Abstract
Nanoparticles labeled with radiometals enable whole-body nuclear imaging and therapy. Though chelating agents are commonly used to radiolabel biomolecules, nanoparticles offer the advantage of attaching a radiometal directly to the nanoparticle itself without the need of such agents. We previously demonstrated that direct radiolabeling of silica nanoparticles with hard, oxophilic ions, such as the positron emitters zirconium-89 and gallium-68, is remarkably efficient. However, softer radiometals, such as the widely employed copper-64, do not stably bind to the silica matrix and quickly dissociate under physiological conditions. Here, we overcome this limitation through the use of silica nanoparticles functionalized with a soft electron-donating thiol group to allow stable attachment of copper-64. This approach significantly improves the stability of copper-64 labeled thiol-functionalized silica nanoparticles relative to native silica nanoparticles, thereby enabling in vivo PET imaging, and may be translated to other softer radiometals with affinity for sulfur. The presented approach expands the application of silica nanoparticles as a platform for facile radiolabeling with both hard and soft radiometal ions.
Keywords: radiolabeling, surface functionalization, silica nanoparticles, PET imaging
Graphical abstract
Radionuclides are often used for noninvasive, whole body quantitative or semiquantitative imaging using positron emission tomography (PET) or single photon emission computed tomography (SPECT). Unless the radionuclide itself distributes to areas of interest, such as 223Ra, which accumulates in the bone and is in use for palliative treatment of metastatic bone pain, the radionuclide requires attachment to a ligand for delivery to the area of interest. Radiolabeling of targeting entities (e.g., antibodies, peptides, or small molecules) typically involves a prosthetic group for radiohalogens or chelating groups for radiometals such as 89Zr.1 In contrast to most biomolecules, radiolabeling strategies that use the nanoparticle itself for radionuclide attachment are available.2 Generally, three radiolabeling strategies are employed: hot-plus-cold precursor synthesis, direct activation, and surface absorption. Although hot-plus-cold synthesis involves the addition of radioactive (hot) and nonradioactive (cold) precursors during de novo nanoparticle synthesis or assembly,3 direct activation involves irradiation of nanoparticles with high-energy beams to convert atoms within the nanoparticle lattice into radiotracers.4 However, the most commonly employed and straightforward approach is surface adsorption, which involves trapping the radionuclide ions or exchange of ions for radionuclides at the nanoparticle surface,5 and is based on the affinity of a radiometal for the nanomaterial.6
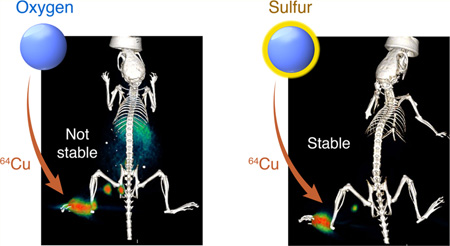
We and others recently demonstrated that silica nanoparticles (SNP) stably bind a variety of clinically relevant radiometal ions to enable whole-body PET imaging of the nanoparticles in vivo.7,8 The stability of the radiometal ion binding to the SNP correlated with the oxophilicity of the metal. Although the hard oxophilic 89Zr and 68Ga radiometal ions remained stably bound to SNP throughout a variety of challenges and in vivo experiments, the softer PET tracer 64Cu dissociated in less than 4 h. We hypothesized that poor stability was likely because 64Cu is a softer cation than 89Zr and 68Ga, and copper chelators typically contain a mixture of nitrogen, oxygen, and sulfur donors.9 Herein, we report a means to expand the palette of radionuclides used to label SNP to include softer metals by introducing thiol functionality onto the surface, because thiols are known to stably bind softer metal ions (Figure 1).10,11
Figure 1.
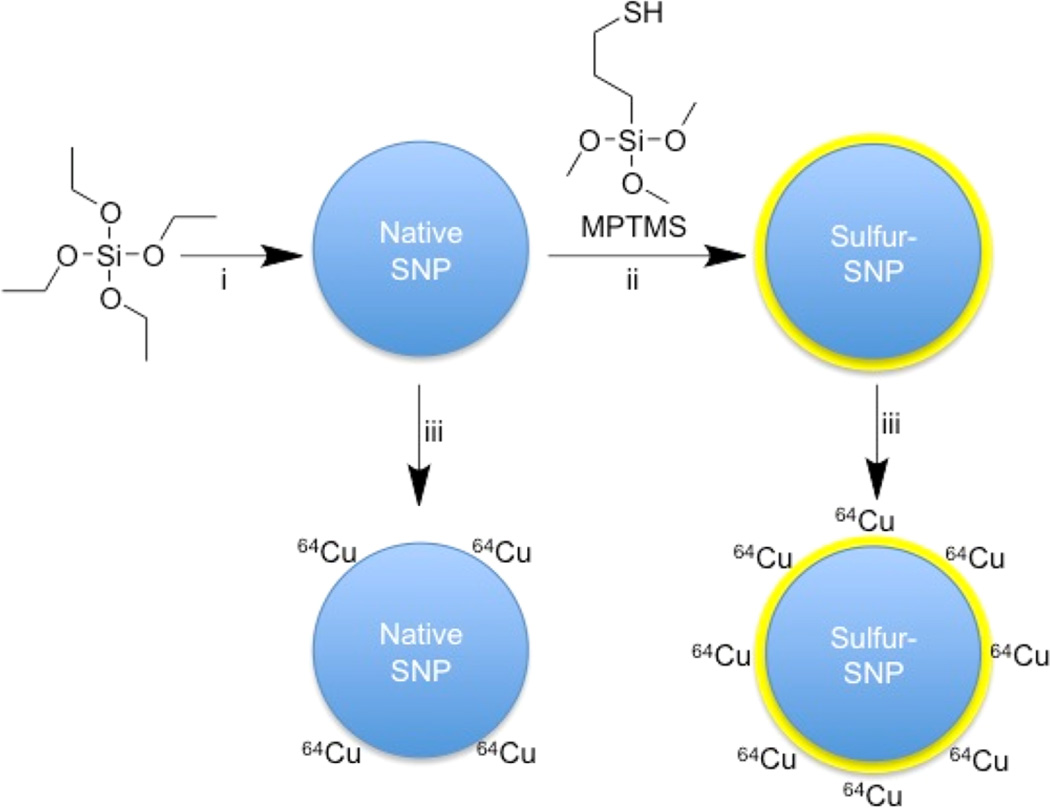
Reaction schematic. Although native SNP (blue) stably bind hard oxophilic radiometals such as zirconium-89 and gallium-68, thiol-functionalization (yellow) of SNP allows stable retention of soft, sulfur-avid copper-64. (i) NH4OH, ethanol, H2O 45 min, RT; (ii) 0.5–33% (v/v) MPTMS in ethanol, 90 min, 70 °C; (iii) 64Cu (200 µCi), pH 7.3, 2 h, 70 °C (See also Supporting Information).
First, SNP with a mean diameter of 130 nm were prepared via the Stöber method.12 The surfaces of the SNP were functionalized with thiols by reacting the SNP (7.5 nM) with 3-mercaptopropyltrimethoxysilane (MPTMS) at various concentrations (% v/v) in ethanol. Following washing steps with ethanol, Ellman’s reagent was used to quantify the thiol-to-SNP ratio of the sulfur-functionalized SNP (Figure S1). Dynamic light scattering (DLS) showed that thiol functionalization had no detectable effect on the hydrodynamic radius, zeta potential, or polydispersity index (<0.05) (Table S1). For subsequent radiolabeling experiments, sulfur-SNP with 1.8 × 105 thiols per nanoparticle were used, a value between previous reported data of 2.7 × 104 and 5.6 × 106 thiol per SNP.13,14
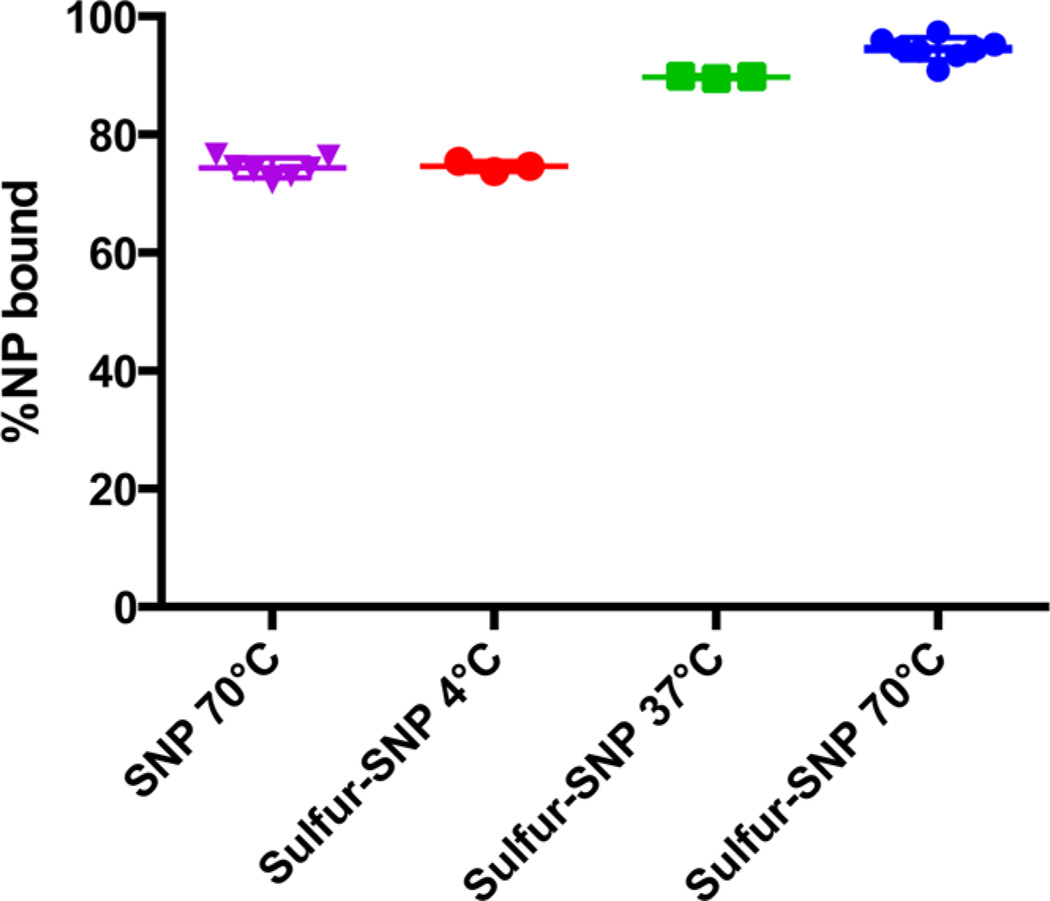
We then evaluated the binding affinity of the sulfur-SNP for 64Cu relative to that of the native SNP. First 64Cu (200 µCi, 7.4 MBq) was added to sulfur-SNP dispersions (100 µL; 10 nM) in 10 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 7.3) at 4 °C, 37 °C, and 70 °C, or to the control SNP radiolabeled at 70 °C, and a reaction time of 2 h. A total of 5 µL of ethylenediaminetetraacetic acid (EDTA) (50 mM, pH 7.1) was then added to scavenge weakly bound 64Cu. After 15 min, the nanoparticles were pelleted via centrifugation (10 500g, 5 min, 25 °C) and the supernatant containing unbound 64Cu was removed. Relative to the native SNP, sulfur-SNP demonstrated a significantly higher radiochemical yield of the soft radiometal 64Cu (sulfur-SNP, 94.5 ± 1.9%, SNP, 74.4 ± 1.9%). Radiolabeling of the sulfur-SNP with 64Cu is temperature-dependent, similar to our previous report for labeling of native SNP with hard radiometal ions (Figure 2).7 Although radiolabeling at 37 °C results in radiochemical yields >85%, the 64Cu detaches from the sulfur-SNP in serum, demonstrating that elevated temperatures (70 °C) are necessary for stable radiolabeling.
Figure 2.
Radiolabeling of native SNP at 70 °C versus sulfur-SNP at various temperatures. 64Cu-sulfur-SNP heated at 70 °C showed the highest stability to EDTA and serum challenge. Shown are individual data points, means, and standard deviations.
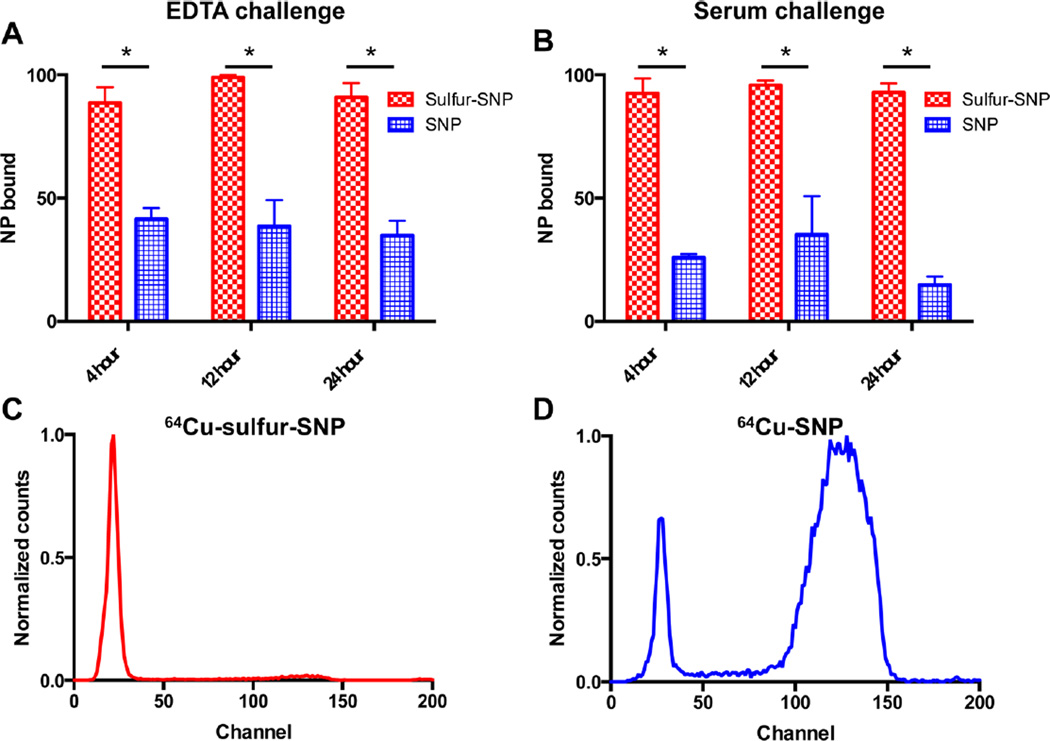
Although the radiochemical yield was greater for sulfur-SNP compared to native SNP, the stability of each nanoparticle construct to an EDTA and serum challenge are essential for in vivo use. Therefore, we assessed the binding stability of 64Cu to sulfur-SNP relative to native SNP by EDTA challenge and we evaluated serum stability. As seen in Figure 3A–B, the sulfur-SNP are significantly more stable compared to native SNPs. Out to 24 h for both the EDTA challenge (90.9 ± 5.8% versus 34.9 ± 5.8%) and the serum stability tests (93.0 ± 3.6% versus 14.8 ± 3.4%), the difference in stability is clearly seen by instant thin-layer chromatography (Figure 3C–D). After 10 half-lives of 64Cu, nanoparticle characterization was performed with TEM, showing that our 64Cu radiolabeling protocol did not affect the nanoparticle morphology, integrity, or diameter (Figure S2).
Figure 3.
Sulfur-SNP show significantly increased binding of 64Cu compared to native SNP. 64Cu shows significantly more stable binding to sulfur-SNP compared to SNP in an (A) EDTA challenge over 24 h, and (B) in a serum stability study, a prerequisite for in vivo use. (Data presented as mean with standard deviations; p < 0.05, unpaired Student’s t-test). Instant thin-layer-chromatography at 24 h shows (C) 64Cu binds stably to sulfur-SNP, whereas (D) 64Cu dissociates from native SNP when challenged in serum, respectively.
Importantly, the addition of thiol groups to the SNP surface does not affect radiolabeling of 89Zr in the silica matrix, as shown by a serum stability of >95% over 120 h (Figure S3A). Therefore, thiol-functionalized SNP can still be radiolabeled with 89Zr and used in vivo, allowing targeting and surface-coating opportunities. This finding is important for future studies that advance ideas in this field.
To prolong circulation times, nanoparticles are commonly coated with polyethylene glycol (PEG). We therefore also determined whether conjugation of PEG would affect 64Cu radiolabeling and stability. PEG (2 kDa; 1% (w/v)) was conjugated to the sulfur-SNP in 10 mM MES buffer (pH 7.3) via straightforward maleimide-chemistry postradiolabeling to determine the effect of PEGylation on 64Cu binding stability. It was found that PEGylated sulfur-SNP demonstrated comparable 64Cu-binding to sulfur-SNP (Figure S3B). The PEG-sulfur-SNP showed comparable 64Cu stability to the sulfur-SNP without PEG, which further enables in vivo use.
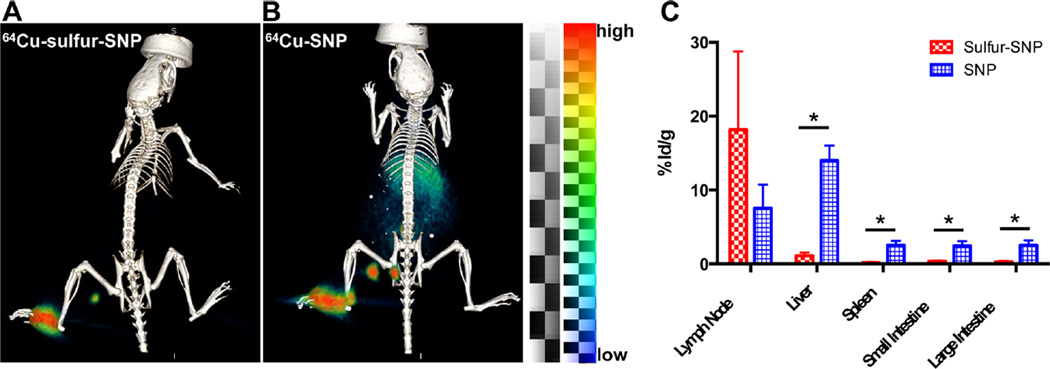
Because the radiochemical results supported the stability of 64Cu-sulfur-SNP, we tested both 64Cu labeled native SNP and sulfur-SNP in vivo for lymph node (LN) imaging capabilities. There is no significant difference in the diameter and zeta potential between the sulfur-SNP and SNP (Table S1), so transport through the lymph node system is expected to be similar. If 64Cu dissociates from the nanoparticle, it naturally accumulates in the liver, spleen, and intestines at later time points. We injected the footpad of one group of nude athymic mice with 64Cu-SNP and one group with 64Cu-sulfur-SNP. After 14 h, PET/CT was conducted on both groups (Figure 4A–B) and organs were collected to determine the biodistribution of 64Cu in both groups (Figure 4C). Whereas PET signal was only appreciable observed in the LNs and footpad after 64Cu-sulfur-SNP, relatively high signal in the liver, spleen, and intestines was found for 64Cu-SNP indicating dissociation and redistribution of 64Cu. Notably, the 64Cu-sulfur-SNP showed similar in vivo stability as our previously optimized 89Zr-labeled native SNP (Figure S4).
Figure 4.
PET/CT and biodistribution of 64Cu-sulfur-SNP and 64Cu-SNP. (A) 64Cu-sulfur-SNP injected into the footpad allow lymph node imaging with little systemic uptake at 14 h postinjection, whereas (B) 64Cu-SNP show appreciable liver, spleen, and intestinal uptake. (C) Quantitative ex vivo biodistribution values show significant differences for all organs except lymph node (* p < 0.05, unpaired t test). Mean with standard deviation shown.
In summary, the addition of thiol functionality—a soft electron donor—to SNP results in a significant increase in binding of 64Cu. As 64Cu is neither a soft nor hard cation, it is likely coordinating with both oxygen (hard) and sulfur (soft) to form a thermodynamically stable bond. This method is likely applicable to soft radiometals for facile radiolabeling of SNP as well. This approach enables the possibility of near-term clinical benefit, Because silica-based nanoparticles such as the smaller silica-based C-dots are already in clinical trials.15 As smaller SNP have been functionalized with similar sulfur densities per surface area, this method should be translatable to these systems. This strategy with cold Cu(II) was recently reported with bulk mesoporous silica for purification purposes, showing an increase in copper retention through the addition of thiol groups.16 Sulfur-SNP radiolabeled with the sulfur-avid 72As has also been reported.13
Because the stability of the sulfur-SNP is not affected by coating with PEG after radiolabeling, other ligands such as targeting peptides or antibodies may be attached after radiolabeling and this should also not affect 64Cu stability. The effect of sulfurs when radiolabeling sulfur-SNP with chelators conjugated to the SNP should be taken into account, because the sulfur-functionalized nanoparticles itself can stably bind various radiometals. With this work, we build forth on our previous work on labeling native SNP with hard oxophilic radiometals. Herein, we demonstrate a straightforward radiolabeling procedure for softer radiometals such as 64Cu, opening new avenues for stable radiolabeling of nanoparticles with a variety of hard and soft radiometals.
Supplementary Material
Acknowledgments
This work was supported by the following grants: NIH R01EB014944 and R01CA183953 (to J.G.), National Science Foundation Integrative Graduate Education and Research Traineeship Grant IGERT 0965983 (to C.M.D.), and R01 EB017748 (to M.F.K.). Technical services provided by the MSKCC Small-Animal Imaging Core Facility were supported in part by the MSKCC NIH Core Grant (P30-CA008748) and NIH Shared Instrumentation Grant No 1 S10 OD016207-01, which provided funding support for the purchase of the Inveon PET/CT. In part supported by a grant from the Center for Molecular Imaging and Nanotechnology (J.G.).
ABBREVIATIONS
- SNP
silica nanoparticle
- iTLC
instant thin layer chromatography
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- MPTMS
3-mercaptopropyltrimethoxysilane
- DLS
dynamic light scattering
- EDTA
ethylenediaminetetraacetic acid
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Experimental procedures, synthesis of silica nanoparticles, sulfur coating of silica nanoparticles, radiotracer production, radiolabeling protocols, radiochemical techniques, PET/CT, and biodistribution details. (PDF)
Author Contributions
T.M.S., S.H., and E.K. synthesized the silica nanoparticles. T.M.S. prepared the surface-coated nanoparticles, conducted DLS and zeta characterization, and conducted all radiochemical and in vivo studies. S.H. completed TEM and concentration determination of the nanoparticles. T.M.S. and S.H. wrote the manuscript. M.K., C.M.D., and J.G. supervised the project and edited the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. Nucl. Med. Biol. 2013;40:3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pratt EC, Shaffer TM, Grimm J. WIREs Nanomed. Nanobiotechnol. 2016 doi: 10.1002/wnan.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Sultan D, Detering L, Cho S, Sun G, Pierce R, Wooley KL, Liu Y. Angew. Chem., Int. Ed. 2014;53:156–159. doi: 10.1002/anie.201308494. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Campana C, Gomez-Vallejo V, Puigivila M, Martin A, Calvo-Fernandez T, Moya SE, Ziolo RF, Reese T, Llop J. ACS Nano. 2013;7:3498–3505. doi: 10.1021/nn400450p. [DOI] [PubMed] [Google Scholar]
- 5.Boros E, Bowen AM, Josephson L, Vasdev N, Holland JP. Chemical Science. 2015;6:225–236. doi: 10.1039/c4sc02778g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel S, Chen F, Ehlerding EB, Cai W. Small. 2014;10:3825–3830. doi: 10.1002/smll.201401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaffer TM, Wall MA, Harmsen S, Longo VA, Drain CM, Kircher MF, Grimm J. Nano Lett. 2015;15:864–868. doi: 10.1021/nl503522y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen F, Goel S, Valdovinos HF, Luo H, Hernandez R, Barnhart TE, Cai W. ACS Nano. 2015;9:7950–7959. doi: 10.1021/acsnano.5b00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeglis BM, Houghton JL, Evans MJ, Viola-Villegas N, Lewis JS. Inorg. Chem. 2014;53:1880–1899. doi: 10.1021/ic401607z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu T, Shi S, Liang C, Shen S, Cheng L, Wang C, Song X, Goel S, Barnhart TE, Cai W, Liu Z. ACS Nano. 2015;9:950–960. doi: 10.1021/nn506757x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi DD, Lane M, Zhou Y, Singh AP, Nayak S, Tisch U, Eizenberg M, Ramanath G. Nature. 2007;447:299–302. doi: 10.1038/nature05826. [DOI] [PubMed] [Google Scholar]
- 12.Stöber W, Fink A, Bohn EJ. Colloid Interface Sci. 1968;26:62–69. [Google Scholar]
- 13.Ellison PA, Barnhart TE, Chen F, Hong H, Zhang Y, Theuer CP, Cai W, Nickles RJ, DeJesus OT. Bioconjugate Chem. 2016;27:179–188. doi: 10.1021/acs.bioconjchem.5b00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jokerst JV, Miao Z, Zavaleta C, Cheng Z, Gambhir SS. Small. 2011;7:625–633. doi: 10.1002/smll.201002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gonen M, Kalaigian H, Schoder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Sci. Transl. Med. 2014;6:260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao QL, Zeng LM, Li LL, Guo F, Wu LH, Le S. Desalin. Water Treat. 2015;56:2154–2159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.