SUMMARY
Oro-pharyngeal dysphagia is frequently present during the acute phase of stroke. The aim of the present study was to evaluate whether the recording of surface EMG using a nasopharyngeal (NP) electrode could be applied to evaluation of pharyngeal muscle activity in acute stroke patients and if this neurophysiological measure is related with clinical assessment of swallowing. Patients were examined and clinical severity was assessed with the National Institute of Health Stroke Scale (NIHSS) score; dysphagia was evaluated through bedside screening test using the Gugging Swallowing Scale (GUSS). Extension of the ischaemic lesion was measured by quantitative score, based on CT scan [Alberta Stroke Programme Early CT Score (ASPECTS)]. We analysed 70 patients; 50 were classified as dysphagic (Dys+), and 20 as non-dysphagic (Dys–). Each participant underwent a surface NP EMG recording performed with a NP electrode, made of a Teflon isolated steel catheter, with a length of 16 cm and a tip diameter of 1.5 mm. The electrode was inserted through the nasal cavity, rotated and positioned approximately 3 mm anteroinferior to the salpingo-palatine fold. At least four consecutive swallowing-induced EMG bursts were recorded and analysed for each participant. Swallowing always induced a repetitive, polyphasic burst of activation of the EMG, lasting around 0.25 to 1 sec, with an amplitude of around 100-600mV. Two parameters of the EMG potentials recorded with the NP electrode were analyzed: duration and amplitude. The duration of the EMG burst was increased in Dys+ patients with a statistically significant difference compared to Dys- patients (p < 0.001). The amplitude was slightly reduced in the Dys+ group, but statistically significant differences were not observed (p = 0,775). Nevertheless, the burst amplitude showed a significant inverse correlation with NIHSS [r(48) = –0.31; p < 0.05] and ASPECTS scores [r(48) = –0.27; p < 0.05], meaning that the burst amplitude progressively reduced with an increase of clinical severity (NIHSS) and topographic extension of brain lesions in CT (ASPECTS). These results suggest that NP recordings can give a semi-quantitative measure of swallowing difficulties originating from pharyngeal dysfunction, in fact, electromyographic findings suggest reduced pharyngeal motility.
KEY WORDS: Dysphagia, Acute stroke, Nasopharyngeal electrode, Surface EMG, GUSS
RIASSUNTO
La disfagia orofaringea è spesso presente durante la fase acuta di un ictus. Lo scopo di questo lavoro è stato quello di valutare se la registrazione elettromiografica di superficie tramite un elettrodo nasofaringeo può essere impiegata per testare l'attività muscolare del faringe nei pazienti con ictus acuto e se queste misurazioni elettrofisiologiche possono essere correlate con la valutazione clinica della deglutizione. Dal punto di vista clinico la severità del quadro è stata valutata mediante l'utilizzo della scala del National Institute of Health Stroke (NIHSS); la disfagia è stata valutata mediante il test di screening Gugging Swallowing Scale (GUSS); l'estensione della lesione ischemica alla TAC è stata misurata attraverso l'Alberta Stroke Programme Early CT Score (ASPECTS). Abbiamo valutato 70 pazienti di cui 50 disfagici (Dys+), e 20 non disfagici (Dys–). Ciascun partecipante è stato sottoposto a un'elettromiografia di superficie registrata mediante un elettrodo NP costituito da un catetere di Teflon isolato in acciaio (lungo 16 cm e con un diametro in punta di 1,5 mm). L'elettrodo è stato inserito attraverso la cavità nasale, ruotato e posizionato approssimativamente 3 mm antero-inferiormente rispetto alla volta salpingo-palatina. Per ogni partecipante sono state registrate ed analizzate le risposte elettromiografiche di almeno quattro deglutizioni volontarie ripetute. La deglutizione induce sempre all'elettromiografia burst ripetitivi e polifasici di durata compresa fra 0,25 e 1 secondo, con un'ampiezza intorno ai 100-600mV. I disfagici hanno mostrano una maggiore durata del burst rilevato all'elettromiografia rispetto ai non disfagici, con una differenza statisticamente significativa (p < 0,001), ma non hanno mostrano differenze in termini di ampiezza del burst stesso (p = 0,775); quest'ultima invece era inversamente correlata con lo NIHSS score [r(48) = –0,31; p < 0,05)] e con lo ASPECTS score [r(48) = –0,27; p < 0,05]. Questi risultati suggeriscono che le registrazioni nasofaringee possono rappresentare un indice semi-quantitativo delle difficoltà deglutitorie secondarie a disfunzione faringea ed in particolare, i risultati dell'elettromiografia sarebbero indicativi di una ridotta motilità faringea durante la fase acuta di un ictus.
Introduction
Dysphagia is a common consequence of ischaemic stroke. Oropharyngeal dysphagia is frequently present during the acute phase of stroke: clinically evident dysphagia, evaluated through bedside screening techniques, is reported in 37-45% of patients in the first three days after stroke onset, largely varying depending on the diagnostic method applied and the dimension and site of stroke lesion 1. The presence of dysphagia increases the risk of aspiration pneumonia, malnutrition and dehydration, and can therefore be potentially life-threatening deserving rapid identification and treatment 2. Dysphagia has generally a favourable course, and its prevalence declines significantly within the first week after stroke 3-5. At three months, most patients recover completely 4 6.
Methods for detecting dysphagia include non-instrumental bedside screening and instrumental methods such as videofluoroscopic study of swallowing or flexible endoscopic evaluation with sensory testing, which are regarded as the gold standard for evaluation of swallowing, although not always feasible in the acute setting. Even though bedside screenings are not as sensitive and standardised as instrumental methods, they are commonly used in daily clinical practice for their ease and repeatability 7.
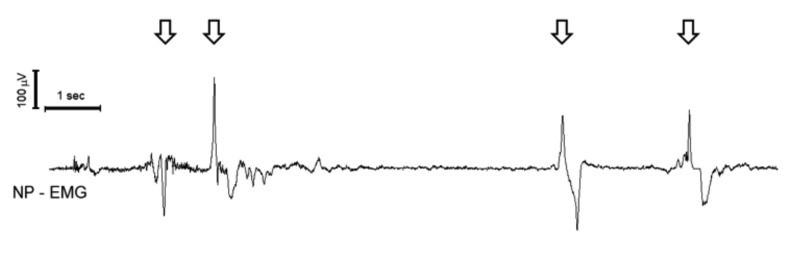
In a previous paper 8 we used a nasopharyngeal (NP), surface electrode, applied trans-nasally, to record the electromyographic (EMG) activity of pharyngeal muscles during swallowing (Fig. 1). We observed that the NP electrode was able to record polyphasic reproducible EMG potentials during swallowing, with duration of approximately 0.5-1.5 sec and average amplitude of 100-600 mV. The polyphasic response recorded by the NP electrode is characterised by an initial downward deflection (when the potential wave travels towards the recording electrode) followed by an upward deflection (when the potential moves away from the electrode) (Fig. 2); thus, it reflects a wave of muscular activation travelling in the pharynx.
Fig. 1.

The naso-pharyngeal electrode.
Fig. 2.
Naso-pharyngeal EMG recording. The arrows indicate swallowing-induced EMG bursts. Time scale and amplitude calibration are in the upper left corner.
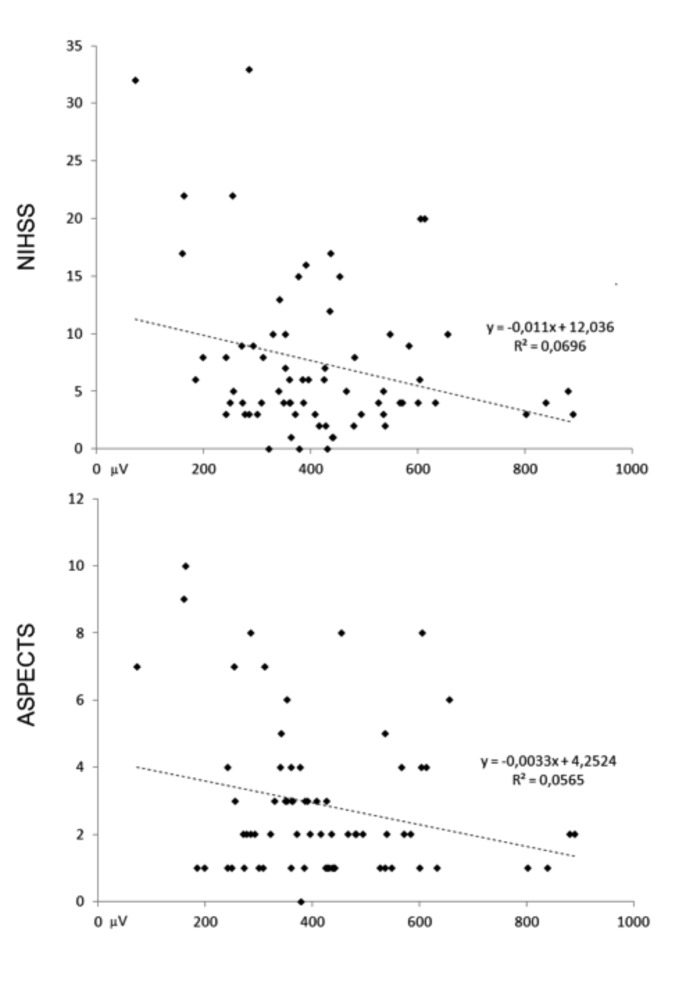
Fig. 3.
Correlation plots between EMG mean burst amplitude and NIHSS (upper panel) and ASPECT score (lower panel).
In the present study, our aim was to evaluate whether this recording technique may be applied to the evaluation of pharyngeal muscle activity in acute stroke patients and if this neurophysiological measure is related with clinical assessment of swallowing.
Materials and methods
A cohort of acute ischaemic stroke patients was prospectively enrolled in this study. All patients were evaluated within 48 hours of stroke onset. The diagnosis of stroke was always confirmed by both computed tomography (CT) and magnetic resonance imaging (MRI). Extension of the ischaemic lesion was measured by quantitative score based on CT scan [Alberta Stroke Programme Early CT Score (ASPECTS) 9]. Clinical evaluation included neurological examination and bedside clinical assessment for dysphagia. Clinical severity was assessed with the National Institute of Health Stroke Scale (NIHSS) 10. As the neurophysiological method used in this study was based on the EMG measure of voluntary swallowing, patients unable to produce a voluntary pharyngeal contraction (i.e. patients comatose, or patients with severe aphasia, unable to understand the request) were excluded from the study. Patients with a history of head and neck damage, neurologic disease other than cerebrovascular disorders or current dysphagia were also excluded from the study. The ethical committee of the hospital approved the study, and patients gave their consent to participate.
Clinical and neurophysiological measure of pharyngeal muscle activity
Clinical evaluation of dysphagia and neurophysiologic recording were performed in the same session, in the first 48 hours following stroke presentation. For the evaluation of dysphagia, the Gugging Swallowing Score (GUSS) 11 was applied. The GUSS is a validated clinical bedside test for dysphagia, which includes a preliminary indirect four step assessment of swallowing followed by a direct swallowing test that consists of three sequentially performed subtests, starting with semisolid, liquid and finally solid textures. The GUSS score ranges from 0 to 20; patients with GUSS = 20 were classified as non dysphagic (Dys–), patients with a GUSS equal or below 19 were considered dysphagic (Dys+) 11.
Each participant underwent a surface NP EMG recording performed with a NP electrode, made of a Teflon isolated steel catheter, with a length of 16 cm, tip diameter 1.5 mm (Fig. 1). The electrode was inserted through the nasal cavity, rotated, and positioned approximately 3 mm anteroinferior to the salpingo-palatine fold, as described by Su et al. 12 In this way, it was placed in a site that allowed recording the activity of the posterior oropharyngeal muscles. The outer part of the electrode was fixed with a tape to the nostril to reduce movement artifacts. A reference electrode was placed on the mastoid bone. The EMG signal was acquired witha Micromed® SystemPlus digital polygraph; sampling rate was 1024 Hz, high pass filter was 10 Hz, low pass filter was 500 Hz and gain was 5 mV/mm. Patients were lying in a supine position during the recording. After electrode placement, subjects were invited to swallow repeatedly, and the position of the electrode was slightly moved, until the maximum amplitude of the swallowing-related potentials was obtained. Then, the patients were asked to swallow repeatedly, at least four times per recording. The parameters measured were the duration of the recorded potential (Fig. 2, from the initial deflection from baseline to the return to baseline) and the peak-to-peak amplitude. For each patient, we measured the average duration and amplitude of the four best responses.
Statistical analysis
The following variables were analysed: age, gender, NIHSS, ASPECTS and GUSS score, amplitude (Amp) and duration of the EMG potential (Tdur). Statistical analysis was performed in multiple steps. In a first step, we evaluated the normality of the distribution of the Amp and Tdur of the EMG potentials, using the Shapiro-Wilk test, with a significance level of p < 0.05. When the distribution was normal in both the samples (Dys+ groupand Dys– group) comparison was made using a Student's t-test. When the distribution was not normal, a non-parametric test was applied (Mann-Whitney U-test). Significance level was set at p < 0.05. Categorical variables were compared with Fisher's exact test. Within the Dys+ group, correlations were tested between Amp and Tdur of the EMG potentials versus age, NIHSS, ASPECTS and GUSS score, using Pearson's correlation coefficient. The critical value of Pearson's correlation coefficient was set to r(48) = 0.28, corresponding to a significance level of p < 0.05.
Results
Seventy patients were enrolled, 46 men and 24 women. Mean age was 67.30 ± 13.1 years; mean scores of NIHSS, ASPECTS and GUSS was 7.44 ± 6.86, 2.90 ± 2.26 and 12.51 ± 7.14, respectively; 50 patients were classified as Dys+ and 20 as Dys–. Mean and SD of NIHSS, ASPECTS and GUSS in the two groups are reported in Table I.
Table I.
Clinical features of the study population, Dys+ and Dys- groups, and results of statistical comparison.
| Condition | Age years |
Gender n |
NIHSS n |
ASPECTS n |
GUSS n |
Amp mV |
Tdur msec |
|
|---|---|---|---|---|---|---|---|---|
| All | Mean | 67.30 | 46M 24W | 7.44 | 2.89 | 12.51 | 418.80 | 674.40 |
| SD | 13.14 | 6.87 | 2.27 | 7.14 | 165.20 | 479.90 | ||
| Dys+ (n = 50) | Mean | 70.10 | 32M 18W | 9.20 | 3.20 | 9.52 | 424.2 | 809.0 |
| SD | 11.79 | 7.31 | 2.54 | 6.31 | 190.1 | 505.1 | ||
| Dys- (n = 20) | Mean | 60.30 | 14M 6W | 3.05 | 2.10 | 20.00 | 405.3 | 337.9 |
| SD | 14.00 | 2.28 | 1.07 | 0.00 | 75.4 | 107.3 | ||
| Mann Whitney | U-test | 293.0 | 182.0 | 423.5 | 522.0 | 127.0 | ||
| p | 0.007 | < 0.001 | 0.307 | 0.775 | < 0.001 | |||
| Fisher | 0.78 |
Each recording session lasted 5 to 15 min. At least four consecutive swallowing-induced EMG bursts were recorded and analysed for each participant. Swallowing always induced a repetitive polyphasic burst of activation of the EMG, lasting around 0.25 to 1 sec, with amplitude around 100-600mV (Fig. 2).
The Shapiro-Wilk test showed a normal distribution for Amp and Tdur of the EMG potentials in the Dys– group, whereas the distribution was not normal in the Dys+ group either for the duration (p < 0.001) or for the amplitude (p = 0.040). Consequently, comparison between Dys+ and Dys– group was performed with a non-parametric test (Mann-Whitney U-test). Compared to Dys– patients, Dys+ patients showed older age (Dys+ = 70.1 ± 11.8 years; Dys– = 60.3 ± 14.0 years; U-test = 293.0; p = 0.007), while no difference was observed in gender composition (Fisher's exact test p = 0.782). Dys+ patients showed a greater NIH score (Dys+ = 9.2 ± 7.3; Dys– = 3.1 ± 2.3; U-test = 182.0; p < 0.001), while no difference was observed in the ASPECTS score (Dys+ = 3.2 ± 2.5; Dys– = 2.1 ± 1.1; U-test = 423.5; p = 0.307). Regarding EMG data, the Dys+ group showed longer EMG burst duration (Dys+ = 809 ± 505 msec; Dys– = 338 ± 107 msec; U-test = 127.0; p < 0.001), but no differences in amplitude (Dys+ = 424 ± 190 mV; Dys– = 405 ± 75 msec; Utest = 522.0; p = 0.775). Finally, in the correlation analysis the EMG burst amplitude was inversely correlated with NIHSS [r(48) = –0.31; p < 0.05] and the ASPECTS score [r(48) = –0.27; p < 0.05].
Discussion
We studied a population of acute ischaemic stroke patients in which surface EMG evaluation of pharyngeal muscles was performed using a surface nasopharyngeal electrode. In our sample, stroke-related dysphagia was present in 71% of patients, and was associated with older age, greater clinical severity (higher NIHSS score) and larger brain lesions (higher ASPECT score). These findings are in agreement with data from the literature, which suggest that swallowing impairment in stroke is more frequent and severe in patients with extensive brain damage and severe clinical deficits 4 13 14.
The NP recording results suggest that the EMG nasopharyngeal burst recorded in dysphagic patients are different from those observed in non-dysphagic ones. Two parameters of the EMG potentials recorded with the NP electrode were analysed: duration and amplitude. The duration of the EMG burst was increased in Dys+ patients with a statistically significant difference compared to Dys- patients. The amplitude was slightly reduced in the Dys+ group, but statistically significant differences were not observed. When we analysed correlations between clinical parameters and EMG data, the burst amplitude showed a significant inverse correlation with NIHSS and ASPECTS scores, meaning that the burst amplitude progressively reduced with the increase of clinical severity (NIHSS) and topographic extension of brain lesions by CT scan (ASPECTS). The increased duration of the EMG burst with a slightly reduced amplitude suggests a temporal dispersion of neuromuscular inputs and a loss of synchrony between muscle cells, which results in less efficient muscular activation.
Swallowing difficulties in stroke patients may be due to several mechanisms. Physiologically, the swallowing sequence is triggered by sensory inputs and completed by a complex bilateral reflex muscular response, organised by a brain stem central pattern generator and modulated by various peripheral and supranuclear controls 14-18. Epidemiological studies in stroke populations have outlined three major mechanism at the basis of neurogenic dysphagia: 1) impaired pharyngeal motility due to hypoactivation of the tongue, incomplete closure of the laryngeal sphincter and/or delayed opening of upper oesophagus sphincter; 2) sensory pharyngeal and laryngeal impairment; 3) altered tongue coordination 19 20. The surface EMG recordings described in the present study, which estimate oropharyngeal muscle activity, can only give information concerning pharyngeal motility. With these limitations, we suggest that NP recordings can give a semi-quantitative measure of swallowing difficulties originating from pharyngeal dysfunction. In particular, increased duration of the EMG burst suggests reduced pharyngeal motility, and burst amplitude is related with the extension and clinical severity of the stroke. If confirmed, these data suggest that surface NP-EMG recording can be helpful in bedside, non-invasive assessment of stroke patients with swallowing impairment.
References
- 1.Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 2.Yeh SJ, Huang KY, Wang TG, et al. Dysphagia screening decreases pneumonia in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci. 2011;306:38–41. doi: 10.1016/j.jns.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16:7–18. doi: 10.1007/pl00021290. [DOI] [PubMed] [Google Scholar]
- 4.Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry. 1989;52:236–241. doi: 10.1136/jnnp.52.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng NH, Wang TG, Lien IN. Dysphagia in patients with brainstem stroke: incidence and outcome. Am J Phys Med Rehabil. 2000;79:170–175. doi: 10.1097/00002060-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Broadley S, Croser D, Cottrell J, et al. Predictors of prolonged dysphagia following acute stroke. J Clin Neurosci. 2003;10:300–305. doi: 10.1016/s0967-5868(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 7.Umay EK, Unlu E, Saylam GK, et al. Evaluation of dysphagia in early stroke patients by bedside, endoscopic, and electrophysiological methods. Dysphagia. 2013;28:395–403. doi: 10.1007/s00455-013-9447-z. [DOI] [PubMed] [Google Scholar]
- 8.Picciotti PM, Della Marca G, Restuccia D, et al. Tensor veli palatini electromyography with surface electrode applied transnasally. Acta Otorhinolaryngol Ital. 2005;25:120–124. [PMC free article] [PubMed] [Google Scholar]
- 9.Finlayson O, John V, Yeung R, et al. Interobserver agreement of ASPECT score distribution for noncontrast CT, CT angiography, and CT perfusion in acute stroke. Stroke. 2013;44:234–236. doi: 10.1161/STROKEAHA.112.665208. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46:660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 11.Trapl M, Enderle P, Nowotny M, et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38:2948–2952. doi: 10.1161/STROKEAHA.107.483933. [DOI] [PubMed] [Google Scholar]
- 12.Su CY, Hsu SP, Chee CY. Electromyographic study of tensor and levator veli palatini muscles in patients with nasopharyngeal carcinoma. Implications for eustachian tube dysfunction. Cancer. 1993;71:1193–1200. doi: 10.1002/1097-0142(19930215)71:4<1193::aid-cncr2820710404>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Carrion S, Cabre M, Monteis R, et al. Oropharyngeal dysphagia is a prevalent risk factor for malnutrition in a cohort of older patients admitted with an acute disease to a general hospital. Clin Nutr. 2015;34:436–442. doi: 10.1016/j.clnu.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Celifarco A, Gerard G, Faegenburg D, et al. Dysphagia as the sole manifestation of bilateral strokes. Am J Gastroenterol. 1990;85:610–613. [PubMed] [Google Scholar]
- 15.Cola MG, Daniels SK, Corey DM, et al. Relevance of subcortical stroke in dysphagia. Stroke. 2010;41:482–486. doi: 10.1161/STROKEAHA.109.566133. [DOI] [PubMed] [Google Scholar]
- 16.Daniels SK, Corey DM, Barnes CL, et al. Cortical representation of swallowing: a modified dual task paradigm. Percept Mot Skills. 2002;94:1029–1040. doi: 10.2466/pms.2002.94.3.1029. [DOI] [PubMed] [Google Scholar]
- 17.Daniels SK, Corey DM, Fraychinaud A, et al. Swallowing lateralization: the effects of modified dual-task interference. Dysphagia. 2006;21:21–27. doi: 10.1007/s00455-005-9007-2. [DOI] [PubMed] [Google Scholar]
- 18.Daniels SK, Corey DM, Hadskey LD, et al. Mechanism of sequential swallowing during straw drinking in healthy young and older adults. J Speech Lang Hear Res. 2004;47:33–45. doi: 10.1044/1092-4388(2004/004). [DOI] [PubMed] [Google Scholar]
- 19.Aviv JE, Martin JH, Sacco RL, et al. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Ann Otol Rhinol Laryngol. 1996;105:92–97. doi: 10.1177/000348949610500202. [DOI] [PubMed] [Google Scholar]
- 20.Daniels SK, Brailey K, Foundas AL. Lingual discoordination and dysphagia following acute stroke: analyses of lesion localization. Dysphagia. 1999;14:85–92. doi: 10.1007/PL00009592. [DOI] [PubMed] [Google Scholar]