among groups with high rates of active TB is recommended [8]. These groups include (1) persons with human immunodeficiency virus (HIV) who have a >10 times risk of progressing to active TB and (2) foreign-born and homeless persons who have much higher active TB rates than the overall US population (15 and 36–47 per 100 000 population, respectively) [3–5].
A major hurdle in TB prevention is getting persons to accept and complete LTBI treatment. In general, rates of LTBI completion in the United States have been low, with most studies finding completion rates <60%, which is far below the Centers for Disease Control and Prevention (CDC) target completion rate of 79% [9–11]. The long treatment duration for LTBI regimens is believed to be a major adherence issue, and thus the approval of a once-weekly, 3-month course of rifapentine plus isoniazid regimen (3HP) in 2011 has provided much hope for improving compliance [12]. Most of the evidence for approval of this regimen came from the PREVENT-TB trial, which found a higher treatment completion rate among patients on 3HP versus those taking a standard 9-month course of isoniazid (82% vs 69%, P < .01) [13]. This shorter and well tolerated regimen may allow more people to start and finish LTBI treatment; however, to date, there is limited data on its performance (adherence, tolerability, and effectiveness) in a nontrial setting [14].
The Fulton County Health Department (FCHD) in Atlanta, Georgia serves the inner city population of downtown Atlanta, which consists of many foreign-born persons, HIV-infected persons, and also many persons residing at local shelters or rehabilitation programs. Our study aims were to determine the completion rate and predictors of completion among this population at high risk for TB amidst a few notable happenings. These include (1) a TB outbreak in metropolitan Atlanta associated with downtown homeless shelters and (2) the introduction of 3HP in early 2012. By identifying the groups at high risk for noncompletion of LTBI treatment, our results may help to inform future interventions.
METHODS
A retrospective cohort study design was used to evaluate adult patients (≥18 years) who accepted treatment for LTBI at the FCHD TB clinic in Atlanta, Georgia. All patients who accepted LTBI treatment from January 2012 through December 2013 were included. The clinic provides screening and treatment services for both active TB and LTBI Monday through Friday and predominantly serves an inner city population. Of note, during the study period, there was an ongoing cluster of isoniazid (INH)-resistant active TB cases associated with Fulton County homeless shelters. The clinic is staffed with a full-time physician, volunteer physicians from a local academic university and the CDC, nurses, program managers, and 5 outreach staff who provide directly observed therapy (DOT) for active TB and patients receiving the 3HP for LTBI. The 3HP regimen was initiated as an additional treatment option for LTBI in April 2012, along with existing regimens: 9 months of daily INH (9H) and 4 months of daily rifampin (4R). The 3HP doses of weekly rifapentine and INH were 900 mg each with dose reduction for weight <50 kg. Either 25 or 50 mg of vitamin B6 was provided for regimens containing INH. A reminder call was made before every appointment, and each patient was assigned a case worker to assist with adherence. All medications and DOT visits were provided at no cost. Additional services were provided at either low cost or at no cost for patients unable to pay, and incentives including transportation and food vouchers were provided to patients when available. Language interpretation services were also available. Patient education was provided by treating clinicians, and TB information pamphlets were available in English and Spanish for all patients.
All patients received a diagnostic test for LTBI, with most receiving a tuberculin skin test (TST) using 5 IU of purified protein derivative and/or a QuantiFERON Gold interferon-gamma release assay. Tests were interpreted according to CDC guidelines for LTBI [11]. All patients with a positive test for LTBI were evaluated for active TB with chest radiography and clinical exam, and, when indicated, sputum samples were collected for acid-fast bacilli smear and culture. If active TB was ruled out, the final decision to offer treatment for LTBI was made by clinic physicians. In general, LTBI treatment was offered to patients with a positive test for LTBI and/or for patients who were contacts of active TB cases. In persons previously treated for LTBI, the decision of whether to retreat was at the discretion of the evaluating clinician and based on timing of prior treatment, likelihood of re-exposure, and risk of progression. Human immunodeficiency virus testing was offered for all patients not known to be HIV positive. It was standard for each of the 3 available treatment regimens (3HP, 4R, and 9H) to be discussed with patients offered LTBI treatment; however, there were certain preferences for a regimen in specific populations. The 4R regimen was preferred for patients with a known exposure to a case of INH-resistant TB or homeless patients staying in local shelters given an ongoing outbreak of INH-resistant TB. In accordance with CDC guidelines, the 3HP regimen was contraindicated for HIV-positive patients receiving antiretroviral therapy (ART) and for patients presumed to be infected with INH-resistant TB, as noted above [12]. Given the possible drug-drug interactions with rifamycins, the 9H regimen was preferred for HIV-positive patients receiving ART [11, 12]. Standard follow-up consisted of a monthly physician visit. Patients on 9H and 4R regimens were given 1 month of medication at a time. Baseline complete blood counts (CBCs) and chemistry panels were standard for all patients on all regimens. Follow-up CBCs and chemistry panels were done at the discretion of the treating clinician, directed by symptoms and for chemistry panels influenced by the presence of underlying risk factors for hepatotoxicity. In addition, the decision to hold treatment or change regimens due to an adverse event was up to the treating physician.
Definitions
For the 9H and 4R regimens, treatment completion was defined as a patient taking ≥88% of his/her doses as prescribed within 12 months and 6 months, respectively. Adherence for these 2 regimens was assessed by patient self-report and monthly medication refills. For the 3HP regimen, treatment completion was defined as receiving ≥11 of 12 scheduled doses via DOT within a 16-week period. Treatment completion rates were based on those used in a clinical trial of 3HP [13]. Alcohol and tobacco use was defined by any current use as recorded in the medical chart. Illicit drug use was defined by any current use of crack cocaine or heroin. Psychiatric illness was defined by the receipt of any psychiatric medication or reported mental illness including but not limited to depression, schizophrenia, and bipolar disease. An interruption of treatment was defined by missing ≥7 days of medication for any regimen. Hepatotoxicity was identified by a ≥3 times increase in liver function tests (LFTs) plus symptoms or a ≥5 times increase in LFTs [15].
Data Collection
A brief medical chart review was performed for all persons without active TB seen in the FHCD TB clinic during the study period. A further detailed review was performed for those accepting LTBI treatment. A standardized data collection form was used to abstract data on demographics, medical history, test results, and LTBI treatment course and outcomes. All symptoms developing during LTBI treatment were recorded as indicated in the medical charts. All data were entered into an online REDCap database [16].
Data Analysis
Data analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC). For descriptive statistics, differences in categorical variables were tested using either the χ2 or Fisher′s exact test, and for continuous variables, a 2-sample t test was used. A 2-sided P < .05 was considered significant throughout analyses. A logistic regression model was used to estimate the independent association of predictors of completing LTBI treatment. Logistic model building and covariate selection were based on the purposeful selection of patient-level factors as previously described [17]. In brief, variables with a P < .20 in univariate analysis were included in an initial multivariate model, and the final model included variables with a P ≤ .10. We also checked for confounders, defined by a change in an odds ratio of ≥20% upon removal of a variable, and we rechecked all variables for inclusion in the final model. Approval for this study was received from the FCHD and the Emory University Institutional Review Board.
RESULTS
A total of 1984 adults were evaluated for LTBI during the 2-year study period. The reasons for screening included for employment or shelter clearance (1252, 63%), contact to an active TB case (343, 17%), referred for a positive LTBI test (327, 17%), or other (62, 3%). As shown in Figure 1, 547 patients (28%) were offered treatment, and, of those, 424 patients (78%) accepted treatment. Of those accepting treatment, 298 completed their LTBI treatment, representing 55% of those offered treatment and 70% of those accepting treatment. There was no significant difference among the completion rates among patients receiving 9H (75 of 115, 65%) vs 4R (181 of 256, 71%) vs 3HP (42 of 53, 79%), P = .18. Among patients receiving 3HP, 38 (72%) received DOT at their place of residence or work, and 2 switched regimens after it was found out they were a contact to an INH-resistant TB case.
Figure 1.
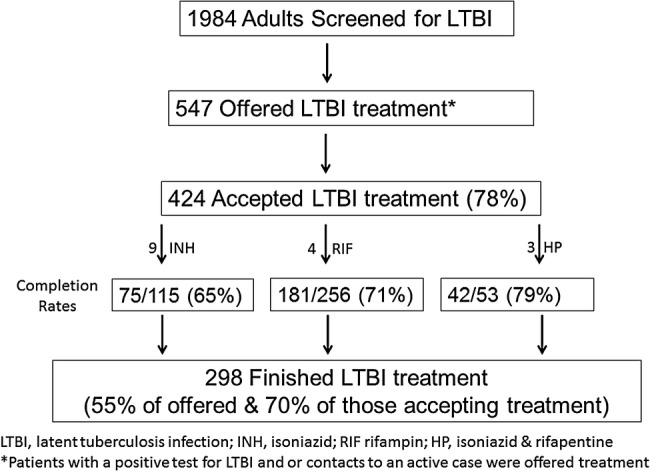
Study cohort diagram.
The mean age of the 424 patients accepting treatment was 42 years, and most patients were black (77%) and male (65%). One third of patients were foreign born. Although most patients had stable housing (68%), only a minority were currently employed (27%). The rates of HIV infection, diabetes mellitus, and hepatitis C infection were 15%, 7%, and 4%, respectively. The rates of current alcohol (33%) and tobacco (29%) use were similar. One fourth of patients were a contact to an active TB case. A total of 345 patients had a TST performed (344 positive), and 102 patients had a QuantiFERON-TB Gold test (QFT) performed (97 positive), with only 25 patients having both tests performed (25 TST positive, 20 QFT positive). The most common LTBI treatment regimen was 4R (60%) followed by 9H (27%) and 3HP (13%). Further cohort characteristics are shown in Table 1.
Table 1.
Characteristics of Patients Accepting Treatment for Latent Tuberculosis Infection by Treatment Completion Status (N = 424)a
| Characteristic | N (n = 424) | Completed Treatment (n = 298) | Did Not Complete (n = 126) | P Value |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 41.6 (12.9) | 41.7 (13.2) | 41.1 (12.3) | .63 |
| Mean weight, lbs (SD) | 180 (48) | 181 (51) | 178 (42) | .60 |
| Male | 275 (65) | 186 (62) | 89 (71) | .11 |
| Race | .30 | |||
| Black | 327 (77) | 223 (75) | 104 (83) | |
| Hispanic | 50 (12) | 37 (12) | 13 (10) | |
| Asian | 29 (7) | 24 (8) | 5 (4) | |
| White | 18 (4) | 14 (5) | 4 (3) | |
| Foreign born | 138 (33) | 107 (36) | 31 (25) | .02 |
| Non-English speaking | 38 (9) | 29 (10) | 9 (7) | .39 |
| Married | 83 (20) | 68 (23) | 15 (12) | <.01 |
| Employed | 114 (27) | 92 (31) | 22 (18) | <.01 |
| Stable housing | 289 (68) | 219 (74) | 70 (56) | <.01 |
| HIV positive | 64 (15) | 41 (14) | 23 (18) | .24 |
| Diabetes | 31 (7) | 18 (6) | 13 (10) | .12 |
| Psychiatric illness | 18 (4) | 9 (3) | 9 (7) | .05 |
| Alcohol use | 141 (33) | 94 (32) | 47 (37) | .25 |
| Tobacco use | 122 (29) | 74 (25) | 48 (38) | <.01 |
| Illicit drug use | 40 (9) | 23 (8) | 17 (154 | .06 |
| Contact to active TB case | 106 (25) | 84 (28) | 22 (18) | .02 |
| Positive QFT result | 97 (23) | 71 (24) | 26 (21) | .48 |
| Treatment | ||||
| Regimen | .18 | |||
| 9 mo INH | 115 (27) | 75 (65)b | 40 (35)b | |
| 4 mo RIF | 256 (60) | 181 (71)b | 75 (29)b | |
| 3HP | 53 (13) | 42 (79)b | 11 (21)b | |
| Changed regimen | 43 (10) | 24 (8) | 19 (15) | .03 |
| Adverse Events | ||||
| Any adverse event | 61 (14) | 30 (10) | 31 (25) | <.01 |
| Adverse event interrupting treatment | 35 (8) | 14 (5) | 21 (17) | <.01 |
| Hepatotoxicity | 7 (2) | 3 (1) | 4 (3) | .11 |
Abbreviations: HIV, human immunodeficiency virus; INH, isoniazid; lbs, pounds; QFT, QuantiFERON-TB Gold test; RIF, rifampin; SD, standard deviation; TB, tuberculosis; 3HP, 3-month course of rifapentine plus isoniazid regimen.
a Values in parentheses refer to percentage unless otherwise noted.
b All percentages are calculated by column with exception of values denoted by b, which are calculated by row.
In comparing patients receiving different LTBI treatment regimens, those who were employed, had stable housing, and were a contact to an active TB were each significantly more likely to receive the 3HP regimen compared with the 9H and 4R regimens (all P < .05). Human immunodeficiency virus-positive patients were more likely to receive the 9H regimen (44%) compared with 4R (5%) and 3HP (2%) regimens (P < .01). Further comparisons are show in Table 2.
Table 2.
Characteristics of Patients Accepting Treatment for Latent Tuberculosis Infection by Initial Treatment Regimen (N = 424)a
| Characteristic | LTBI Treatment Regimen |
|||
|---|---|---|---|---|
| 9 INH (n = 115) | 4 RIF (n = 256) | 3HP (n = 53) | P Value | |
| Mean age, years (SD) | 38.3 (12.3) | 43.0 (12.2) | 41.2 (16.0) | |
| Mean weight, lbs (SD) | 172.2 | 186.8 | 161.4 | |
| Male | 82 (71) | 167 (65) | 26 (49) | .02 |
| Race | .02 | |||
| Black | 83 (72) | 204 (80) | 40 (76) | |
| Hispanic | 16 (14) | 30 (12) | 4 (8) | |
| Asian | 6 (5) | 15 (6) | 8 (15) | |
| White | 10 (9) | 7 (3) | 1 (2) | |
| Foreign born | 35 (30) | 87 (34) | 16 (30) | .74 |
| Non-English speaking | 11 (10) | 23 (9) | 4 (8) | .92 |
| Married | 15 (13) | 55 (22) | 13 (25) | .10 |
| Employed | 21 (18) | 71 (28) | 22 (42) | <.01 |
| Stable housing | 96 (84) | 144 (56) | 49 (93) | <.01 |
| HIV positive | 51 (44) | 12 (5) | 1 (2) | <.01 |
| Diabetes | 10 (9) | 20 (8) | 1 (2) | .26 |
| Psychiatric illness | 9 (8) | 8 (3) | 1 (2) | .07 |
| Alcohol use | 32 (28) | 83 (32) | 26 (49) | .02 |
| Tobacco use | 37 (32) | 70 (27) | 15 (28) | .64 |
| Illicit drug use | 14 (12) | 21 (8) | 5 (9) | .48 |
| Contact to active TB case | 24 (21) | 61 (24) | 21 (40) | .03 |
Abbreviations: HIV, human immunodeficiency virus; INH, isoniazid; lbs, pounds; LTBI, latent tuberculosis infection; RIF, rifampin; SD, standard deviation; TB, tuberculosis; 3HP, once-weekly, 3-month course of rifapentine plus isoniazid regimen.
a Values in parentheses refer to percentage unless otherwise noted.
In regards to adverse events, a total of 61 patients (14%) had any adverse event recorded in the medical chart (Table 3). The most common experienced symptom was nausea (5%) followed by rash (4%), fatigue (3%), and abdominal pain (3%). Although there was no significant difference in treatment interruption among the different regimens, significantly more patients who received 9H (16%) had their treatment regimen changed due to an adverse event compared with those who received 4R (4%) and 3HP (6%) (P < .01).
Table 3.
Adverse Events Among Patients Accepting Treatment for Latent Tuberculosis Infection by Initial Treatment Regimen (N = 424)
| Adverse Event | Total | 9 INH (n = 115) | 4 RIF (n = 256) | 3HP (n = 53) | P Valuea |
|---|---|---|---|---|---|
| Any adverse Event | 61 (14) | 25 (22) | 25 (10) | 11 (21) | <.01 |
| Nausea | 20 (5) | 5 (4) | 10 (4) | 5 (10) | .22 |
| Loss of appetite | 5 (1) | 3 (3) | 2 (1) | 0 | .22 |
| Fatigue | 12 (3) | 4 (4) | 7 (3) | 1 (2) | .84 |
| Abdominal pain | 11 (3) | 3 (3) | 1 (3) | 1 (2) | .94 |
| Rash | 16 (4) | 5 (4) | 7 (3) | 4 (8) | .23 |
| Numbness | 10 (2) | 9 (8) | 1 (1) | 0 | <.01 |
| Other symptomsb | 28 (7) | 12 (10) | 9 (4) | 7 (13) | <.01 |
| Hepatotoxicity | 7 (2) | 4 (4) | 3 (1) | 0 | .16 |
| Treatment interruption due to adverse event | 37 (9) | 12 (10) | 18 (7) | 7 (13) | .26 |
| Regimen change due to adverse event | 31 (7) | 18 (16) | 10 (4) | 3 (6) | <.01 |
| Regimen change due to hepatotoxicity specifically | 4 (1) | 2 (2) | 2 (1) | 0 | .51 |
Abbreviations: INH, isoniazid; RIF, rifampin; 3HP, once-weekly, 3-month course of rifapentine plus isoniazid regimen.
a χ2 statistic.
b Includes dizziness (4), headaches (3), diarrhea (3), edema (3), arthralgias (3), itching (2), fever (1); remaining symptoms with only 1 occurrence.
Among patients who completed LTBI treatment, significantly more were married (23% vs 12%), employed (31% vs 18%), a contact to an active case (28% vs 18%), and had stable housing (74% vs 56%) than those who did not complete treatment (all P < .05) (Table 1). In addition, the completion rate among those with stable housing was significantly higher than among those without stable housing (76% vs 59%, P < .01). Patients who did not complete treatment were more likely to report current tobacco use (38% vs 25%, P < .01) and experience any adverse event (25% vs 10%, P < .01) including events leading to an interruption in treatment (17% vs 5%, P < .01). In multivariate logistic regression analysis, having stable housing (adjusted odds ratio [aOR], 2.46; 95% confidence interval [CI], 1.46–4.12) was associated with completing LTBI treatment. In contrast, current tobacco use (aOR, 0.57; 95% CI, .35–.91) and any adverse event (aOR, 0.26; 95% CI, .14–.46) were associated with a lower likelihood of completing LTBI treatment (Table 4). Treatment regimen was not found to be significantly associated with LTBI treatment completion and hence was not included in the final model.
Table 4.
Multivariate Analysis of Predictors for Completing Latent Tuberculosis Infectiona
| Variable | aOR | 95% CI | P Value |
|---|---|---|---|
| Age (per year) | 1.02 | 1.00–1.04 | .06 |
| Employed | 1.53 | .86–2.71 | .15 |
| Stable housing | 2.46 | 1.46–4.12 | <.01 |
| Tobacco use | 0.57 | .35–.91 | .02 |
| Any adverse event | 0.26 | .14–.46 | <.01 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; LTBI, latent tuberculosis infection.
a Other variables considered but not retained in the final model included sex, LTBI treatment regimen, weight, foreign born, English speaking, HIV status, contact to active tuberculosis case, treatment change due to an adverse event, diabetes, psychiatric illness.
DISCUSSION
We found a high treatment completion rate (70%) among patients accepting LTBI treatment, including ≥65% for all 3 regimens used. These completion rates are higher than most reported in the United States and are likely a result of a multifaceted approach to LTBI care at the FHCD. Although the high completion rate is encouraging, we also demonstrate certain groups are at increased risk for not completing LTBI treatment, including persons with unstable housing, tobacco use, and those experiencing an adverse drug event. Further measures are needed to improve care in these persons and also to decrease the rate of LTBI treatment refusal (22%).
Although our overall completion rate of 70% was below the target CDC completion rate, it was much higher than that found in other inner city US populations, including in New York City (45%) and Los Angeles (51%), and higher than the completion rate (47%) found in a recently published study of 12 sites in the United States and Canada [10, 18–20]. Our relatively high completion rate may be due to the comprehensive case management system used at the FCHD, which has similarities to those from the RISE TB clinic in Rhode Island [21]. To accommodate patients′ schedules, the clinic is open from Monday through Friday, with a morning and afternoon session each day, including a longer session Monday afternoon until 6:30 PM. Calls are made to remind all patients about their upcoming visits, and each LTBI case is assigned a case worker to follow up with the patient if a visit is missed. Case workers will also make field visits for patients who are having difficulty with adherence, and for patients who are lost to follow up, case workers will perform searches of local jails to attempt to locate the patients. An onsite translator is available for Spanish-speaking patients, and a language line is available for other languages. Depending on availability, public transportation vouchers, nutritional supplement drinks, and grocery vouchers are provided to patients in need.
Our results demonstrate the feasibility and high completion rates of the 3HP regimen in an inner city population. Under nonclinical trial conditions, we had a completion rate of 79% compared with 82% found in the PREVENT TB study [13], and, if discounting the 2 patients switching regimens due to contact with an INH-resistant case, our completion rate would have been the same at 82%. A recently published study from the New York City Health Department found a completion rate of 65% among patients accepting 3HP treatment [14]. In contrast to this study, we offered DOT at the patient's place of residence or work, which was used by most patients receiving the 3HP regimen (72%). Offering DOT outside of the clinic was done in an effort to limit the inconvenience of travel and may have led to a higher rate of completion. Overall, 3HP was well tolerated, with the most common reported adverse events being nausea (10%) and rash (8%), as also seen in the New York City study [14]. The rate of permanent discontinuation of the 3HP regimen due to an adverse event was similar to that seen in the PREVENT TB study (5.7% vs 4.9%, respectively) [13].
In contrast to our overall completion rate, the completion rate among persons without stable housing was much lower at 59%. The importance of housing was further confirmed in multivariate analysis in which persons with stable housing had a 2.5 times odds of completing treatment compared to those without stable house (most of whom were either residing in a shelter or on the streets). Homelessness has been found to be associated with not completing LTBI treatment in prior studies and speaks to the role of social factors in treatment adherence [10, 18, 22]. Given the high rate of TB in the homeless population and the low rates of LTBI treatment adherence among this population, it is imperative to develop interventions that improve completion rates among this group [3, 8]. Among the limited data in this area, a randomized control trial among homeless patients in Los Angeles found intensive case management, education sessions, and a financial incentive increased completion rates substantially (62% vs 39%), whereas another trial in San Francisco found that low cost cash incentives improved adherence to LTBI treatment among homeless adults [20, 23]. In an effort to improve completion rates among homeless patients in Fulton County and utilizing information from the above studies, there is a current program offering 4R treatment via DOT to shelter patients and providing transportation vouchers to all patients for monthly visits and weekly grocery vouchers. Although we were prevented from using the 3HP regimen among most of the homeless population in our study due to possible exposure to INH-resistant TB, whether the implementation of this shorter regimen among homeless populations improves adherence rates warrants further study.
A few other points to highlight based on our results are the significance of adverse events in treatment adherence and limited treatment options for persons infected with HIV. A multisite study found the presence of adverse events to predict noncompletion of LTBI treatment and emphasize the importance of clinical surveillance and management of adverse events [10]. The majority of HIV-infected persons (80%) in our study received a 9H regimen, and current guidelines do not recommend the 3HP regimen for HIV-infected persons receiving ART due to unknown drug interactions [12]. Given the many interactions of rifampin with ART, this leaves 9H as the remaining regimen in most cases. Shorter and better tolerated regimens for HIV-infected patients are needed.
The retrospective design of our study prevented us from (1) assessing reasons for not accepting LTBI treatment, (2) performing a rigorous evaluation of adverse events, and (3) controlling for unknown confounders. For further insight into adverse events, a recent analysis from the PREVENT TB study team found that significant drug reactions, including a flu-like syndrome, were more common in the 3HP versus 9H regimen [24]. In regards to lack of randomization, there may have been confounding factors, such as clinician or patient preference for a certain treatment regimen, that were unaccounted for and may have affected our results.
CONCLUSIONS
Utilizing a multifaceted intensive case management approach, we found high rates of LTBI treatment tolerability and completion, including among those receiving a newly introduced shortened 3HP regimen. Further work is needed to improved adherence rates among the high-risk homeless population.
Acknowledgments
We thank the following contributors for their tremendous support in putting this study together: Terry Chorba, Daniel VanderEnde, David Holland, Andrew Vernon, Jaunita Martin, Ruby Hardy, Trung Le, and the dedicated staff of TB program, Fulton County Health Department.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Scott C, Kirking HL, Jeffries C et al. . Tuberculosis trends--United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:265–9. [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Global tuberculosis report 2015. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 1 October 2015.
- 3. Bamrah S, Yelk Woodruff RS, Powell K et al. . Tuberculosis among the homeless, United States, 1994–2010. Int J Tuberc Lung Dis 2013; 17:1414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cain KP, Benoit SR, Winston CA, Mac Kenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA 2008; 300:405–12. [DOI] [PubMed] [Google Scholar]
- 5. Selwyn PA, Hartel D, Lewis VA et al. . A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med 1989; 320:545–50. [DOI] [PubMed] [Google Scholar]
- 6. Miramontes R, Hill AN, Yelk Woodruff RS et al. . Tuberculosis infection in the United States: prevalence estimates from the National Health and Nutrition Examination Survey, 2011–2012. PLoS One 2015; 10:e0140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geng E, Kreiswirth B, Driver C et al. . Changes in the transmission of tuberculosis in New York City from 1990 to 1999. N Engl J Med 2002; 346:1453–8. [DOI] [PubMed] [Google Scholar]
- 8. Azevedo MJ, Conwill DE, Lawrence S et al. . Tuberculosis Containment among the Homeless in Metropolitan Jackson, Mississippi. J Miss State Med Assoc 2015; 56:243–8. [PubMed] [Google Scholar]
- 9. Hirsch-Moverman Y, Daftary A, Franks J, Colson PW. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada. Int J Tuberc Lung Dis 2008; 12:1235–54. [PubMed] [Google Scholar]
- 10. Hirsch-Moverman Y, Shrestha-Kuwahara R, Bethel J et al. . Latent tuberculous infection in the United States and Canada: who completes treatment and why? Int J Tuberc Lung Dis 2015; 19:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep 2000; 49:1–51. [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention (CDC). Recommendation for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650–3. [PubMed] [Google Scholar]
- 13. Sterling TR, Villarino ME, Borisov AS et al. . Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. [DOI] [PubMed] [Google Scholar]
- 14. Stennis NL, Burzynski JN, Herbert C et al. . Treatment for tuberculosis infection with 3 months of isoniazid and rifapentine in New York City health department clinics. Clin Infect Dis 2016; 62:53–9. [DOI] [PubMed] [Google Scholar]
- 15. Saukkonen JJ, Cohn DL, Jasmer RM et al. . An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006; 174:935–52. [DOI] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R et al. . Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd ed Hoboken, NJ: John Wiley & Sons, Inc., 2000. [Google Scholar]
- 18. Hirsch-Moverman Y, Bethel J, Colson PW et al. . Predictors of latent tuberculosis infection treatment completion in the United States: an inner city experience. Int J Tuberc Lung Dis 2010; 14:1104–11. [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Munsiff SS, Tarantino T, Dorsinville M. Adherence to treatment of latent tuberculosis infection in a clinical population in New York City. Int J Infect Dis 2010; 14:e292–7. [DOI] [PubMed] [Google Scholar]
- 20. Nyamathi AM, Christiani A, Nahid P et al. . A randomized controlled trial of two treatment programs for homeless adults with latent tuberculosis infection. Int J Tuberc Lung Dis 2006; 10:775–82. [PubMed] [Google Scholar]
- 21. Kwara A, Herold JS, Machan JT, Carter EJ. Factors associated with failure to complete isoniazid treatment for latent tuberculosis infection in Rhode Island. Chest 2008; 133:862–8. [DOI] [PubMed] [Google Scholar]
- 22. Malejczyk K, Gratrix J, Beckon A et al. . Factors associated with noncompletion of latent tuberculosis infection treatment in an inner-city population in Edmonton, Alberta. Can J Infect Dis Med Microbiol 2014; 25:281–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tulsky JP, Hahn JA, Long HL et al. . Can the poor adhere? Incentives for adherence to TB prevention in homeless adults. Int J Tuberc Lung Dis 2004; 8:83–91. [PubMed] [Google Scholar]
- 24. Sterling TR, Moro RN, Borisov AS et al. . Flu-like and other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT Tuberculosis Study. Clin Infect Dis 2015; 61:527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


