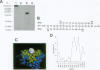
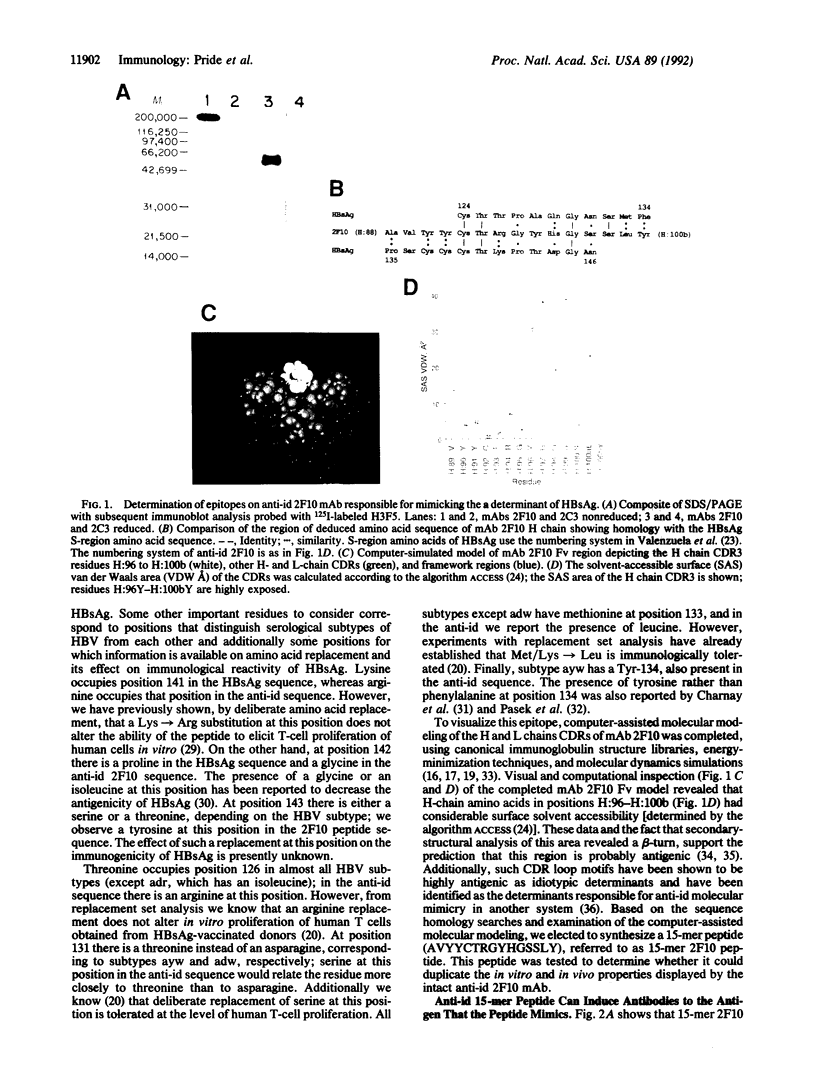
Abstract
Monoclonal antibody 2F10 is an "internal-image" anti-idiotype (anti-id) antibody capable of mimicking the group-specific "a" determinant of human hepatitis B surface antigen (HBsAg). By mRNA sequencing and computer-assisted molecular modeling of monoclonal antibody 2F10, we identified a 15-amino acid region of the heavy-chain hypervariable region that has partial residue homology with sequences of the "a" determinant epitopes of HBsAg. We have established that a linear 15-mer peptide from a contiguous region on the anti-id antibody can (i) generate anti-HBsAg-specific antibodies when injected into mice, (ii) prime murine lymph node cells for in vitro HBsAg-specific T-cell proliferative responses, and (iii) stimulate in vitro human CD4+ T cells that were primed in vivo to HBsAg by natural infection with hepatitis B virus or vaccination with a commercially available HBsAg vaccine. Significantly, this peptide could also stimulate CD4+ T cells of human hepatitis B virus carriers. We conclude that a 15-mer peptide derived from the anti-id sequence can duplicate the B- and T-cell stimulatory activity of the intact anti-id antibody and the antigen that is mimicked, HBsAg.
Full text
PDF

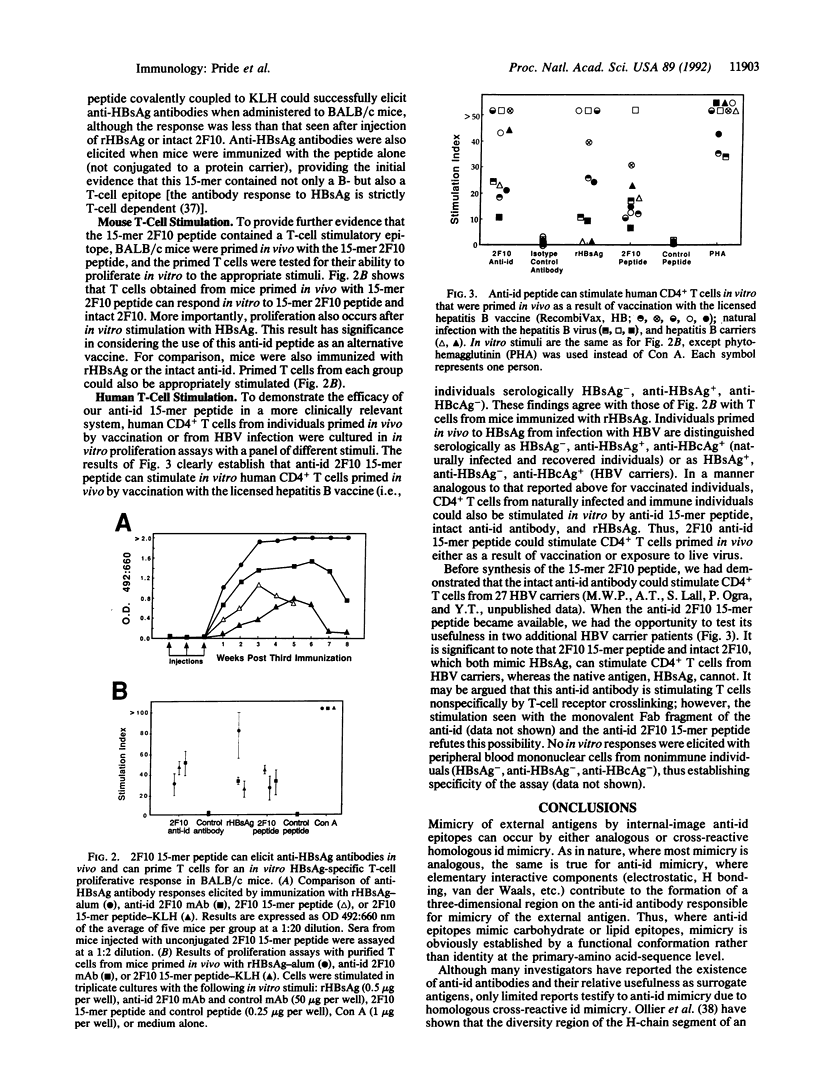

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton-Rickardt P. G., Murray K. Mutants of the hepatitis B virus surface antigen that define some antigenically essential residues in the immunodominant a region. J Med Virol. 1989 Nov;29(3):196–203. doi: 10.1002/jmv.1890290310. [DOI] [PubMed] [Google Scholar]
- Bhatnagar P. K., Papas E., Blum H. E., Milich D. R., Nitecki D., Karels M. J., Vyas G. N. Immune response to synthetic peptide analogues of hepatitis B surface antigen specific for the a determinant. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4400–4404. doi: 10.1073/pnas.79.14.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruccoleri R. E., Haber E., Novotný J. Structure of antibody hypervariable loops reproduced by a conformational search algorithm. Nature. 1988 Oct 6;335(6190):564–568. doi: 10.1038/335564a0. [DOI] [PubMed] [Google Scholar]
- Bruck C., Co M. S., Slaoui M., Gaulton G. N., Smith T., Fields B. N., Mullins J. I., Greene M. I. Nucleic acid sequence of an internal image-bearing monoclonal anti-idiotype and its comparison to the sequence of the external antigen. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6578–6582. doi: 10.1073/pnas.83.17.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman W. F., Zanetti A. R., Karayiannis P., Waters J., Manzillo G., Tanzi E., Zuckerman A. J., Thomas H. C. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990 Aug 11;336(8711):325–329. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- Charnay P., Mandart E., Hampe A., Fitoussi F., Tiollais P., Galibert F. Localization on the viral genome and nucleotide sequence of the gene coding for the two major polypeptides of the hepatitis B surface antigen (HBs Ag). Nucleic Acids Res. 1979 Sep 25;7(2):335–346. doi: 10.1093/nar/7.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Emmrich F., Kaufmann S. H. Idiotypic vaccinations: consideration towards a practical application. Crit Rev Immunol. 1987;7(3):193–227. [PubMed] [Google Scholar]
- Ferrari C., Penna A., DegliAntoni A., Fiaccadori F. Cellular immune response to hepatitis B virus antigens. An overview. J Hepatol. 1988 Aug;7(1):21–33. doi: 10.1016/s0168-8278(88)80503-8. [DOI] [PubMed] [Google Scholar]
- Hiernaux J. R. Idiotypic vaccines and infectious diseases. Infect Immun. 1988 Jun;56(6):1407–1413. doi: 10.1128/iai.56.6.1407-1413.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu-Matiu I., Kennedy R. C., Sparrow J. T., Culwell A. R., Sanchez Y., Melnick J. L., Dreesman G. R. Epitopes associated with a synthetic hepatitis B surface antigen peptide. J Immunol. 1983 Apr;130(4):1947–1952. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kussie P. H., Anchin J. M., Subramaniam S., Glasel J. A., Linthicum D. S. Analysis of the binding site architecture of monoclonal antibodies to morphine by using competitive ligand binding and molecular modeling. J Immunol. 1991 Jun 15;146(12):4248–4257. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- McMahon G., Ehrlich P. H., Moustafa Z. A., McCarthy L. A., Dottavio D., Tolpin M. D., Nadler P. I., Ostberg L. Genetic alterations in the gene encoding the major HBsAg: DNA and immunological analysis of recurrent HBsAg derived from monoclonal antibody-treated liver transplant patients. Hepatology. 1992 May;15(5):757–766. doi: 10.1002/hep.1840150503. [DOI] [PubMed] [Google Scholar]
- McMillan S., Seiden M. V., Houghten R. A., Clevinger B., Davie J. M., Lerner R. A. Synthetic idiotypes: the third hypervariable region of murine anti-dextran antibodies. Cell. 1983 Dec;35(3 Pt 2):859–863. doi: 10.1016/0092-8674(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Pride M. W., Strick N., Thanavala Y. M. Toleration of amino acid substitutions within hepatitis B virus envelope protein epitopes established by peptide replacement set analysis. I. Region S(139-147). Pept Res. 1990 May-Jun;3(3):116–122. [PubMed] [Google Scholar]
- Nisonoff A., Lamoyi E. Implications of the presence of an internal image of the antigen in anti-idiotypic antibodies: possible application to vaccine production. Clin Immunol Immunopathol. 1981 Dec;21(3):397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Ollier P., Rocca-Serra J., Sommé G., Thèze J., Fougereau M. The idiotypic network and the internal image: possible regulation of a germ-line network by paucigene encoded Ab2 (anti-idiotypic) antibodies in the GAT system. EMBO J. 1985 Dec 30;4(13B):3681–3688. doi: 10.1002/j.1460-2075.1985.tb04135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Pride M. W., Thanavala Y. M., Strick N., Houghten R. A., Neurath A. R. Toleration of amino acid substitutions within hepatitis B virus envelope protein epitopes established by peptide replacement set analysis. II. Region S(122-136). Pept Res. 1992 Jul-Aug;5(4):217–226. [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I. M., Bernard C. C., Vyas G. N., Mackay I. R. T-cell dependence of immune response to hepatitis B antigen in mice. Nature. 1975 Apr 17;254(5501):606–607. doi: 10.1038/254606a0. [DOI] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Harley E. J., Zang E. A., Alter H. J., Taylor P. E., DeVera A., Chen G. T., Kellner A. Hepatitis B vaccine in medical staff of hemodialysis units: efficacy and subtype cross-protection. N Engl J Med. 1982 Dec 9;307(24):1481–1486. doi: 10.1056/NEJM198212093072403. [DOI] [PubMed] [Google Scholar]
- Tedder R. S., Guarascio P., Yao J. L., Lord R. B., Eddleston A. L. Production of monoclonal antibodies to hepatitis B surface and core antigens, and use in the detection of viral antigens in liver biopsies. J Hyg (Lond) 1983 Feb;90(1):135–142. doi: 10.1017/s0022172400063932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanavala Y. M., Bond A., Tedder R., Hay F. C., Roitt I. M. Monoclonal 'internal image' anti-idiotypic antibodies of hepatitis B surface antigen. Immunology. 1985 Jun;55(2):197–204. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Gray P., Quiroga M., Zaldivar J., Goodman H. M., Rutter W. J. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature. 1979 Aug 30;280(5725):815–819. doi: 10.1038/280815a0. [DOI] [PubMed] [Google Scholar]
- Van Cleave V. H., Naeve C. W., Metzger D. W. Do antibodies recognize amino acid side chains of protein antigens independently of the carbon backbone? J Exp Med. 1988 Jun 1;167(6):1841–1848. doi: 10.1084/jem.167.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., Guy H. R., Rubin D. H., Robey F., Myers J. N., Kieber-Emmons T., Weiner D. B., Greene M. I. Sequences of the cell-attachment sites of reovirus type 3 and its anti-idiotypic/antireceptor antibody: modeling of their three-dimensional structures. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6488–6492. doi: 10.1073/pnas.85.17.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W. V., London S. D., Weiner D. B., Wadsworth S., Berzofsky J. A., Robey F., Rubin D. H., Greene M. I. Immune response to a molecularly defined internal image idiotope. J Immunol. 1989 Jun 15;142(12):4392–4400. [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]