Abstract
With rapid advances in technology, wearable devices have evolved and been adopted for various uses, ranging from simple devices used in aiding fitness to more complex devices used in assisting surgery. Wearable technology is broadly divided into head-mounted displays and body sensors. A broad search of the current literature revealed a total of 13 different body sensors and 11 head-mounted display devices. The latter have been reported for use in surgery (n = 7), imaging (n = 3), simulation and education (n = 2) and as navigation tools (n = 1). Body sensors have been used as vital signs monitors (n = 9) and for posture-related devices for posture and fitness (n = 4). Body sensors were found to have excellent functionality in aiding patient posture and rehabilitation while head-mounted displays can provide information to surgeons to while maintaining sterility during operative procedures. There is a potential role for head-mounted wearable technology and body sensors in medicine and patient care. However, there is little scientific evidence available proving that the application of such technologies improves patient satisfaction or care. Further studies need to be conducted prior to a clear conclusion.
Keywords: Academic medicine, medical education, medical informatics
Introduction
Wearable technology may be defined as any wearable compact device that presents information to users and enables user interaction, either through voice-command or physical input. It often comes as a ‘clothing accessory’.1 With rapid advances in technology, wearable devices have evolved and been adopted for various uses in medicine, ranging from simply aiding in fitness to more complex devices used in surgery.
Currently available devices can be broadly divided into head-mounted displays and body sensors. The latter category may be defined as any wearable and portable device with the ability to detect or record any physiological mechanism of the human body, whereas head-mounted displays are visual devices, which have hands-free capabilities and are mounted to the user’s head.
Although there is an increasing reliance on wearable technology, the extent to which these devices actually benefit patient health remains unanswered due to paucity of the available literature. The aim of this article is to review the current literature in order to:
Highlight the latest forms of wearable technology utilised in healthcare;
Explore their potential uses and drawbacks;
Understand the potential costs of implementation of such devices; and
Provide a step-by-step guide on production and implementation of new wearable devices.
Methods
A broad search was performed using EMBASE and MEDLINE in the date range of 1980 to March 2016 using a combination of search terms including ‘wearable technology’, ‘head-mounted display’, ‘body sensors’ and ‘portable devices’. Original research articles describing innovative and potential uses of wearable technology were included in this review. Any poster or oral presentations from conferences, letters, bulletins and comments were excluded in addition to non-English articles. The references of the included studies were reviewed and relevant studies were subjected to the same selection criteria.
Head-mounted displays
Several head-mounted display devices have been identified (Table 1), which are currently utilised in surgery, imaging, simulation and education and as a navigation tool.
Table 1.
An overview of the available head-mounted display devices and their utilisation.
| Device/Sensors | Use of device | Benefits | Drawbacks | Study |
|---|---|---|---|---|
| Surgery | ||||
| MicroOptical™(MicroOptical™ Corporation, USA) | Orthopaedic fluoroscopy | Good image quality, reduction in number of times the surgeon turned away from surgical site, prevents radiation through turn exposure | Lag time, difficulties focusing with HMD | Ortega et al.2 |
| Opti-Vu HDVD (Stryker Endoscopy, USA) | Laparoscopy HMD vs. Video monitor | Improved motion smoothness | Weight imbalance of the HMD (shifting one side slightly lower), unable to use various surgical goggles at the same time, limited image use | Maithel et al.3 |
| ANC HD1080P CMOS & Google GLASS | Visual image-guided cancer resection surgery | Light (approx. 36 g), easy to wear, wireless connection, minimal interference with surgery | Costs, significant ‘lagging' effect, poor mobility of CMOS camera, requires wireless data transport | Shao et al.4 |
| (Richard Wolf GmbH, Germany) | Trans-anal endoscopic microsurgery | Comfortable with adequate peripheral view, surgeon remains upright in posture and relaxed | Poor image quality | van Koesveld et al.5 |
| Nomad ND2000™ (Microvision, USA) | Vital signs presented on display for general anaesthesia | Quicker response time when difficulty in seeing normal monitor | Similar to normal monitor when no difficulty in seeing | Liu et al.6 |
| HMZ-T2 (Sony Corporation, Japan) | TURP | Cheaper than conventional surgery, more commercially available, comfortable to wear | Endoscope was only of 10 mm diameter limiting view | Yoshida et al.12 |
| Medical education | ||||
| HMM-3000MT (Sony Corporation, Japan) | Vision-based index finger tracking | Maintain sterility during teaching, easy to use, comfortable to wear, low cost and compact size | Lag between endoscopic image and pointer device | Yoshida et al.11 |
| Imaging | ||||
| HMZ-T2 & Wrap1200 (Sony Corporation, Japan) | Sonography for patient education | Comfortable, improved understanding of patient disease, high quality images | Lag time, battery issues | Inoue et al.7 |
| Google GLASS (Google Inc. USA) | Ultrasound-guided central venous access | Comfortable | Restricted size of image, required pan and zoom tool | Wu et al.8 |
| Other | ||||
| Google GLASS (Google Inc. USA) | Evaluation in forensic medicine | Easy to use and wear | Poor quality images | Albrecht et al.9 |
| eMagin Z800 3D Visor | Driving simulator following SC injury, patient rehabilitation | Improved behind the wheel performance | More ‘off road’ collisions | Carlozzi et al.13 |
| Primesense 1080/Xtion (ASUS, Taiwan) | Navigation aid for partially sighted patients | Easy to use and understand, provides depth and distance | No studies conducted | Hicks et al.14 |
HMD, head-mounted displays; TURP, transurethral resection of the prostate.
Use in surgery
A case series reported the use of a head-mounted display, MicroOptical™ (MicroOptical™ Corporation, USA), within orthopaedic surgery to view intraoperative fluoroscopy images during the procedure.2 The study analysed head-mounted display use in 50 cases and found significant reduction in the number of times the surgeon looked away from the operative field to the mobile display compared to the standard monitor (0 vs. 19), thereby reducing the exposure of unprotected body areas to radiation. However, several users noticed significant imbalance of weight with the head-mounted displays along with the limitation of only allowing one image to be viewed at a time.
Maithel et al.3 investigated the use of Opti-Vu HDVD (Stryker Endoscopy, USA) to help reduce muscle fatigue and improve performance during laparoscopic tasks on a Computer-Enhanced Laparoscopic Training System (CELTS) in comparison to a standard monitor display. A total of 30 participants were recruited into the study consisting of 15 junior and 15 senior surgeons. The results showed a significant improvement in motion smoothness (3.01 vs. 2.79, p < 0.03) when using head-mounted displays. However, the head-mounted displays resulted in more muscle fatigue (11.21 vs. 12.42, p = 0.01) compared to the standard monitor. Interestingly, 66% of the junior participants preferred the head-mounted displays compared to 20% of seniors,3 indicating a potential use within training.
Shao et al.4 used a head-mounted display device and the Google Glass (Google Inc., USA) to provide surgeons with clear image-guided margins for cancer resection. The study used an ex-vivo tissue model injected with fluorescence, where the margins were identified and made visible on the Glass. This was used to identify residual tumour foci and, hence, reduce the risk of recurrent pathology following surgery. This surgical navigation system described has the potential to improve clinical results and outcomes following cancer resection surgery. However, it is limited to superficial tissue rather than deep, due to the properties of the tissue itself. Validation and optimisation work is still required for the software used on the Glass.
A head-mounted display in the form of a helmet with two displays (Richard Wolf GmbH, Germany) was used to perform trans-anal endoscopic microsurgery in assisting tumour removal.5 Surgeons reported that the device was comfortable to wear with adequate peripheral view. Using a standard monitor requires surgeons to move into unpleasant postures from extreme positioning due to handling of the endoscope, in contrast to the head-mounted displays, where the surgeon remains upright and in a relaxed posture. However, the quality of display was limited within the helmet due to low resolution.5
Liu et al.6 used the Nomad ND2000™ (Microvision, USA) head-mounted display to display vital signs along with other parameters to allow anaesthetists to spend more time monitoring the patient without needing to constantly refer to individual scanners. The results highlighted that the detection time between the head-mounted displays and standard vital signs monitor was not significantly different; however, numerical data are not included within the study.
Use in imaging
Inoue et al.7 provided patients with a head-mounted display during their sonography examinations. A total of 56 patients with genitourinary disease were included in the study, where each wore the HMZ-T2 (Sony Corporation, Japan) head-mounted display during their sonography examinations, allowing them to view their own live sonography image. Among them, 75% (n = 42) of patients reported a good-quality image, with only 4% (n = 2) reporting mild eye fatigue. When questioned about the wearability of the head-mounted display, 70% (n = 39) stated it was comfortable. Patients welcomed the head-mounted displays, with 64% (n = 36) stating it had increased their understanding of the disease.7 However, use was restricted by battery life.
Wu et al.8 explored the use of Google Glass during ultrasound imaging in order to determine whether it helped decrease unintentional hand movements in novices. A total of 40 participants were randomised to use either the Glass or standard ultrasound display. Participants using the Glass took a longer time to perform the procedure compared to those without (207 vs. 86 s, p > 0.05) and required more central wire needle directions (3.9 vs. 2.2, p < 0.05). The Glass also exhibited a lag of 1 s during transmission, which made it difficult to use in real time. Furthermore, multiple breaks between subjects to allow for re-charging, due to low battery-life, further complicated the ultrasound tasks.
Albrecht et al.9 compared the Glass with a digital single-lens reflex (DSLR) camera in forensic medicine. The DSLR camera was found to be far superior in the quality of pictures taken in an autopsy setting. Although the Google Glass has been very promising as hands-free technology, several issues such as poor image quality have been repeatedly identified. Such limitations need to be addressed prior to implementation within clinical practice.
Uses in simulation and education
Wu et al.10 investigated the use of incorporating the Glass in a simulation-based training programme, comparing medical students to postgraduate trainees. All participants wearing the Glass reported it comfortable to wear. Furthermore, it did not impede the simulation programme, and the recordings obtained from the simulation helped provide feedback in debriefing participants.
Yoshida et al.11,12 conducted two studies in which the authors reported the use of HMM-3000MT (Sony Corporation, Japan) and HMZ-T2 (Sony Corporation) head-mounted displays have a valid role within medical education. Using these head-mounted displays as a vision-based finger tracking system during transurethral resection of the prostate procedures, surgeons were able to point areas of interest, which can then be streamed live to trainees and medical students. This allows surgeons to maintain sterility while communicating and instructing colleagues and teaching students.11
A virtual reality simulator was tested in a head-mounted display format and has been proven to have significant positive relationship between the simulator and behind the wheel performance in rehabilitation of patients with spinal cord injury.13 However, the study reports that, due to limitations of the head-mounted display display, participants using it had more ‘off road’ collisions (0.4 vs. 0.25, p > 0.05) during the simulation and stopped for longer durations. Some participants also reported acute simulator sickness during the simulation.
Use as a navigation tool
A head-mounted display, Primesense 1080/Xtion (ASUS, Taiwan), with the ability to detect surrounding objects and provide a visual guide to partially blind patients’ residual vision was developed to be tested around an obstacle track.14 The head-mounted display showed the size and position of nearby objects but excluded any finger surface details. The distance the object was away from the head-mounted display was represented using the brightness of the display. The study concluded that the use of a head-mounted display to aid navigation in partially sighted individuals has a potential future application; individuals easily understood the system and the ability to determine the depth and distance of the object was hugely advantageous.
Body sensors
Several body sensors have been identified (Table 2) to monitor vitals signs including patient electrocardiography (ECG) and as posture and fitness devices.
Table 2.
An overview of the available body sensors and their utilisation.
| Device name | Use of device | Location | Benefits | Drawbacks | Study |
|---|---|---|---|---|---|
| ViSi Mobile System (Sotera Wireless Inc., USA) | Vital signs monitoring | Chest, shoulder, wrist, abdomen | ECG, RR, HR, SpO2, pulse rate and skin temperature measured, pressure ulcer risk stratification, low cost | Limited results | Welch et al.15 |
| Aingeal (Intelesens Ltd., N. Ireland) | Vital signs monitoring including arrhythmia detection | Upper abdomen | Small device, preferred by patients, low false-positive rate, automated device | Limited results | Donnelly et al.16 |
| Vitalsens VS100 (Intelesens Ltd., N. Ireland) | Vital signs monitoring | Chest | Increased automated detection of abnormal vital signs, high accuracy reported | Limited results in one hospital setting | Harper et al.17 |
| QUASAR | ECG monitor | Chest, head | Clear ECG results, small device | No viable P-waves | Matthews et al.18 |
| BioHarness (Zephyr Technology Corp., USA) | Heart rate, respiratory rate, activity and posture monitor | Chest | Various clinical trials with positive results, high sensitivity and specificity | $400 per device, requires external monitor | Bianchi et al.19 |
| PhysioDroid | Vital signs | Chest | Easy to use, several parameters recorded | Requires smartphone for use, no evidence for use in studies | Banos et al.20 |
| EQ02 LifeMonitor (Equivital Inc., USA) | Vital signs, sleep monitoring | Chest | Records core temperature, multipoint temperature, ability to connect with various devices, long-term data recorder | Expensive ($75 per single-use sensor) | Liu et al.21 Welles et al.22 |
| LifeVest/LifeShirt (VivoMetrics Inc, USA) | Ambulatory inductive plethsysmography, pulse oximeter, ECG monitoring | Chest | Validated in literature with good clinical evidence | Complicated to use and not patient friendly | Hollier et al.23 |
| (Microstain, Inc. G-Link & Eagle System) | Posture and gait – rehabilitation | Ankle, sternum | Provides posture guidance for various types of falls, more focused physiotherapy | Limited results and various limitations of study | Aziz et al.26 |
| Vitaport 3 (Temec BV, Netherlands) | Assessing rehabilitation in stroke survivors | Finger, thumb, trunk | More objective diagnosis then current | Limited results | Patel et al.27 |
| PERFORM | Posture monitoring for Parkinson’s disease | Wrist, ankle | Provides excellent feedback on various PD symptoms, very accurate | Few studies in literature | Cancela et al.28 |
| Robot Suit HAL (University of Tsukuba, Japan) | Posture aid – gait cycle | Shoe-embedded sensors and walking cane | Various aid in gait | Limited data | Hassan et al.29 |
| Zensor (Intelesens Ltd., N. Ireland) | ECG, arrhythmia detection, Holter monitor, cardiac condition assessment, sleep apnoea | Chest | Wireless transmission of data, possibly reduction of costs, small device | No clinical evidence available | N/A |
ECG, electrocardiography.
Vital signs monitoring
Continuous vital signs monitoring is a significant part of basic care of patients admitted to hospital. ViSi Mobile System (Sotera Wireless Inc., USA)15 can continuously measure heart rate, respiratory rate, oxygen saturation level and blood pressure. Welch et al.15 report that such devices have the ability to reduce alarm fatigue, often a result of constant monitoring of patient vital signs. The device has also been used in assessment and risk stratification for pressure ulcers. However, there are limited data available in the literature to support its accuracy or clinical benefit. It was developed to constantly monitor vital signs and alert medical professionals of abnormalities, instead of nurse-led intermittent measurements of vital signs. However, it may not be suitable for daily patient use outside a hospital setting due to positioning of the sensors on the chest, wrist, shoulder and abdomen.
A similar, but less intrusive, device is the Aingeal (Intelesens Ltd., Northern Ireland), an automated wearable device with a wireless monitor to continuously observe patient respiratory and heart rates as well as skin surface temperature. It has the ability to detect arrhythmias and alert medical staff about the possibility of a cardiac arrest. It has proved to have low false-positive results in a study among 19 participants,16 which demonstrated strong preference (4.6/5) for the Aingeal device over the standard capnograph and other devices typically used for monitoring similar parameters. Similarly, the Vitalsens VS100 (Intelesens Ltd.)17 has also been developed with similar aims and demonstrated similar results.
Intelesens have also developed Zensor which is more effective in detecting arrhythmias. The device can also act as a Holter monitor and be used for prolonged periods of time. The device alerts patients when abnormal physiological signs are detected, ensuring patients have peace of mind knowing that they are under care. It can allow cardiologists to have more information, possibly cutting down the number of hospital visits and, subsequently, reducing hospital admissions. However, no numerical data are available as yet.
QUASAR have developed various biosensors, which have the capability of recording ECGs without artefacts, affected by walking or moving rapidly. Matthews et al.18 recruited a total of six participants to wear the QUASAR, mounted on a belt around the torso for 30 days. ECGs were then collected and over 90% of the segments were classified as excellent and clear without artefacts. However, it was noted that when patients were walking, the p-wave could be affected or was absent.
BioHarness (Zephyr Technology Corp., USA)19 uses a thoracic pressure sensor attached to a chest band to detect respiratory and heart rate. It has been validated in various studies for high accuracy and user-friendliness and can also be paired with a smart phone to view live physiological data wirelessly or onto a central display monitor. Bianchi et al.19 analysed 44 patients with tachypnoea, in an emergency department setting, with sensitivity results of 91% and specificity of 97% compared to standard equipment (23% sensitivity and 99% specificity). Each BioHarness device costs approximately $400, possibly resulting in substantial costs for implementation.
The PhysioDroid is also a phone application, developed to work alongside a physical chest band and is cable of monitoring physiological data such as heart rate, respiratory rate and temperature, wirelessly transmitting these data to the user’s smartphone.20
EQ02 LifeMonitor (Equivital Inc., USA) belt has also been demonstrated to accurately monitor respiratory and heart rates, temperature, biometrics, activity, sleep, fitness and psychophysiology through various animal and human studies.21,22 The EQ02 LifeMonitor has various advantages over other physiological sensors such as the BioHarness as it has the ability to detect and record core temperature along with multipoint temperature at various locations. It also allows data recording and communication with various devices to analyse data. However, it requires single-use sensors, which cost approximately $77 per use.
Other wearable devices such as the LifeShirt (VivoMetrics Inc., USA) have been used in studies to measure various parameters. It has been validated for use to detect tidal volume, minute ventilation and respiratory frequency.23 It has also been described to accurately monitor ECG in animal studies without restricting movement.24 However, the device is complicated to set up and use but, with further development, has the potential to be used by medical professionals for diagnosing sleep disorders, heart disease, pulmonary disorders and pre- and post operative monitoring.25
Posture-related uses
A 2014 study26 reported a wearable device with the ability to automatically detect falls. Twelve adults participated in the study, in which experimental falls were detected and classified into categories such as slips, trips, fainting and standing up from sitting. The sensor’s results indicated a sensitivity of 83% and specificity of 89% at detecting the type of fall. Hence, it may be used to aid better prevention strategies for falls in older adults with more directed physiotherapy.
The Vitaport 3 (Temec BV, the Netherlands) is an accelerometer device attached on six different locations on the body to deduce the severity of symptoms in stroke patients by calculating the Functional Ability Scale (FAS). Patel et al.27 used the Vitaport 3 for 24 post-stroke survivors with upper limb weakness (hemiparesis), where FAS was subjectively calculated by clinicians and compared to the FAS determined by the Vitaport 3. The results indicated an error rate of 5.76% (p < 0.05) between the two sets of data.
The PREFORM system is a wearable device designed to detect and quantify (0–4) symptoms of Parkinson’s disease in order to provide objective measurements to monitoring symptoms.28 The system has been reported to show an accuracy of 93.7% for the classification of levodopa-induced dyskinesia severity, 86% for bradykinesia severity and 87% for tremors; furthermore, all 24 Parkinson’s patients recruited in the study reported that the wearable devices was comfortable to wear.
Human exoskeleton robots have been demonstrated in gait analysis in a study published in 2014.29 The Robot Suit HAL (University of Tsukuba, Japan) exoskeleton has body sensors, which consist of shoe-embedded force sensors and a walking cane. The system receives input from sensors and can then provide feedback to the user on whether they should start, stop or continue walking. However, the device is in early development but could have huge potential in aiding patients with gait difficulties.
Discussion
Health technology assessment
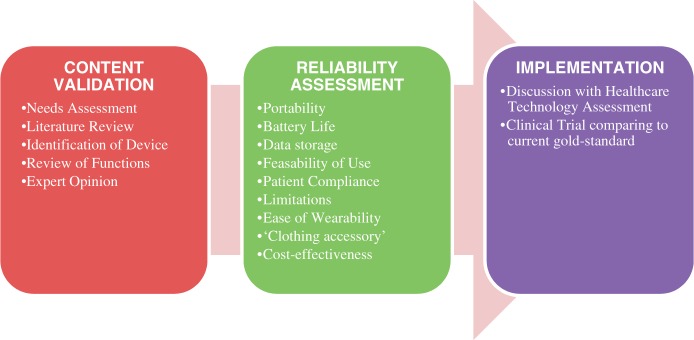
Health technology assessment is a multidisciplinary method to systematically analyse technology based on safety and clinical effectiveness. We recommend that, prior to any implementation, devices are assessed in several domains (Figure 1). Devices should first demonstrate (1) content validity followed by (2) reliability assessment and (3) implementation.
Figure 1.
Recommended validation process for wearable healthcare devices. Step 1 – Content Validation: There needs to be a needs assessment for a particular device, which can be identified from the literature, based upon review of its functions. Expert opinion can be used to determine whether the device will be suitable for its intended use. Step 2 – Reliability Assessment should take place with evaluation of the device features, limitations and cost effectiveness. Step 3 – Implementation should follow after discussions with Healthcare Technology Assessment and clinical trials comparing the device to the gold standard method.
Future developments
A 2014 study26 analysed optimal sensor location, to ensure accuracy of sensors and minimise visibility. The authors concluded that patients have higher preference and compliance for devices placed on the wrist, as such devices can often be perceived to be clothing accessories. It has been noted that large devices which are placed in visible locations often lead to poor compliance when required for long periods of time, such as the PREFORM body sensor, which has reported that patients felt anxious and uncomfortable wearing the device, especially in public places.28
Smart watches are the latest development in wearable devices, highlighted by the release of Apple Watch and Fit Bit Flex. The new ‘Kardia Band’ allows monitoring of ECG through the Apple Watch. These devices are designed to be user-friendly, enchaining the information provided to clinicians while allowing patients to monitor their own conditions.
Limitations
As with all reviews, relevant devices and articles may have been missed despite broad search terms across a variety of databases. Furthermore, there is a lack of high-level evidence within studies included in this review, with no randomised controlled trials present. This reflects the limited availability of the literature on this topic. Nevertheless, despite limited results, the possibility of enriching patients with devices to monitor their own wellbeing and facilitating clinicians with more superior and capable methods of monitoring, only highlights the potential of such devices. With technology improving drastically, we can expect such devices to be involved in both patient care and in medical settings.
Conclusions
Wearable technology has a potential role within medicine. Many uses have been identified within the medical field. Body sensors have excellent functionality in aiding patient posture and rehabilitation and head-mounted displays can provide an array of uses within the medical field. However, it is difficult to assess the true usefulness of such devices in medicine due to limited trials and literature. We suggest a validation pathway for each wearable device, prior to implementation in the clinical setting.
Declarations
Competing Interests
None declared.
Funding
None declared.
Guarantor
KA
Ethical approval
Not applicable
Contributorship
MHI and AA were involved in the acquisition of data and writing the manuscript. OB assisted in writing the manuscript. PD and KA provided critique of the content and were involved in the review of the manuscript.
Acknowledgements
None.
Provenance
Not commissioned; peer-reviewed by Frank Portelli.
References
- 1.Tehrani K. Wearable Technology and Wearable Devices: Everything You Need to Know. Wearable Devices Magazine 2014. See http://www.wearabledevices.com/what-is-a-wearable-device/ (last checked July 2015).
- 2.Ortega G, Wolff A, Baumgaertner M, Kendoff D. Usefulness of a head mounted monitor device for viewing intraoperative fluoroscopy during orthopaedic procedures. Archive Orthopaedic Trauma Surg 2008; 128: 1123–1126. [DOI] [PubMed] [Google Scholar]
- 3.Maithel SK, Villegas L, Stylopoulos N, Dawson S and Jones DB. Simulated laparoscopy using a headmounted display vs traditional video monitor: an assessment of performance and muscle fatigue. Surg Endosc 2005; 19: 406–411. [DOI] [PubMed]
- 4.Shao P, Ding H, Wang J, Liu P, Ling Q, Chen J, et al Designing a wearable navigation system for image-guided cancer resection surgery. Ann Biomed Eng 2014; 42: 2228–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Koesveld JJ, Tetteroo GW, de Graaf EJ. Use of head-mounted display in transanal endoscopic microsurgery. Surg Endosc 2003; 17: 943–946. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Jenkins SA, Sanderson PM, Fabian P and Russell WJ Monitoring with head-mounted displays in general anesthesia: a clinical evaluation in the operating room. Anesth Analg 2010; 110: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 7.Inoue M, Kihara K, Yoshida S, Ito M, Takeshita H, Ishioka J, et al A novel approach to patient self-monitoring of sonographic examinations using a head-mounted display. J Ultrasound Med 2015; 34: 29–35. [DOI] [PubMed] [Google Scholar]
- 8.Wu TS, Dameff CJ, Tully JL. Ultrasound-guided central venous access using google glass. J Emerg Med 2014; 47: 668–675. [DOI] [PubMed] [Google Scholar]
- 9.Albrecht UV, von Jan U, Kuebler J, Zoeller C, Lacher M, Muensterer OJ, et al. Google Glass for documentation of medical findings: evaluation in forensic medicine. J Med Internet Res 2014; 16: e53–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu T, Dameff C, Tully J. Integrating Google GLASS into simulation-based training: experinces and future directions. J Biomed Graphic Comput 2014, pp. 4: 49–54–4: 49–54. [Google Scholar]
- 11.Yoshida S, Kihara K, Takeshita H and Fujii Y Instructive head-mounted display system: pointing device using a vision-based finger tracking technique applied to surgical education. Videosurgery Other Miniinvasive Technique 2014; 9: 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida S, Kihara K, Takeshita and Fujii Y A head-mounted display-based personal integrated-image monitoring system for transurethral resection of the prostate. Wideochir Inne Tech Malo Inwazyjne 2014; 9: 644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlozzi NE, Gade V, Rizzo AS and Tulsky DS Using virtual reality driving simulators in persons with spinal cord injury: three screen display versus head mounted display. Disabil Rehabil Assist Technol 2013; 8: 176–180. [DOI] [PubMed] [Google Scholar]
- 14.Hicks SL, Wilson I, Muhammed L, Worsfold J, Downes SM and Kennard C A depth-based head-mounted visual display to aid navigation in partially sighted individuals. PLoS ONE 2013; 8: e67695–e67695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch J, Kanter B, Skora B, McCombie S, Henry I, McCombie D, et al. Multi-parameter vital sign database to assist in alarm optimization for general care units. J Clin Monitor Comput 2015, pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnelly N, Hunniford T, Harper R, Flynn A, Kennedy A, Branagh D, et al. Demonstrating the accuracy of an in-hospital ambulatory patient monitoring solution in measuring respiratory rate. Conf Proc IEEE Eng Med Biol Soc 2013; 2013: 6711–6715. [DOI] [PubMed]
- 17.Harper R, Donnelly N, McCullough I, Francey J, Anderson J, McLaughlin JA, et al. Evaluation of a CE approved ambulatory patient monitoring device in a general medical ward. Conf Proc IEEE Eng Med Biol Soc 2010; 2010: 94–97. [DOI] [PubMed]
- 18.Matthews R, McDonald J, Hervieux P, Turner PJ and Steindorf MA. A wearable physiological sensor suite for unobtrusive monitoring of physiological and cognitive state. Conf Proc IEEE Eng Med Biol Soc 2007; 2007: 5276–5281. [DOI] [PubMed]
- 19.Bianchi W, Dugas AF, Hsieh YH, Saheed M, Hill P, Lindauer C, et al. Revitalizing a vital sign: improving detection of tachypnea at primary triage. Ann Emerg Med 61: 37–43. [DOI] [PubMed]
- 20.Banos O, Villalonga C, Damas M, Gloesekoetter P, Pomares H and Rojas I. PhysioDroid: combining wearable health sensors and mobile devices for a ubiquitous, continuous, and personal monitoring. Scientific World J 2014; 2014: 490824. [DOI] [PMC free article] [PubMed]
- 21.Liu Y, Zhu SH, Wang GH, Ye F and Li PZ Validity and reliability of multiparameter physiological measurements recorded by the Equivital LifeMonitor during activities of various intensities. J Occup Environ Hyg 2013; 10: 78–85. [DOI] [PubMed] [Google Scholar]
- 22.Welles AP, Buller MJ, Margolis L, Economos D, Hoyt RW and Richter MW Thermal-work strain during Marine rifle squad operations in Afghanistan. Mil Med 2013; 178: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 23.Hollier CA, Harmer AR, Maxwell LJ, Menadue C, Willson GN, Black DA, et al Validation of respiratory inductive plethysmography (LifeShirt) in obesity hypoventilation syndrome. Respiratory Physiol Neurobiol 2014; 194: 15–22. [DOI] [PubMed] [Google Scholar]
- 24.Heilman KJ, Porges SW. Accuracy of the LifeShirt (Vivometrics) in the detection of cardiac rhythms. Biol Psychol 2007; 75: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman P. The LifeShirt: a multi-function ambulatory system monitoring health, disease, and medical intervention in the real world. Stud Health Technol Inform 2004; 108: 133–141. [PubMed] [Google Scholar]
- 26.Aziz O, Park EJ, Mori G and Robinovitch SN Distinguishing the causes of falls in humans using an array of wearable tri-axial accelerometers. Gait Posture 2014; 39: 506–512. [DOI] [PubMed] [Google Scholar]
- 27.Patel S, Hughes R, Hester T, Stein J, Akay M, Dy J, et al. Tracking motor recovery in stroke survivors undergoing rehabilitation using wearable technology. Conf Proc IEEE Eng Med Biol Soc 2010; 2010: 6858–6861. [DOI] [PubMed]
- 28.Cancela J, Pastorino M, Tzallas AT, Tsipouras MG, Rigas G, Arredondo MT, et al. Wearability assessment of a wearable system for Parkinson's disease remote monitoring based on a body area network of sensors. Sensors (Basel) 2014; 14: 17235–17255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan M, Kadone H, Suzuki K and Sankai Y Wearable gait measurement system with an instrumented cane for exoskeleton control. Sensors (Basel) 2014; 14: 1705–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]