Abstract
Background:
The aim of this study was to analyse registry data of seizure outcome and adverse events (AEs) for perampanel as add-on therapy in patients with focal epilepsy since its approval in 2012 for adjunctive treatment of focal epilepsy in patients ⩾12 years.
Method:
A retrospective 2-year chart review of all patients receiving perampanel was carried out.
Results:
A total of 122 patients received perampanel [median treatment length: 20.1 (range: 3.4–26.8) months]; 71 (58%) remained on treatment at last follow up. Overall, 33 patients (27%) were seizure-free for ⩾3 months at last follow up; of these, eight were seizure free for ⩾3 times the longest interictal interval before perampanel therapy; 18 (15%) had reduced seizure frequency ⩾50%. A total of 58 (47%) had an AE and 34 (28%) withdrew from treatment because of AEs. AEs included dizziness (33%), fatigue (12%), psychiatric symptoms (8%), cognitive deficits (7%), speech problems (5%), nausea (4%) and gait problems (4%). AEs subsided in 17/18 patients (94%) following a 2 mg dose reduction. A total of 43 (35%) took a concomitant enzyme inducer. Patients not taking enzyme inducers were more likely to be seizure free (p = 0.002); there were no other between-group differences.
Conclusions:
Perampanel was well tolerated and improved seizure control in 42% of patients (50– 100% reduction), with higher rates in those not receiving a concomitant enzyme inducer. AEs, particularly dizziness, were common but often disappeared with a slight dose reduction. The results are consistent with those from randomized controlled trials.
Keywords: clinical experience, efficacy, focal epilepsy, perampanel, tolerability
Introduction
Perampanel is a first-in-class orally active antagonist of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. It has been approved as an adjunctive therapy in patients aged ⩾12 years with drug-resistant focal epilepsy with and without secondary generalization. Its efficacy and tolerability have been evaluated in an extensive clinical trial programme including three randomized, double-blind, placebo-controlled phase III studies (E2007-304, -305, -306) in patients experiencing focal seizures despite receiving up to three antiepileptic drugs (AEDs), and one extension study (E2007-307) with a planned duration of 5 years [Krauss et al. 2012; French et al. 2012, 2013; Krauss, 2013].
In placebo-controlled trials, perampanel 4, 8 and 12 mg/day consistently and significantly reduced the frequency of focal seizures compared with placebo, and improved responder and seizure freedom rates in all controlled studies [Krauss et al. 2012; French et al. 2012, 2013]. Individual study data have been supported by a post-hoc analysis of pooled data from 1480 patients [Steinhoff et al. 2013]. During open-label follow up of up to 3 years, seizure responses remained stable, with marked reductions in seizures, particularly in secondarily generalized seizures [Krauss et al. 2012]. Perampanel was associated with a predictable and acceptable adverse event (AE) profile in both short-term [French et al. 2012, 2013; Krauss, 2013] and long-term (up to 3.3 years) trials [Krauss, 2013; Krauss et al. 2014].
Clinical trials provide important data for regulatory approval but do not provide all the information required for doctors to know how well the drug will work in clinical practice [Berger et al. 2012; Brodie, 2013; Brodie et al. 2014]. Thus, real-world experience provides clinical data that complement investigational data, providing valuable insight into epilepsy treatment in different patient populations and informing the use of newer AEDs. Real-world experience of perampanel is accumulating, with some results already published [Steinhoff et al. 2014a, 2014b]. These studies have shown efficacy and safety that is consistent with results of the core phase III studies and the long-term phase III open-label extension study [French et al. 2012, 2013; Krauss, 2013; Krauss et al. 2012; Steinhoff et al. 2013].
Adding to this body of real-world data, here we report an analysis of registry data of seizure outcome and AEs for perampanel as add-on therapy in patients with focal epilepsy.
Methods
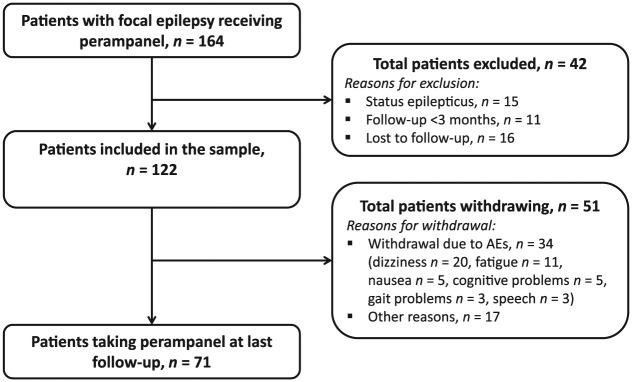
We retrospectively identified all patients with focal epilepsy who received perampanel as add-on therapy between September 2012 and January 2015 in the Department of Neurology, Paracelsus Medical University Salzburg, Austria, using the hospital information system ‘ORBIS’. Patients were excluded if they had status epilepticus (SE), if they were followed up for less than 3 months, or if they were lost to follow up. As there were differences in the dose titration and assessments of patients with SE, data for these patients have been reported separately [Rohracher et al. 2015].
Patients received once-daily perampanel at bedtime, at a starting dose of 2 mg (range: 2–6 mg), which was on average increased by 2 mg weekly to a median target dose of 6 mg (range: 2–12 mg) or a maximum dose of 12 mg, if needed. All patients were instructed to take perampanel at bedtime to minimise AEs and increase tolerability.
Baseline seizure frequency was assessed within a 12-week period before treatment initiation as reported by the patient or caretaker and accurately documented in a ‘seizure diary’. Clinical course before enrolment, including seizure onset, remission and treatment history, as well as aetiology and seizure types, was retrospectively extracted from patient records. Seizure frequency and responder rates were assessed at every outpatient visit, which were done on a clinical basis, usually at 4 and 12 weeks and then every three months. Seizure frequency was assessed by patient and caretaker reports as well as seizure documentation in ‘seizure diaries’ at every clinical visit. Response was assessed at the last visit as the percentages of seizure-free patients (⩾3 months) and percentage of patients with reduction in seizure rate of >75%, 50–75% and <50%, compared with baseline seizure frequency. Seizure freedom for ⩾3 times the longest interictal interval before perampanel therapy was regarded as ‘true’ seizure freedom.
Tolerability was assessed through AE reporting by the patients and specific questioning about ‘common’ AEs associated with perampanel (as identified in the literature). No standardized AE questionnaire was used. Perampanel dose and concomitant AEDs were controlled for during statistical analysis. According to the Austrian Law on Research, no ethics committee approval was required for this retrospective noninvasive study.
Statistical analysis was performed using the statistical software SPSS. Patients were grouped according to their co-medication, into those taking concomitant enzyme inducers, which were found to influence perampanel plasma levels in previous studies (namely carbamazepine, oxcarbazepine, phenytoin or topiramate) and those who did not. Descriptive statistical analysis was performed for the whole sample as well as for both groups separately. We used the Kolmogorov–Smirnov test to test all interval-scaled variables for normal distribution. Group comparison was performed using the nonparametric Mann–Whitney U test for the variable ‘seizure control’. All other variables were compared using the Chi-square test. Significance levels were set at p < 0.05; in the case of multiple comparisons, significance levels were set at p < 0.008 after Bonferroni correction.
Results
Of 164 patients with focal epilepsy who received add-on treatment with perampanel since September 2012, 122 patients [median age: 40.1 (14.3–90.5) years; 52% women] were followed up until January 2015.
Baseline characteristics for these individuals are presented in Table 1. Patients had previously failed a median of 4 AEDs (range: 1–14; seizure free patients: median 3, range: 1–14; not seizure free patients: median 4.5, range 1–12) before enrolment. Of the 42 (26%) patients who were omitted from the sample, 15 were excluded due to SE, 16 were lost to follow up, as they did not attend any further check-ups after treatment initiation, and 11 were followed up for less than 3 months. Patients received perampanel for a median of 20.1 months (range: 3. 4–26.8 months) at a median maintenance dose of 6 mg. Overall, 11 patients (9%) received perampanel 2 mg; 32 (26%) received 4 mg; 30 (25%) received 6 mg; 28 (23%) received 8 mg; 8 (7%) received 10 mg; and 13 received 12 mg (11%) as a maintenance dose (Figure 2). The initial dose of perampanel was 2 mg in 116 patients (95%), 4 mg in five patients, and 6 mg in one patient, according to the treating physician’s choice. Titration rates were 2 mg weekly in the majority of patients (86 patients, 71%) or 2 mg every 2 weeks (16 patients, 13%), though faster up-titration was performed in 20 patients (16%) based on individual choices of the treating physician. At final follow up, 71 patients (58%) were still on perampanel. The median duration of observation for these patients was 19.42 months (range: 3.1–26.0 months). The 12-month retention rate for all patients was 55%.
Table 1.
Baseline characteristics of 122 patients with focal epilepsy taking perampanel.
| Baseline characteristic | Patients (N = 122) |
|---|---|
| Age, years, mean (SD) | 45.1 (18.7) |
| Median (range) | 40 (14–91) |
| Sex, n (%) | |
| Male | 58 (48) |
| Female | 64 (52) |
| Number of failed AEDs, median (range) | 4 (1–14) |
| Number of concomitant AEDs, median (range) | 2 (1–4) |
| 1 concomitant AED, number of patients | 33 |
| 2 concomitant AEDs, number of patients | 62 |
| 3 concomitant AEDs, number of patients | 25 |
| 4 concomitant AEDs, number of patients | 2 |
| Patients with enzyme-inducing drugs, n (%) | 43 (35) |
| Number of concomitant enzyme inducers (EIs) | |
| 1 concomitant EI, number of patients | 38 |
| 2 concomitant EIs, number of patients | 5 |
| Outcome | |
| Duration of epilepsy, years, median (range) | 17 (0–61) |
| Seizure frequency per month (12 weeks before therapy) | |
| Simple focal and complex focal seizures, median (range) | 3 (0–90) |
| Secondary generalized tonic–clonic seizures, median (range) | 0.3 (0–45) |
| Longest remission before therapy, months, median (range) | 3 (0 –120) |
AED, antiepileptic drug; EI, enzyme inducer; SD, standard deviation.
Figure 2.
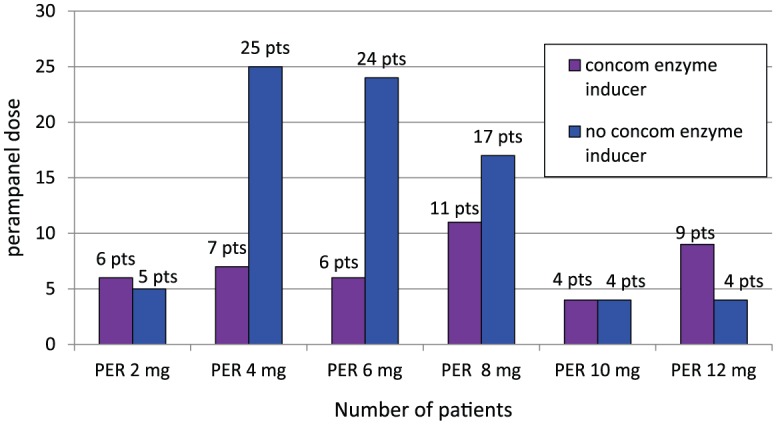
Maintenance doses of perampanel in patients with or without a concomitant enzyme Inducer.
Concom, concomitant; PER, perampanel; pts, patients.
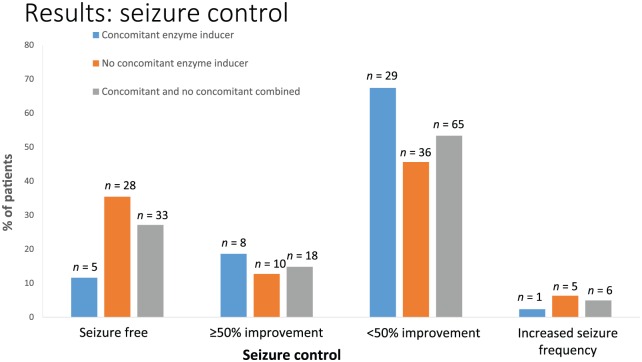
A total of 33 patients (27%) were seizure-free for 3 months at last follow up; of these, eight patients (7%) experienced seizure freedom for ⩾3 times the longest interictal interval in the individual’s entire course of disease before perampanel therapy, according to the ‘rule of three’ [Hanley and Lippman-Hand, 1983]. Median duration of the longest pretreatment interictal interval in these eight patients was 1.75 months (range: 0.5–3 months), median duration of seizure freedom with perampanel was 13 months (range: 7.0–24.6 months). A further 18 patients (15%) were responders with a reduction in seizure frequency of ⩾50% [3% (n = 4) had >75% improvement and 12% (n = 14) had 50–75% improvement]. Overall, 65 patients (53%) had a seizure reduction of <50% and six patients (5%) had an increase in seizure frequency. Median overall seizure frequency before add-on therapy with perampanel was 3 (range: 0–90) per month; median number of secondary generalized tonic–clonic seizures was 0.3 (range: 0.3–45). At last follow up, overall seizure frequency was 2 seizures per month (range: 0–60; p = 0.071), median frequency of secondary generalized tonic–clonic seizures was 0 (range: 0–30; p = 0.083). Treatment response was compared between patients with and without concomitant enzyme inducers for every outcome.
A total of 43 patients (35%) took a concomitant enzyme inducer: carbamazepine, oxcarbazepine, phenytoin or topiramate. There was no difference in perampanel dose (p = 0.515) in patients taking a concomitant enzyme inducer compared with those who did not (see Figure 2). However, treatment response differed significantly between these two groups. Patients not taking a concomitant enzyme inducer were significantly more likely to be free of seizures than those receiving a concomitant enzyme inducer (p = 0.002).
Figure 3.
Response rates in patients receiving perampanel with or without a concomitant enzyme inducer.
Overall, 58 of 122 patients (48%) experienced at least one AE, of which dizziness was the most common (33%, n = 40/122); followed by fatigue (12%, n = 15/122); psychiatric symptoms (8%, n = 10/122) namely, depression (2/10), aggression (3/10), irritability (4/10), and one suicide attempt not related to perampanel (1/10); and cognitive deficits (7%, n = 8/122; subjective concentration problems, no neuropsychological evaluation was performed). A total of 34 patients (28% of total) withdrew from perampanel treatment because of AEs (some with more than one AE). In seven of these patients (6% of total), lack of improvement of seizure control was an additional reason for withdrawal; nine patients (7% of total) withdrew due to lack of seizure control alone. Dizziness (20/34; 59%) was the most common AE that led to withdrawal (Figure 1), followed by fatigue (11/34; 32%), nausea (5/34; 15%), cognitive problems (5/34; 15%), gait problems (3/34; 9%) and speech problems (3/34; 9%). Overall, 18 patients (15%) had a reduction in perampanel dose because of AEs. In 17 of these patients, the perampanel dose was reduced by 2 mg resulting in remission of AEs. There was no statistically significant difference in the frequency of AEs between patients taking an enzyme inducer and patients not taking an enzyme inducer (p = 0.246, Table 2). Because of the small sample size of each AE, sensitivity of the Chi-square test was limited and a contradictory trend towards a higher frequency of AEs in patients taking concomitant enzyme inducers was observable. However, the appearance of AEs cannot easily be attributed to a single added new drug, but may also be caused by different combinations, and by the total drug load.
Figure 1.
Patient flow.
Table 2.
Adverse events experienced in 122 patients taking perampanel either with (n = 43) or without (n = 79) a concomitant enzyme inducer.
| Adverse event | Total, n (%) | Concomitant enzyme inducer, n (%) | No concomitant enzyme inducer, n (%) |
|---|---|---|---|
| Any AE | 58 (48) | 24 (56) | 34 (43) |
| Dizziness | 40 (33) | 17 (40) | 23 (29) |
| Fatigue | 15 (12) | 8 (19) | 7 (9) |
| Psychiatric AEsa | 10 (8) | 5 (12) | 5 (6) |
| Cognitive deficits | 8 (7) | 3 (7) | 5 (6) |
| Speech | 6 (5) | 3 (7) | 3 (4) |
| Nausea | 5 (4) | 3 (7) | 2 (3) |
| Gaitb | 5 (4) | 2 (5) | 3 (4) |
Psychiatric AEs, depression 2/10, aggression 3/10, irritability 4/10, suicide attempt not related to perampanel 1/10.
Gait AEs, insecurity while walking; no clinically objective ataxia could be observed.
AE, adverse event.
Discussion
In this real-life population, 27% (33/122) of patients that were prescribed perampanel were seizure-free for 3 months at follow up; with 7% (9/122) experiencing seizure freedom for ⩾3 times the longest interictal interval before perampanel therapy, and 15% (18/122) experiencing a seizure reduction ⩾50%. Patients also demonstrated a reduction in median seizure frequency. Patients not taking a concomitant enzyme inducer were significantly more likely to be seizure-free than those using a concomitant enzyme inducer. Perampanel was well tolerated, with commonly expected AEs. The percentage of patients who were seizure free (27%) in the current study was slightly higher than that reported in other real-life studies: 14% in the single-centre German study and 15% in the multicentre study in Germany and Austria [Steinhoff et al. 2014a, 2014b].
Possible causes for the observed differences can only be hypothesized. Explanations may include differences in patient populations between the studies (e.g. baseline seizure counts and types, epilepsy type, duration and type of previous treatments). In line with this reasoning, the populations appear to be more refractory in the German series compared with our population. Although a period of seizure freedom for 3 months does not yet meet the criteria for ‘remission’ (defined as a seizure-free period of more than 3 times the longest seizure freedom [Hanley and Lippman-Hand, 1983] lasting for a minimum of 12 months), considering the time of follow up, a seizure-free period of 3 months is already a substantial improvement in this highly drug-resistant population. However, retention rates were similar among the studies suggesting that the tolerability was similar. Another explanation may be differences in how parameters were measured (e.g. differences in interval of assessments).
Overall, real-world data have shown efficacy that is consistent with that demonstrated in the core phase III studies and the long-term phase III open-label extension study [Steinhoff et al. 2013; French et al. 2012, 2013; Krauss, 2013]. Retention rates were high and similar among all three real-life studies (58% in our study versus 60% in the study in Germany and Austria, and 70% in the German study) [Steinhoff et al. 2014a, 2014b]. Furthermore, retention has been shown to be highest (100%) in a real-life study using a very slow titration (<2 mg every 2 weeks) [Lawthom et al. 2014]. In reality, patients are likely to stop using a drug when the overall perceived side effects outweigh the overall perceived benefits. If patients did not take an enzyme inducer, they had a higher chance of being seizure-free. However, there were no statistical differences between the two groups in perampanel dose or AEs. Enzyme inducers may interfere with the metabolism of perampanel and reduce serum drug concentrations [Steinhoff et al. 2013]. In phase III studies, three enzyme inducers (carbamazepine, oxcarbazepine and phenytoin) increased perampanel clearance, reducing serum levels by as much as 30% [Laurenza et al. 2012; Rheims and Ryvlin, 2013]. Furthermore, carbamazepine has been shown to reduce mean exposure to perampanel by approximately 70% [Laurenza et al. 2012; Gidal et al. 2013]. Therefore, carbamazepine may be expected to affect both efficacy and AEs in perampanel-treated patients. Clinical data show that perampanel efficacy was reduced but remained significantly superior to placebo if carbamazepine was among the AEDs to which perampanel had been added [Steinhoff et al. 2013]. However, data for the effect of enzyme inducers on perampanel vary in real-life settings. Whereas, the current study suggests reduced efficacy of perampanel with a concomitant enzyme inducer, the prospective observational study from Germany showed no difference in responder rates in patients taking enzyme inducer AEDs (42%; n = 18) compared with those taking non-enzyme inducer AEDs (48%; n = 15) [Steinhoff et al. 2014a]. Therefore, physicians should be aware that low serum concentrations of perampanel may occur in some patients receiving concomitant enzyme inducers, necessitating an increase in perampanel dosage [Steinhoff et al. 2013]. However, some patients receiving perampanel and concomitant enzyme inducers will have a good response.
Perampanel was well tolerated with common AEs being dizziness, fatigue, psychiatric symptoms (irritability, aggression, depression), cognitive deficits, speech problems, nausea, and gait problems (described as insecurity while walking; no ataxia was reported). Dizziness was the most common AE leading to withdrawal. This profile is similar to that of other published real-world studies, in which somnolence and dizziness were the most common AEs. For example, in the study from Germany and Austria, 52% of patients experienced AEs, the most common of which were somnolence (24.6%), dizziness (19.6%), ataxia (3.9%), aggression (2.8%), nausea (2.5%) and irritability (2.1%) [Steinhoff et al. 2014b]. Ataxia, irritability, falls, cognitive slowing and depression occurred in single cases [Steinhoff et al. 2014a]. Unexpected AEs (e.g. dermatological, cardiological or laboratory findings) were not found in either study [Steinhoff et al. 2014a, 2014b]. Both dizziness and somnolence are commonly found with the use of many AEDs, with eslicarbazepine acetate, lacosamide, pregabalin, retigabine, tiagabine and zonisamide being associated with increased rates of dizziness versus placebo, and pregabalin, retigabine and zonisamide being associated with increased rates of somnolence versus placebo [Zaccara et al. 2008; Martyn-St James et al. 2012]. Somnolence and dizziness can generally be prevented or reduced by taking perampanel at bedtime or by reducing the perampanel dose [Steinhoff et al. 2014a].
Psychiatric AEs in this study were in the range that would be expected in these difficult-to-treat patients [Steinhoff et al. 2014b]. In the pooled analysis of phase III studies, hostility/aggression were more common in the perampanel groups compared with the placebo group (primarily driven by irritability), and the rate increased with increasing dose (5%, 12%, and 20% with perampanel 4, 8, and 12 mg, respectively, versus 6% with placebo) [Steinhoff et al. 2013]. Perampanel should be used with caution in patients with anger management issues or hostile or aggressive behaviour. The possibility of irritability, impulsivity, anger and aggression should be discussed with patients and family members, and these AEs monitored during dose titration. Slower titrations should be considered in patients with a personal or family history of psychiatric disorders. Results from real-life studies were similar to those reported in clinical trials [French et al. 2012, 2013; Rugg-Gunn, 2014; Zaccara et al. 2013], in which perampanel was associated with a predictable and acceptable AE profile. Most AEs were mild or moderate in intensity [Krauss et al. 2012; French et al. 2012, 2013], were dose-dependent, and occurred in the central nervous system [dizziness (10.0–47.9%) and somnolence (9.3–18.2%)]. Headache, falls, irritability, ataxia and fatigue also occurred in ⩾10% of patients in any treatment group, with vestibulocerebellar AEs (dizziness, ataxia), sedative effects (somnolence), irritability and weight increase being significantly associated with perampanel treatment. There were no serious drug-related AEs and no cases of sudden unexpected death in epilepsy. The AE profile with long-term use of perampanel reflected that seen in placebo-controlled trials [Krauss, 2013].
Overall, these data suggest that, in clinical settings, patients can be retained on perampanel with good outcomes using personalized titration, night-time administration, and reduced dosages if AEs are experienced.
The current study has some limitations. We could not measure perampanel plasma levels; therefore, we could not directly assess the influence of enzyme inducers on plasma levels in relation to seizure control and AEs. Furthermore, we did not use a standardized questionnaire for assessing AEs. As seizure frequency was collected by patient reports, some inaccuracy concerning baseline seizure frequency as well as seizure frequency throughout therapy is inevitable. Hence the reduction of seizure frequency, presented as percent changes compared with baseline seizure frequency, always has to be interpreted with this limitation in mind.
Nonetheless, real-world experience is of importance for complementing clinical trial data. Real-world experience provides additional information required by doctors to help them understand how well a drug will work in clinical practice, especially across different populations, and it gives insight into longer-term safety issues.
Conclusion
The current study adds to the accumulating body of real-world data for perampanel, two studies of which have previously been published [Steinhoff et al. 2014a, 2014b]. Perampanel was well tolerated and resulted in significant improvement of seizure control (50–100% reduction in seizures) in 42% of patients. Greater freedom from seizures was achieved in those who had not received a concomitant enzyme inducer versus those who had, but tolerability did not differ between the two groups. AEs, particularly dizziness, were common but were avoidable using night-time administration or dose reduction of perampanel.
Acknowledgments
Editorial assistance was provided by Margaret Heaslop of Lucid Group UK and funded by Eisai Inc.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Eisai Inc.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A. Rohracher has acted as a paid consultant for Neuroconsult and received travel support from Eisai.
J. Dobesberger has received honoraria from UCB Pharma, Gerot Lanach Pharma GmbH, Eisai, and GlaxoSmithKline and received travel support from UCB Pharma, Gerot Lanach Pharma GmbH, Eisai, GlaxoSmithKline and Neurodata Handels GmbH/Micromed Austria.
C. A. Granbichler received travel support from Cyberonics and Eisai.
J. Höfler has received speaker honoraria from UCB and travel support from UCB and Eisai.
M. Leitinger received travel support from Medtronic.
G. Kalss received travel support from UCB.
I. Deak, G. Kuchukhidze and A. Thomschewski have nothing to disclose.
E. Trinka has acted as a paid consultant for Eisai, Ever Neuropharma, Biogen Idec, Medtronics, Bial, Sanofi-Aventis, Takeda, SAGE, Genzyme and UCB. He has received research funding from UCB, Biogen-Idec, Sanofi-Aventis, Genzyme, FWF, Jubiläumsfond der Österreichischen Nationalbank and Red Bull as well as speakers’ honoraria from Bial, Eisai, Ever Neuropharma, GL Lannacher, Genzyme, Biogen, Glaxo Smith Kline, Sanofi-Aventis, Boehringer, Viropharma, Actavis and UCB.
Contributor Information
Alexandra Rohracher, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Ignaz-Harrer-Straße 79, A-5020 Salzburg, Austria.
Gudrun Kalss, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria.
Markus Leitinger, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria.
Claudia Granbichler, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria.
Ildiko Deak, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria.
Judith Dobesberger, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria.
Giorgi Kuchukhidze, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria; Centre for Cognitive Neuroscience Salzburg, Salzburg, Austria; Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Aljoscha Thomschewski, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria.
Julia Höfler, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria.
Eugen Trinka, Department of Neurology, Christian Doppler Medical Klinik of the Paracelsus Medical University Salzburg, Salzburg, Austria; Centre for Cognitive Neuroscience Salzburg, Salzburg, Austria.
References
- Berger M., Dreyer N., Anderson F., Towse A., Sedrakyan A., Normand S. (2012) Prospective observational studies to assess comparative effectiveness: the ISPOR Good Research Practices Task Force report. Value Health 15: 217–230. [DOI] [PubMed] [Google Scholar]
- Brodie M. (2013) Meta-analyses of antiepileptic drugs for refractory partial (focal) epilepsy: an observation. Br J Clin Pharmacol 76: 630–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie M., Kelly K., Stephen L. (2014) Prospective audits with newer antiepileptic drugs in focal epilepsy: insights into population responses? Epilepsy Behav 31: 73–76. [DOI] [PubMed] [Google Scholar]
- French J., Krauss G., Biton V., Squillacote D., Yang H., Laurenza A., et al. (2012) Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology 79: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J., Krauss G., Steinhoff B., Squillacote D., Yang H., Kumar D., et al. (2013) Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia 54: 117–125. [DOI] [PubMed] [Google Scholar]
- Gidal B., Ferry J., Majid O., Hussein Z. (2013) Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia 54: 1490–1497. [DOI] [PubMed] [Google Scholar]
- Hanley J., Lippman-Hand A. (1983) If nothing goes wrong, is everything all right? JAMA 249: 1743–1745. [PubMed] [Google Scholar]
- Krauss G. (2013). Perampanel: a selective AMPA antagonist for treating seizures. Epilepsy Curr 13: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss G., Perucca E., Ben-Menachem E., Kwan P., Shih J., Clement J., et al. (2014) Long-term safety of perampanel and seizure outcomes in refractory partial-onset seizures and secondarily generalized seizures: results from phase III extension study 307. Epilepsia 55: 1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss G., Serratosa J., Villanueva V., Endziniene M., Hong Z., French J., et al. (2012) Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology 78: 1408–1415. [DOI] [PubMed] [Google Scholar]
- Laurenza A., Ferry J., Hussein Z. (2012) Population pharamacokinetics and pharmacodynamics of perampanel: a pooled analysis of three phase III trials. Epilepsy Curr 12(Suppl. 1): 231. [Google Scholar]
- Lawthom C., Powell R., Hillman E., John K., Talbert A., Hamandi K. (2014) Perampanel in South Wales: a multi-centre clinical evaluation. Epilepsia 55(Suppl. 2): 138. [Google Scholar]
- Martyn-St James M., Glanville J., McCool R., Duffy S., Cooper J., Hugel P., et al. (2012) The efficacy and safety of retigabine and other adjunctive treatments for refractory partial epilepsy: a systematic review and indirect comparison. Seizure 21: 665–678. [DOI] [PubMed] [Google Scholar]
- Rheims S., Ryvlin P. (2013) Profile of perampanel and its potential in the treatment of partial onset seizures. Neuropsychiatr Dis Treat 9: 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohracher A., Höfler J., Kalss G., Leitinger M., Kuchukhidze G., Deak I., et al. (2015) Perampanel in patients with refractory and super-refractory status epilepticus in a neurological intensive care unit. Epilepsy Behav 49: 354–358. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn F. (2014) Adverse effects and safety profile of perampanel: a review of pooled data. Epilepsia 55(Suppl. 1): 13–15. [DOI] [PubMed] [Google Scholar]
- Steinhoff B., Bacher M., Bast T., Kornmeier R., Kurth C., Scholly J., et al. (2014a) First clinical experiences with perampanel–the Kork experience in 74 patients. Epilepsia 55(Suppl. 1): 16–18. [DOI] [PubMed] [Google Scholar]
- Steinhoff B., Ben-Menachem E., Ryvlin P., Shorvon S., Kramer L., Satlin A., et al. (2013) Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia 54: 1481–1489. [DOI] [PubMed] [Google Scholar]
- Steinhoff B., Hamer H., Trinka E., Schulze-Bonhage A., Bien C., Mayer T., et al. (2014b) A multicenter survey of clinical experiences with perampanel in real life in Germany and Austria. Epilepsy Res 108: 986–988. [DOI] [PubMed] [Google Scholar]
- Zaccara G., Gangemi P., Cincotta M. (2008) Central nervous system adverse effects of new antiepileptic drugs. A meta-analysis of placebo-controlled studies. Seizure 17: 405–421. [DOI] [PubMed] [Google Scholar]
- Zaccara G., Giovannelli F., Cincotta M., Verrotti A., Grillo E. (2013) The adverse event profile of perampanel: meta-analysis of randomized controlled trials. Eur J Neurol 20: 1204–1211. [DOI] [PubMed] [Google Scholar]