SUMMARY
Several benzoylphenyl urea-derived insecticides such as diflubenzuron (DFB, Dimilin®) are in wide use to control various insect pests. Although this class of compounds is known to disrupt molting and to affect chitin content, their precise mode of action is still not understood. To gain a broader insight into the mechanism underlying the insecticidal effects of benzoylphenyl urea compounds, we conducted a comprehensive study with the model beetle species and stored product pest Tribolium castaneum (red flour beetle) utilizing genomic and proteomic approaches. DFB was added to a wheat flour-based diet at various concentrations and fed to larvae and adults. We observed abortive molting, hatching defects and reduced chitin amounts in the larval cuticle, the peritrophic matrix and eggs. Electron microscopic examination of the larval cuticle revealed major structural changes and a loss of lamellate structure of the procuticle. We used a genomic tiling array for determining relative expression levels of about 11,000 genes predicted by the GLEAN algorithm. About 6% of all predicted genes were more than 2-fold up-or down-regulated in response to DFB treatment. Genes encoding enzymes involved in chitin metabolism were unexpectedly unaffected, but many genes encoding cuticle proteins were affected. In addition, several genes presumably involved in detoxification pathways were up-regulated. Comparative 2D gel electrophoresis of proteins extracted from the midgut revealed 388 protein spots, of which 7% were significantly affected in their levels by DFB treatment as determined by laser densitometry. Mass spectrometric identification revealed that UDP-N-acetylglucosamine pyrophosphorylase and glutathione synthetase were up-regulated. In summary, the red flour beetle turned out to be a good model organism for investigating the global effects of bioactive materials such as insect growth regulators and other insecticides. The results of this study recapitulate all of the different DFB-induced symptoms in a single model insect, which have been previously found in several different insect species, and further illustrate that DFB-treatment causes a wide range of effects at the molecular level.
Keywords: chitin, diflubenzuron, molting, Tribolium castaneum, tiling array, proteomics
Graphical Abstract
1. INTRODUCTION
Insect growth regulators (IGRs)1 are in wide use to control a variety of insect pests by interfering with their growth and/or development (Yu, 2008). The main IGR categories are distinguished by their mode of action. Ecdysone agonists such as tebufenozide or methoxyfenozide, and juvenile hormone mimics such as fenoxycarb, methoprene or pyriproxyfen interfere with the hormonal control of molting and metamorphosis (Dhadialla et al., 1998). In contrast, benzoylphenyl urea compounds such as DFB [1-(4-chlorophenyl)-3-(2,6-diflubenzoyl)urea] and its derivatives prevent the formation of chitinous structures in many insect orders. They have been shown to disturb cuticle formation causing abortive molting. Ultrastructural analysis revealed abnormal deposition of procuticular layers (Gijwijt et al., 1979; Grosscurt, 1978b; Lim and Lee, 1982; Mulder and Gijswijk, 1973; Verloop and Ferrell, 1977). Moreover, DFB also prevents normal formation of the peritrophic matrix (PM), which protects the midgut epithelium from various harms (Becker, 1978; Clarke et al., 1977; Soltani, 1984). The underlying mechanism(s) of DFB’s insecticidal activity was the subject of several investigations utilizing several different insect species. An early suggestion was that DFB efficiently inhibited chitin synthesis, because the incorporation of radiolabeled precursors into the growing chitin chain was impaired (Clarke and Jewess, 1990; Hajjar and Casida, 1978; Mayer et al., 1980; Post and Vincent, 1973). Chitin is a polymer of β(1,4)-linked N-acetylglucosamine, which is synthesized from UDP-N-acetylglucosamine precursors by chitin synthase (CHS), an integral membrane protein that belongs to the family of β-glycosyltransferases (Merzendorfer, 2006). As the catalytic domain of CHS is predicted to face the cytoplasm, the nascent chitin chain has to be translocated across the plasma membrane before it can be deposited into the cuticle or PM. In contrast to polyoxins or nikkomycins, which as competitive inhibitors directly inhibit CHS in vitro by binding in the active site of this enzyme, DFB inhibits chitin synthesis only in intact cellular systems and tissues, but not in solubilized membrane fractions (Cohen and Casida, 1980; Kitahara et al., 1983; Mayer et al., 1981; Zimoch et al., 2005). It also does not block any of the metabolic reactions that are necessary for the production of UDP-N-acetylglucosamine, nor does it affect chitin synthesis in fungal systems (Cohen, 1987 ; Verloop and Ferrell, 1977). Based on these and other findings, it was suggested that DFB does not act directly on the catalytic step of chitin synthesis (Cohen, 2001). Over time, many suggestions for DFB’s mode of action have been made including direct or indirect effects on the activities of chitinases, phenoloxidases, glycolytic enzymes, and microsomal oxidases, as well as on processes controlled by molting hormones (DeLoach et al., 1981; Ishaaya and Ascher, 1977; Ishaaya and E., 1974; Mitlin et al., 1977; Soltani et al., 1984). DFB is structurally similar to sulfonylureas (SUs), which are used in the treatment of type II diabetes in humans. SUs are known to bind to membrane-bound sulfonylurea receptors (SURs), which act as regulatory subunits of a voltage-gated inward rectifying potassium channel (Kir channel) (Ashcroft, 1988). Indeed, a gene encoding an SUR ortholog was identified in the Drosophila melanogaster genome, and expression of this gene in Xenopus oocytes leads to potassium channel activity, which is sensitive to glibenclamide, an SU derivative (Nasonkin et al., 1999). A more recent study suggested that a SUR may be the target for DFB in insects (Abo-Elghar et al., 2004). This conclusion was deduced from competitive binding assays with radiolabeled glibenclamide, an SU compound and structural analog of DFB, which was displaced from its binding site by the addition of unlabeled DFB. As in the case of vertebrates, the insect SUR may act as a regulatory subunit of ATP-sensitive K+ channels (Akasaka et al., 2006). Modulation of the activity of SUR/Kir could affect the membrane potential in a way that the activity of voltage-gated Ca2+ channels is affected disturbing Ca2+ homeostasis. As a result, Ca2+19-dependent vesicle fusion, which is necessary for the secretion of proteins involved in cuticle and PM formation, might be blocked. In support of this hypothesis, Ca2+ uptake experiments performed with a vesicle preparation from the integument of Blattella germanica demonstrated that Ca2+ uptake by isolated cuticular vesicles is inhibited in the presence of glibenclamide or DFB (Abo-Elghar et al., 2004). However, the significance of this finding remains unclear.
As the target molecule of DFB and other benzoylphenyl urea derivatives is still not identified, we aimed to establish a genetic model to examine the insecticidal effects of benzoylphenyl urea compounds. The model beetle and stored product pest Tribolium castaneum (red flour beetle) is a well-established insect model amenable to genetic and genomic approaches (Brown et al., 2003; Richards et al., 2008). Therefore, we examined DFB effects in T. castaneum by monitoring its toxicity in the course of development, measuring chitin amounts in whole larvae and isolated midguts, and by investigating ultrastructural changes in the cuticle. In addition, we conducted a comprehensive study utilizing genomic tiling arrays to detect changes in transcript levels and computerized 2D gel electrophoresis to document changes in the proteome of larval midguts.
2. MATERIALS AND METHODS
2.1 Insects and DFB treatment
T. castaneum strain GA-1 (Haliscak and Beeman, 1983) and the pu11 enhancer-trap line (Lorenzen et al., 2003) were used in this study. Insects were reared in whole wheat flour containing 5% yeast at 30°C. For various experiments the flour was supplemented with DFB as follows: per 50 g of flour in a beaker, 50 ml of acetone containing the desired amount of DFB (a kind gift from Dr. Kun Yan Zhu, Kansas State University) was added and the suspension was vigorously mixed for 30 min at room temperature. Then the acetone was completely evaporated under a fume hood over four days. Control diet was prepared exactly as described above but using acetone without the inclusion of DFB. Rearing of insects was performed in standard insect glass vials closed with cork stoppers with a maximum of 50 animals per 10 g of diet. For most evaluations T. castaneum larvae with a body weight of 1.50 ± 0.05 mg were fed with the control diet or the diet containing 100 ppm DFB, and feeding was continued until a mortality of 30% was achieved, which usually occurred after 2–3 d. Only live larvae with no obvious signs of intoxication were analysed for transcript measurements and proteomic analyses.
2.2 Array design and probe synthesis
Global gene expression analysis was performed with Roche NimbleGen HD2 whole tiling microarray. The arrays consist of 1050 by 4200 Perfect Matching (PM)-only oligonucleotide probes. The sizes of the probes ranged from 50- mer to 64-mer. The probes were aligned to the latest T. castaneum genome (Tcas3). 30,983 control probes (Random_GC) representing GC-richness of non-coding regions were randomly placed on each of the chips. These random probes were used to measure special bias and to derive background noise by maximum-likelihood estimation.
Total RNA from 3 (replicate 1) or 10 larvae (replicates 2 and 3) was prepared using a combined protocol of Trizol extraction (Invitrogen) and RNAeasy column purification (Qiagen). cDNA synthesis was performed with the Superscript cDNA synthesis kit (Invitrogen) using about 10 μg of total RNA and random hexamers (Shippy et al., 2008). The resulting cDNA was used as the template for Cy3/Cy5 labelling and competitive hybridization (Squazzo et al., 2006). Specifically, control and DFB treated larval cDNA were differentially amplified/labeled with Cy3- and Cy5-coupled nonamers, respectively, for the first two replicates. The third replicate was dye-swapped. Fifteen μg of each of the Cy3/Cy5-labeled cDNA samples were co-hybridized to a single microarray and the array was processed according to the manufacturer’s protocol (http://www.nimblegen.com/products/lit/expression_userguide_v5p0.pdf). The arrays were scanned using an Axon GenePix 4000B microarray scanner from Molecular Devices. The fluorescence intensities of the probes were quantitated and represented in a GFF file.
2.3 Obtaining relative expression values
Model-based analysis was applied (Li and Wong, 2001). However, the Mismatch (MM) signal was replaced by the global background (MMG), so that the actual intensity level for each probe was obtained using the values of PM–MMG, with PM being the perfect match signal. When the PM-MMG difference is less than zero, the negative value was replaced with 1. Finally, the actual intensity levels were log-transformed to the base 2. MMG was derived under the statistical inference on the random probes. The intensity values of the random probes for each array were scattered against the x-axis and y-axis (Figure S1). The plots showed that all intensity values except for a few outliers fall around approximately the same mean with approximately the same standard deviation along the x-axis and y-axis. The result indicated that spatial bias would not significantly affect the analysis and that the global background for MMG would be appropriate for each array being analysed. It also allowed us to assume that the intensity values of the random probes are normally distributed on the probability density function given the mean (μ) and standard deviation (σ):
By using the UNIVARIATE procedure in SAS, fitted normal curves were described on the histograms of the intensity values (x) for each array and the quantiles for each of the curves were estimated (Figure S2 and Table S1). Initially the 99 percentile with a 1% false discovery rate (FDR) and the 95 percentile with a 5% FDR were selected to obtain the best cut-off of the global background. Each of the selected cut-offs was applied to log2(PM-MMG.). The results were visualized through GBrowse (Figure S3). The 95 percentile was adequate for the first and third replicates, which have higher standard deviations, while the 99 percentile was adequate for the second replicate, which has a two-fold lower standard deviation.
The PM-MM intensity scores for each GLEAN gene annotation covering at least four tiles were averaged after removing the minimum and maximum values. The average values for each gene model between the control (no DFB treatment) and DFB-treated samples were compared to each other. The ratio was expressed as the average value obtained for the control group divided by that for the DFB-treated group (relative expression value, rE-value).
2.4 Protein extraction from midguts and 2D gel electrophoresis
Ten midguts were surgically removed from control and DFB-treated larvae. The pooled midguts were frozen in liquid nitrogen and to each sample 100 μl of osmotic lysis buffer (OLB, 10 mM Tris-HCl, pH 7.4, 0.3% SDS, supplemented with nuclease and protease inhibitor solutions purchased from KendrickLabs, Madison, WI) was added. The probes were homogenized in micro-cups using appropriate pestles (Eppendorf). After two freeze/thaw cycles, one volume of SDS boiling buffer (5% SDS, 10% glycerol, 60 mM Tris-HCl, pH 6.8) was added and the samples were incubated for 5 min in a boiling water bath. After insoluble material was removed by centrifugation for 5 min at 12,000 × g and 4°C, the protein concentration was determined using a Micro BCA Protein Assay Kit (Pierce). Two-dimensional gel electrophoresis was performed with 150 μg of midgut proteins using the carrier ampholine method of isoelectric focusing by Kendrick Labs, Inc. (Madison, WI) (O’Farrell, 1975). Isoelectric focusing was carried out in glass tubes of inner diameter 2.0 mm, using 2% pH 3.5–10 (GE Healthcare, Piscataway, NJ) for 20,000 volt-h. 100 ng of an IEF internal standard, tropomyosin (MW 33 kDa and pI 5.2), was added to each sample prior to loading. The enclosed pH gradient plot for these ampholines was determined using a surface pH electrode. After equilibrium in SDS sample buffer (10% glycerol, 50 mM dithiothreitol, 2.3% SDS and 0.0625 M Tris-HCl, pH 6.8), each tube gel was sealed to the top of a stacking gel that overlays a 10% acrylamide slab gel (1.0 mm thick). SDS slab gel electrophoresis was carried out for about 5 h at 25 mA/gel. The following proteins (Sigma Chemical Co, St. Louis, MO) were added as molecular weight markers: myosin (220 kDa), phosphorylase A (94 kDa), catalase (60 kDa), actin (43 kDa), carbonic anhydrase (29 kDa), and lysozyme (14 kDa). The gels were stained with silver for computerized comparison and with Coomassie blue for mass spectrometric protein identification. Duplicate gels were obtained from each sample and were scanned with a laser densitometer (Model PDSI, Molecular Dynamics Inc., Sunnyvale, CA). The images were analyzed using Progenesis PG240 software with TT900 (version 2006, Nonlinear Dynamics). Spot % is equal to spot integrated density above background (volume) expressed as a percentage of total density above background of all spots measured. Difference is defined as the fold-change of spot percentages.
2.5 MALDI-MS Analysis
Gel spots were transferred to clean tubes and completely hydrated by adding deionized water. After washing the gel spots twice for 20 min with 100 μl 50 mM Tric-HCl (pH 8.5)/30% (v/v) acetonitrile and for 1–2 min with 100% acetonitrile, they were dried in a Speed-Vac concentrator. Gels were digested overnight at 32°C after adding 15 μl 25 mM Tris-HCl (pH 8.5) supplemented with 0.06 μg trypsin (sequencing grade, Roche). Peptides were extracted with 2 × 50 μl 50% acetonitrile/2 % TFA, and the combined extracts were dried and resuspended in matrix solution (4-hydroxy-α-cyanocinnamic acid in 50% acetonitrile/0.1% TFA with two internal standards, angiotensin and ACTH 7–37 peptide). The dried gel samples were dissolved in matrix solution and 0.5 μl aliquots were spotted onto the sample plate, completely dried and washed twice with water. MALDI mass spectrometry was performed using an Applied Biosystems Voyager DE Pro mass spectrometer in linear mode. Peptide masses were entered into search programs to identify proteins in the NCBI or GenPept databases. Programs used were ProFound (http://prowl.rockefeller.edu/prowl-cgi/profound.exe) and MS-Fit (http://prospector.ucsf.exe). Cysteine residues were modified by acrylamide. Error tolerance was set to 0.5 Da for average masses.
2.6 Other Methods
Measurements of chitin content were performed as described previously (Arakane et al., 2005). In brief, whole larvae or isolated midguts were precisely weighed, homogenized with zirconium beads and hydrolyzed by incubating the samples at 80°C for 90 min in 6% KOH. After washing, the samples were digested with chitinase from Trichoderma viride (5 mg/ml in PBS, Sigma-Aldrich), and the released GlcNAc was measured by a modified Morgan-Elson assay (Reissig et al., 1955). The chitin content of whole larvae or of isolated midguts from pools of ten control or ten DFB treated larvae were measured separately. The chitin measurements were repeated three times, and the values are given as means ± SD. Cryoprotection, tissue embedding and cryosectioning were performed as described previously (Zimoch and Merzendorfer, 2002). To visualize cuticular chitin in T. castaneum, transverse sections of untreated and DFB treated larvae were stained for 30 min in 0.001 % (w/v) Calcolfuor white (CFW, Fluostain, Sigma-Aldrich, Munich) dissolved in PBS buffer (pH 7.6). Excess CFW was removed by washing with PBS. To detect chitin in the PM, isolated midguts were stained with a fluorescein-conjugated chitin-binding domain probe (FITC-CBD, New England BioLabs) as reported previously (Arakane et al., 2005). Fluorescence was recorded using light of appropriate excitation and emission wavelengths. Ultra-sectioning and transmission electron microscopy was carried out as described elsewhere (Chaudhari et al., 2011). In brief, decapitated larvae were fixed for 1–16 h at room temperature in a 0.1 M sodium cacodylate buffer (pH 7.4) containing 2% (w/v) paraformaldehyde and 2% glutaraldehyde (v/v). Post fixation was performed with 1–2% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.4) at room temperature with constant rotation for 2 h. After washing, the samples were stained with 2% uranyl acetate (w/v; in H2O) for 1 h. After dehydration in an ascending series of ethanol (50–100% ethanol, 10% steps, 3 × 5 min each) the samples were infiltrated with EMBED 812/Araldite resin at room temperature and embedded in flat mold capsule. Samples were thin sectioned and imaged on a CM-100 TEM (FEI Co.). Polymerization of the resin was carried out in a drying oven at 65°C for 24–48 h. RT-PCR for evaluating relative mRNA-amounts in control and DFB treated larvae was performed as described previously (Broehan et al., 2010).
3. RESULTS
3.1 DFB perturbs molting and egg hatching in T. castaneum
To monitor the insecticidal effects of DFB in T. castaneum (GA-1 strain; Haliscak and Beeman, 1983), we fed larvae and adults with a wheat flour-based diet containing DFB at various concentrations. Larvae were significantly impaired by DFB when compared with control animals (Figure 1). Younger larvae (1.5 mg at the start of treatment) were generally highly susceptible to the insecticide and died during the larval-to-larval molts (Figures 2A,B). Only a few of them (about 10%) molted to the pupal stage but these eventually died during the pupal-to-adult molt (Figure 2D). In contrast, older larvae (2.5 mg at the start of treatment) died primarily during the larval-to-pupal molt (Figure 2C). In Figure S4, the typical distribution of larval, pupal, adult and dead insects is shown for various DFB concentrations at different exposure times. Adult beetles that were fed with a DFB containing diet three days after emergence were not affected by this IGR and did not show increased mortality compared to control beetles during a three month period of observation (Figure 2E). Oviposition and subsequent embryonic development appeared to be normal after DFB treatment of adult females (Figure 2F). However, egg hatch was completely inhibited by DFB treatment and the unhatched embryos became twisted and enlarged (Figure 2G). The larval, pupal, adult and embryonic phenotypes generally resembled those described for animals injected with dsRNA for the TcCHS1 (or TcCHS-A) and TcCHT10 genes encoding chitin synthase A and chitinase 10 of T. castaneum, respectively (Arakane et al., 2005; Zhu et al., 2008).
Figure 1. Dose response of mortality of T. castaneum fed a diet containing DFB.
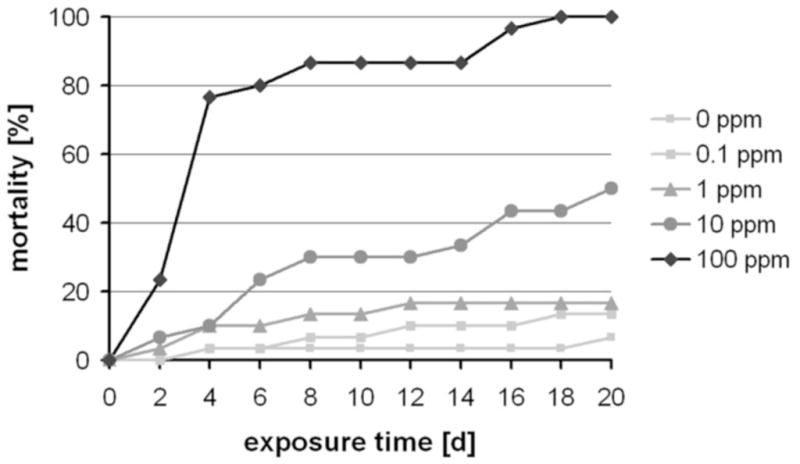
DFB was added to a wheat flour-based diet and fed to larvae at the indicated concentrations. Mortality was monitored at indicated exposure times.
Figure 2. Effects of DFB on molting, egg-laying and hatching of T. castaneum.
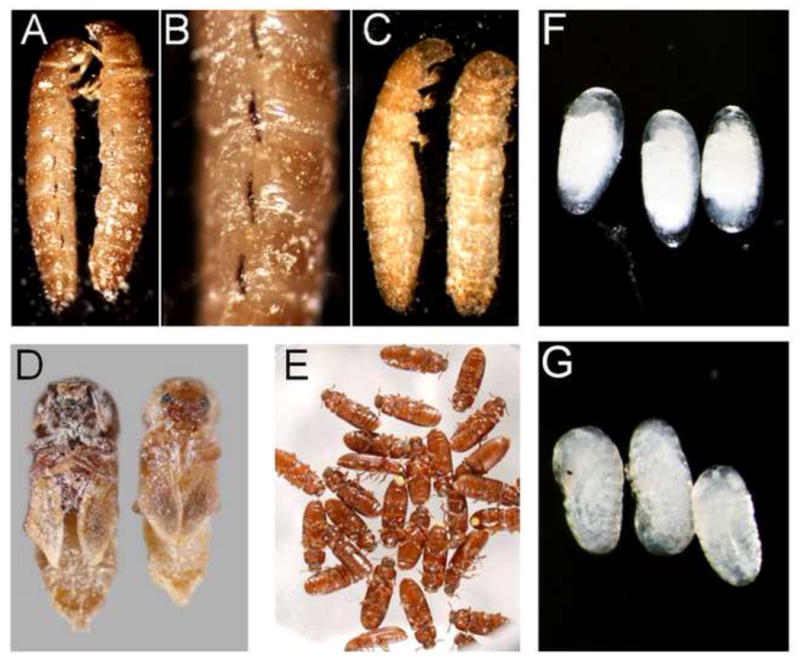
Larvae and adults were fed with a flour-brewer’s yeast diet containing 100 ppm DFB. Whereas particularly younger larvae were significantly impaired by DFB, adults did not exhibit any increase in mortality when compared with control insects. Death was observed during the larval-to-larval molts (A, B), the larval-to-pupal molt (C), and the pupal-to-adult molt (D). Note that the larval spiracles are melanised extensively (B). Adults were not affected by DFB (E). Oviposition and subsequent embryonic development appeared to be normal after DFB treatment of adult females (G). However, egg-hatch was completely inhibited by DFB treatment and the development was arrested (F).
3.2 DFB-treatment reduces chitin levels in the cuticle, PM and eggs, and alters procuticle structure
To analyze the effects of DFB on chitin levels in the larval cuticle, we performed longitudinal cryosectioning of whole body and stained for chitin with Calcofluor white (CWF). As shown in Figure 3A, CFW fluorescence is significantly reduced after DFB treatment, indicating decreased chitin levels in the cuticle (Meissner et al., 2010). Similar results were obtained when we stained midguts isolated from control and DFB treated larvae with a fluorescein-conjugated chitin-binding domain probe which is a more specific probe for detecting chitin present in the PM (Arakane et al., 2005). Midguts from DFB treated larvae showed a lower fluorescence than control larvae (Figure 3B). To quantify the reduction in chitin levels, we determined the chitin content of untreated and DFB treated larvae using a modified Morgan-Elson assay (Arakane et al., 2005; Reissig et al., 1955). In comparison to control insects, DFB-treated larvae showed a significant reduction in whole body chitin content (Table 1). The mean chitin content per insect was equivalent to ~ 70 nmole GlcNAc per larva and hence only about one half of the value for the untreated larvae, which was ~ 150 nmole GlcNAc per larva. Similarly, DFB-treatment led to a reduction in chitin content of the PM from larval midguts by about 40%, and of eggs obtained from female beetles fed a DFB-containing diet by about 60% (Table 1). To examine possible ultrastructural changes within the cuticle in response to DFB-treatment, we performed transmission electron microscopy (TEM). For this purpose, defined abdominal segments (A6) of control larvae and larvae fed with a diet containing either 10 or 100 ppm DFB were fixed, stained with uranyl acetate and subjected to ultrathin sectioning. The dorsal cuticles were examined by TEM analysis. As depicted in Figures 4A & D, control larvae had a characteristic laminar cuticle with numerous layers. While the old procuticle of larvae treated with 10 ppm DFB exhibited a clear reduction in the numbers of layers (Figures 4B & E), the new cuticle of larvae treated with either 10 or 100 ppm DFB appeared completely amorphous (Figures 4C,F). Hence, DFB produces dramatic changes in the lamellar organization of the cuticle.
Figure 3. Labeling of chitin in larval cuticles and midgut peritrophic matrices from T. castaneum.
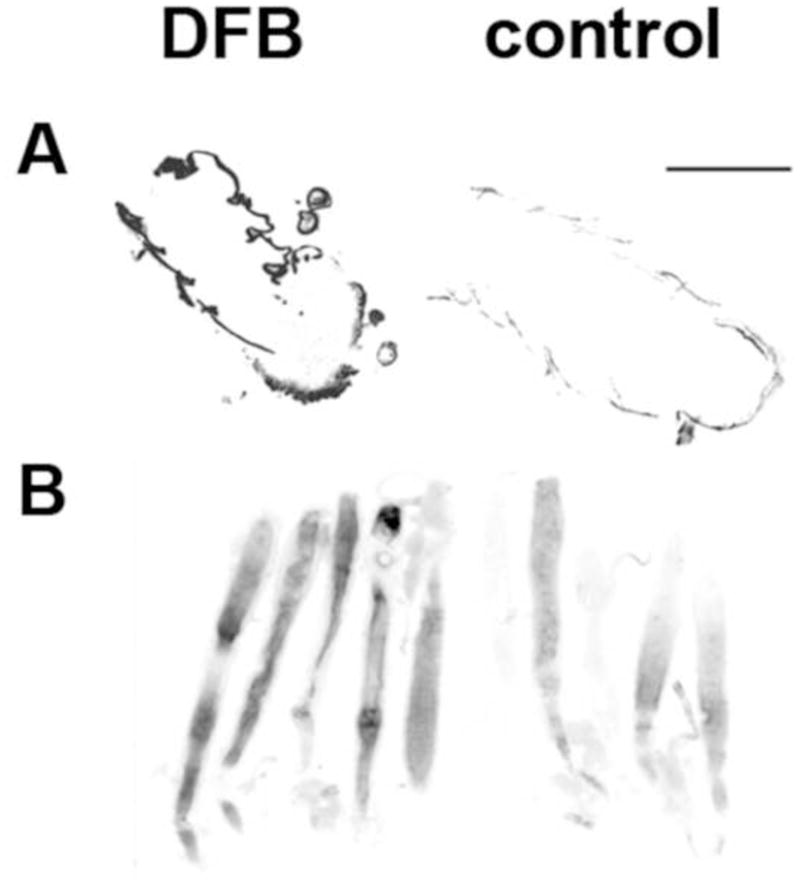
Larvae (1.5 mg) were fed a control diet or a diet containing 100 ppm DFB. (A) Cryosections of larvae were stained with Calcofluor White. (B) Midguts were isolated and stained with a FITC-conjugated chitin binding domain. Gray scales were inverted for better visualization. Bar size = 500 μm.
Table 1.
Chitin amounts in whole larvae, midguts and eggs of T. castaneum
| Exp. # | nmole GlcNAc per larva | nmole GlcNAc per gut | nmole GlcNAc per 100 eggs | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | DFB | Control | DFB | Control | DFB | |
| 1 | 159.68±13.27 | 76.91±8.16 | 4.87±3.54 | 2.15±0.05 | 11.03±6.31 | 7.62±0.19 |
| 2 | 171.92±15.36 | 79.13±8.84 | 4.10±0.07 | 2.99±0.06 | 54.41±6.50 | 19.87±1.98 |
| 3 | 126.45±13.61 | 61.65±17.45 | 3.78±0.10 | 2.57±0.03 | 60.36±1.39 | 24.30±1.41 |
| mean | 152.68±14.08 | 75.56±11.58 | 4.25±1.24 | 2.57±0.05 | 41.93±4.73 | 17.26±1.19 |
| % | 100.00 | 49.49 | 100.00 | 60.47 | 100.00 | 41.16 |
Figure 4. Effects of DFB on the ultrastructure of the epicuticle and procuticle from penultimate instar larvae of T. castaneum.
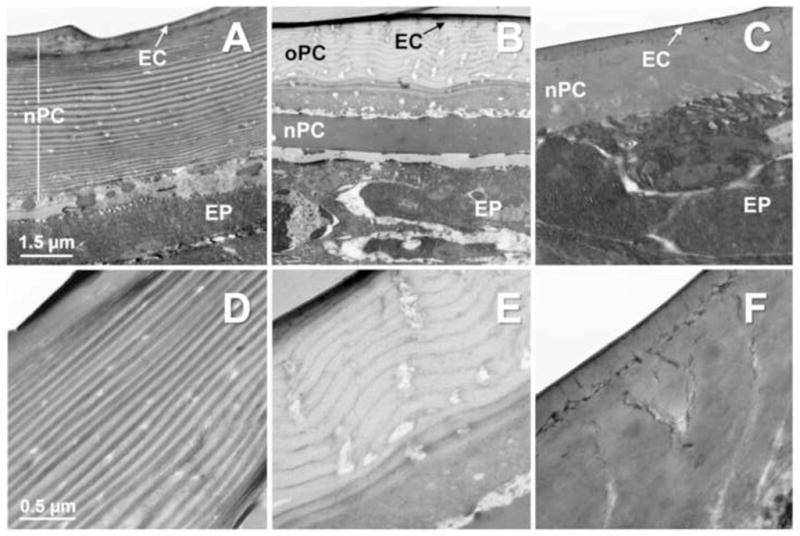
Defined abdominal segments (A6) of control larvae (A & D) and larvae fed with a diet containing either 10 ppm (B & E) or 100 ppm DFB (C & F) were fixed, stained with uranyl acetate and subjected to ultrathin sectioning. The new procuticle (nPC) of larvae treated with either 10 or 100 ppm DFB appears completely amorphous. The number of layers in the old procuticle (oPC) of larvae treated with 10 ppm DFB is reduced when compared to control larvae. EC, epicuticle; EP, epidermis; Magnifications: 25,000x (A–C) and 7,900x (D–F).
3.3 DFB does not significantly accelerate or retard larval development
An important prerequisite for genome-wide analysis of DFB effects is the precise monitoring of larval development. Differences in the developmental rate between control and DFB treated animals could cause significant shifts in global gene expression patterns. As the precise larval stages are difficult to assign in T. castaneum, we used the pu11 enhancer trap line to monitor development by means of a stage-specific marker. The strain pu11 is a transgenic line, which shows onset of EGFP expression in the wing and elytral imaginal discs in the last larval instar as well as constitutive expression in the eye due to the presence of the 3xP3 artificial Pax6 element (Lorenzen et al., 2003; Tomoyasu and Denell, 2004). When we fed pu11 larvae (1.5 mg at the start of treatment) with a flour-based diet containing 100 ppm DFB, these larvae showed the same response to the insecticide as those of the GA-1 strain. Onset of GFP expression in the wing and elytral imaginal discs occurred at about the same time in the last larval instar as in animals fed with the control diet, indicating that larval development is not significantly altered during the three days of observation (Figure S5, left panels). Those larvae that had molted to the pupal stage showed expression of GFP in the wings and elytra, indicating that development was not arrested by DFB treatment during the pupal stage (Figure S5, right panels).
3.4 DFB effects on global gene expression
To conduct an unbiased study of changes in expression of all genes that are potentially affected by DFB treatment, we utilized a genome-wide tiling array for hybridization with differentially labeled cDNA derived from total RNA of control and DFB treated (100 ppm) larvae. Log2 values of fluorescence intensities from three biological replicates were averaged over all tiles (50–64 nucleotides) available for the respective gene. Intensity values were corrected for background fluorescence determined by means of Random-GC oligonucleotides distributed throughout the chips (see Materials and Methods). We predict that these averaged and background-corrected log2 values provide a confident measure for the respective gene expression level, as it is based on values from numerous, individual hybridizations covering the entire gene sequence predicted by GLEAN. To evaluate the effects of DFB on the expression level (as measured by steady-state transcript levels), we calculated relative expression values (rE-values). We categorized rE-values values as follows: <0.5, substantially up-regulated; 0.5–0.9, marginally up-regulated; 0.9–1.1, not affected; 1.1–2.0 marginally down-regulated; >2.0 substantially down-regulated. The frequency distribution indicates that significantly more genes are down-regulated than up-regulated (Figure S6). The expression of most genes (10,491 of 11,213) predicted by the GLEAN algorithm as measured by steady-state transcript levels were not affected at all or only marginally altered by DFB treatment as indicated by rE-values that ranged between 0.5 and 2.0. In total, 722 genes were substantially up- or down-regulated. Hence, about 6% of all of the genes predicted by GLEAN showed significant changes in expression levels in response to the DFB treatment. As illustrated in Figure S7, among the 400 genes that were most strongly up- or down-regulated, there were many involved in protein, lipid, nucleotide and sugar metabolism (7, 10, 4%; total, up-regulated and down-regulated, respectively), replication, transcription and translation (8, 11, 4%), development (9, 9, 8%), signal transduction (6, 6, 5%), cuticle formation (5, 7, 3%), or genes encoding channels, transporters and receptors (5, 4, 5%). In contrast, genes involved in detoxification (2, 3, 0%), energy metabolism (2, 1, 2%), formation of the cytoskeleton and nucleoskeleton (1, 1, 0%), exo-/endocytosis and vesicle transport (2, 2, 1%), and stress and immune responses (1, 1, 2%) constituted only a smaller fraction of all genes that were altered in their expression levels. The group of the 200 genes that were most up-regulated contained also 1% transposon-like genes. The tiling array data can be found in the Excel file provided in the supplementary data (Spreadsheet.xls).
Our measurements of the chitin levels after a DFB challenge indicated that the chitin content is significantly reduced in T. castaneum larvae. Therefore, we focused on the expression levels particularly of those genes encoding enzymes involved in the chitin biosynthetic pathway including hexokinase (TcHEX), trehalase (TcTRE1/2), GlcNAc-6-phosphate deacetylase (TcNAGA), GlcNAc kinase (TcNAGK), glucosamine-6-phosphate deaminase (TcGNPDA), glutamine: fructose-6-P aminotransferase (TcGFAT), glucosamine-6-P acetyltransferase (TcGNPAT), phosphoacetylglucosamine mutase (TcPAGM), UDP-GlcNAc pyrophosphorylase (TcUAP1) and chitin synthase (TcCHS1/2). We considered also chitin modifying and degrading enzymes such as chitin deacetylases (TcCDA1/2), N-acetylglucosaminidases (TcNAG1/2/3 and TcFDL), and several chitinases (TcCHT4/5/7/10). As shown in Table 2, none of these genes is significantly up- or down-regulated, a finding which is in line with the results from RT-PCR performed for several of these genes (Figure S8). Hence, the analysis of global changes in gene expression after a DFB challenge indicates that the transcription of genes involved in chitin metabolism, modification and degradation is not affected significantly by DFB. Most notably, among the genes that were significantly affected by the DFB treatment were numerous genes encoding cuticle structural proteins of the RR1 and RR2 subfamilies (TcCPRs), as were genes that are known to be involved in detoxification of insecticides such as those encoding cytochrome P450 4c3 (TcCYP4c3), glutathione-S-transferase (TcGST), sulfotransferase (TcSULT), glucosyl/glucuronosyl-transferase (TcGLCT) and subfamily C ABC transporter C5K (TcABC-C5K) (Figure S8 and Table 2).
Table 2.
Relative expression levels of various genes in response to DFB treatment as revealed by genomic tiling array chip hybridization.
| Genes | Function | NCBI ref. seq. | Glean | rE-value | Transcript levels |
|---|---|---|---|---|---|
| Chitin biosynthesis | |||||
| TcHEX | Hexokinase | XM_965552.1 | 00318 | 1.064 | ± |
| TcTRE1 | Trehalase | XM_968826.2 | 06698 | 0.999 | ± |
| TcTRE2 | Trehalase | XM_967517.2 | 04791 | 1.062 | ± |
| TcNAGA | GlcNAc-6-phosphate deacetylase | XM_968918.2 | 07801 | 0.849 | + |
| TcNAGK | GlcNAc kinase | XM_967098.2 | 12681 | 0.947 | ± |
| TcGNPDA | glucosamine-6-phosphate deaminase | XM_970016.2 | 02924 | 1.087 | ± |
| TcGFAT | Glutamine: fructose-6-P aminotransferase | XM_961795.1 | 02585 | 0.976 | ± |
| TcPAGM | Phosphoacetylglucosamine mutase | XM_968253.2 | 11920 | 0.932 | ± |
| TcGNPAT | Glucosamine-6-P acetyltransferase | XM_966988.1 | 09619 | 1.081 | ± |
| TcUAP1* | UDP-GlcNAc pyrophosphorylase | XM_963976.2 | 01751 | 1.005 | ± |
| TcUAP2* | UDP-GlcNAc pyrophosphorylase | XM_968988.2 | 05593 | 0.962 | ± |
| TcCHS1* | Chitin synthase | NM_001039402.1 | 14634 | 0.999 | ± |
| TcCHS2* | Chitin synthase | NM_001039403.1 | N.A. | N.A. | N.A. |
| Chitin modifying/degrading | |||||
| TcCHT4* | Chitinase | NM_001080098.1 | 09180 | 1.049 | ± |
| TcCHT5 | Chitinase | NM_001039435.1 | 01770 | 1.054 | ± |
| TcCHT7 | Chitinase | NM_001042570.1 | 15481 | 0.875 | + |
| TcCHT10 | Chitinase | NM_001042602.1 | 12734 | 0.941 | ± |
| TcNAG1 | N-acetylglucosaminidase | NM_001098848.1 | 09808 | 0.964 | ± |
| TcNAG2 | N-acetylglucosaminidase | NM_001098828.1 | 11540 | 1.109 | − |
| TcNAG3 | N-acetylglucosaminidase | NM_001098827.1 | 01116 | 1.310 | − |
| TcFDL | N-acetylglucosaminidase | NM_001098826.1 | 09779 | 0.971 | ± |
| TcCDA1* | Chitin deacetylase | NM_001102476.1 | 14100 | 0.986 | ± |
| TcCDA2* | Chitin deacetylase | NM_001102577.1 | 14101 | 0.984 | ± |
| Cuticle proteins (nomenclature according to Dittmer et al., in preparation) | |||||
| TcCPR12 | RR2 type cuticle protein | XM_001815995.1 | 00853 | 3.083 | − − − |
| TcCPR15 | RR2 type cuticle protein | XM_965862.1 | 03109 | 2.738 | − − − |
| TcCPR17 | RR2 type cuticle protein | NM_001167848.1 | 03363 | 0.643 | ++ |
| TcCPR20 | RR2 type cuticle protein | XM_970249.1 | 02908 | 0.564 | ++ |
| TcCPR25 | RR1 type cuticle protein | XM_963573.1 | 03834 | 1.950 | − − |
| TcCPR26* | RR1 type cuticle protein | XM_963646.2 | 03835 | 0.465 | +++ |
| TcCPR51 | RR2 type cuticle protein | XM_001809938.1 | 13814 | 3.048 | − − − |
| TcCPR54 | RR2 type cuticle protein | XM_963430.1 | 13992 | 0.574 | ++ |
| TcCPR55 | RR2 type cuticle protein | XM_963581.2, | 13810 | 2.425 | − − |
| TcCPR59 | RR1 type cuticle protein | NC_007420.2 | 13306 | 3.733 | − − − |
| TcCPR67 | RR1 type cuticle protein | XM_968570.1 | 13139 | 0.690 | ++ |
| TcCPR79 | RR1 type cuticle protein | XM_968926.1 | 13129 | 0.669 | ++ |
| TcCPR84 | RR1 type cuticle protein | XM_964170.1 | 14770 | 0.653 | ++ |
| TcCPR88 | RR2 type cuticle protein | XM_965123.1 | 15901 | 0.540 | ++ |
| TcCPR89* | RR1 type cuticle protein | XM_961240.2 | 09873 | 0,997 | ± |
| TcCPR96* | RR2 type cuticle protein | XM_001816297.1 | 08767 | 3,769 | − − − |
| TcCPR100 | RR2 type cuticle protein | XM_965802.1 | 11148 | 3,474 | − − − |
| TcCPR103 | RR2 type cuticle protein, Pro-resilin | XM_001806976.1 | 03362 | 1.454 | − |
| TcCPR104 | RR1 type cuticle protein | XM_001811735.1 | 01120 | 0,873 | + |
| Phenoloxidases | |||||
| TcLAC1 | Laccase, diphenol oxidase | NM_001039425.1 | 00821 | 1.210 | − |
| TcLAC2* | Laccase, diphenol oxidase | NM_001039398.1 | 10489 | 1.002 | ± |
| TcTYR1 | Tyrosinase, monophenol oxidase | NM_001039404.1 | N.A. | N.A. | N.A. |
| TcTYR2 | Tyrosinase, monophenol oxidase | NM_001039433.1 | 15848 | 1.011 | ± |
| Detoxification | |||||
| TcSULT* | Sulfotransferase | XM_961924.1 | 12015 | 0.465 | +++ |
| TcGLCT | Glucosyl/glucuronosyl-transferase | XM_967666.2 | 10152 | 0.596 | ++ |
| TcCHE | Carboxy/cholinesterase | XM_962191.2 | 02194 | 4.626 | − − − |
| TcGST | Gluthathione S-transferase | XM_969955.2 | 00522 | 0.673 | ++ |
| TcGS | Glutathione synthetase | XM_962977.2 | 09391 | 0.982 | ± |
| TcCYP4c3 | Cytochrome P450 monoxygenase | XM_968307.2 | 15293 | 0.599 | ++ |
| Others | |||||
| TcRPS6* | Ribosomal protein S6 | NM_001172390.1 | 10830 | 1.054 | ± |
| TcACT2 | Actin-2 | XM_970977.2 | 06296 | 0.991 | ± |
| TcPMP2C* | Peritrophic matrix protein | NM_001168449.1 | 09580 | 0.970 | ± |
| TcSTE24* | Zinc-dependent metalloprotease | XM_963563.1 | 00463 | 1.176 | − |
| TcABC-C9A (TcSur1)* | ABC transporter subfamily C | XM_964261.2 | 12252 | 0.971 | ± |
| TcABC-C5K | ABC transporter subfamily C | XM_966647.2 | 14381 | 0.635 | ++ |
| TcHP12b | Odorant binding protein | XM_962784.2 | N.A. | N.A. | N.A. |
| TcY-e | Yellow protein | NM_001168307.1 | 06227 | 0.608 | ++ |
| TcY-k | Yellow protein | XM_971253.1 | 06225 | 4.715 | − − − |
| TcY-2 | Yellow protein | NM_001168312.1 | 03539 | 5.162 | − − − |
Genes whose expression levels were also analysed by RT-PCR (see Figure S8) are marked with an asterisk. Strong, moderate and weak up or down regulation of transcript levels are indicated with (+++),(++),(+) or (−−−),(−−), (−), respectively, and genes whose expression was unaltered are indicated with (±). Genes that were not predicted by the GLEAN algorithm were not assigned (N.A.).
3.5 DFB effects on the larval midgut proteome
In silico comparison of proteins extracted from midgut of control and DFB treated larvae and resolved by 2D gel electrophoresis revealed changes in the midgut proteome due to DFB treatment. Among 388 proteins that were analyzed in duplicate gels (see Figure 5), only 26 proteins (~7%) were significantly changed as determined by laser densitometry, comprising 21 up-regulated and 5 down-regulated proteins (Table 3 and Figure S9). Of these, ten proteins whose levels changed more than 2.5-fold were identified by MALDI-TOF analysis of tryptic peptides (Table S3). For nine of these proteins high quality mass spectra were obtained. Two of the protein spots are probably due to sample contamination from human keratin. Among the remaining seven midgut proteins from T. castaneum were UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1), glutathione synthetase (GS), two forms of the NADH-ubiquinone oxidoreductase 75 kDa subunit (complex I), paramyosin (PMYO), actin-2 (ACT2) and the 12 kDa hemolymph protein b (TcHP12b). All of these proteins appeared to be up-regulated between 3- and 6-fold. As the corresponding mRNA levels for these proteins were not affected significantly (see Table 2), the observed changes may be due to posttranscriptional regulation. However, transcriptional regulation in the midgut cannot be completely excluded, because mRNA levels were measured using whole body preparations, which blurred any possible changes in the midgut transcriptome.
Figure 5. In silico comparison of larval midgut proteins from T. castaneum resolved by two-dimensional gel electrophoresis.
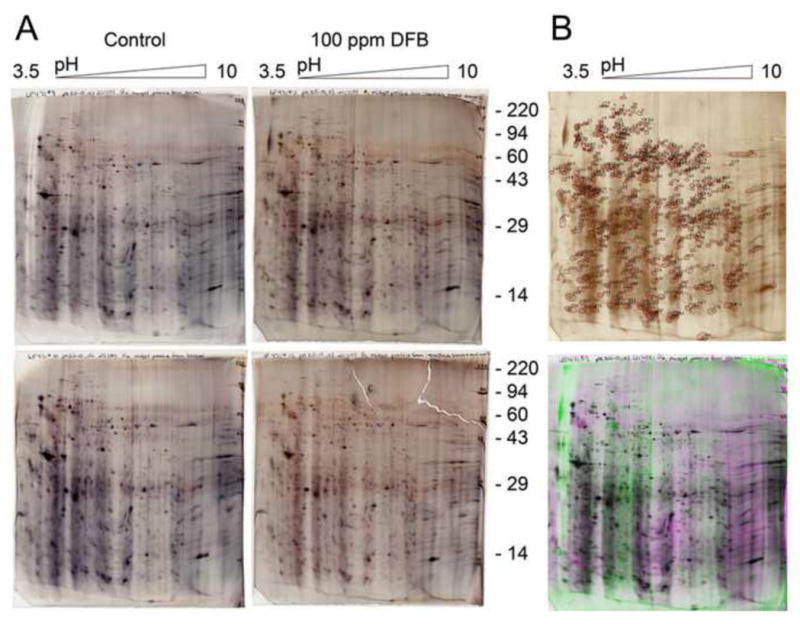
(A) Proteins were SDS extracted from midguts of larvae that were fed a control diet or a diet containing 100 ppm DFB. The proteins were separated in duplicate gels by 2D electrophoresis. The gels were scanned with a laser densitometer, aligned, and compared to each other. (B) Reference gel showing all spot numbering (top). Overlay image showing midgut proteins from DFB treated animals in green overlaying midgut proteins from control in magenta (bottom). Image appears black where spots overlay each other. A total of 388 protein spots were identified, but only the levels of 26 proteins were changed more than 2-fold.
Table 3.
Molecular masses, isoelectric points and MALDI-TOF analysis of midgut proteins from T. castaneum whose amount significantly changed in response to DFB treatment
| Spot# | pI | MW | DFB | Control | DFB/Control | DFB/Control | MALDI-MS |
|---|---|---|---|---|---|---|---|
| Spot % | Spot % | Difference | T-test (p) | Identified protein spots | |||
| 8 | 5.7 | 112,068 | 0.077 | 0.021 | 3.6 | 0.009 | XP_970719.1, paramyosin |
| 12 | 5.9 | 92,018 | 0.012 | 0.006 | 1.9 | 0.029 | N.D. |
| 17 | 6.2 | 83,232 | 0.023 | 0.006 | 3.7 | 0.025 | XP_973797.1, NADH-ubiquinone oxidoreductase 75 kDa subunit |
| 19 | 6.3 | 82,382 | 0.054 | 0.017 | 3.2 | 0.015 | XP_973797.1, NADH-ubiquinone oxidoreductase 75 kDa subunit, modified |
| 30 | 6.2 | 75,584 | 0.099 | 0.042 | 2.3 | 0.024 | N.D. |
| 35 | 6.1 | 71,193 | 0.041 | 0.004 | 9.5 | 0.020 | Keratin contamination |
| 39 | 5.7 | 69,280 | 0.036 | 0.018 | 2.0 | 0.045 | N.D. |
| 56 | 6.8 | 59,520 | 0.095 | 0.018 | 5.3 | 0.182 | NP_001164533.1, UDP-GlcNAc pyrophosphorylase I |
| 82 | 6.8 | 51,143 | 0.055 | 0.010 | 5.7 | 0.002 | XP_968070.1, glutathione synthetase |
| 87 | 6.0 | 50,477 | 0.156 | 0.088 | 1.8 | 0.022 | N.D. |
| 90 | 6.2 | 50,286 | 0.101 | 0.056 | 1.8 | 0.037 | N.D. |
| 119 | 8.1 | 46,875 | 0.037 | 0.016 | 2.4 | 0.039 | N.D. |
| 153 | 7.0 | 43,867 | 0.059 | 0.029 | 2.0 | 0.046 | N.D. |
| 161 | 4.6 | 39,311 | 0.054 | 0.029 | 1.9 | 0.026 | N.D. |
| 168 | 7.6 | 38,283 | 0.172 | 0.096 | 1.8 | 0.027 | N.D. |
| 242 | 7.9 | 27,975 | 0.475 | 0.276 | 1.7 | 0.022 | N.D. |
| 262 | 5.8 | 26,911 | 1.269 | 0.645 | 2.0 | 0.031 | N.D. |
| 267 | 5.9 | 26,573 | 0.222 | 0.116 | 1.9 | 0.020 | N.D. |
| 297 | 6.3 | 22,166 | 0.523 | 0.207 | 2.5 | 0.044 | N.D. |
| 307 | 5.9 | 20,324 | 0.251 | 0.087 | 2.9 | 0.037 | NP_001165843.1, actin-2 |
| 385 | 7.1 | 11,933 | 0.167 | 0.055 | 3.1 | 0.196 | XP_967877.1, 12 kDa hemolymph protein b |
| 89 | 6.1 | 50,286 | 0.008 | 0.020 | −2.6 | 0.025 | Poor spectrum, no ID |
| 294 | 5.3 | 22,495 | 0.283 | 0.563 | −2.0 | 0.019 | N.D. |
| 311 | 7.1 | 19,855 | 0.202 | 0.341 | −1.7 | 0.027 | N.D. |
| 361 | 5.8 | 13,290 | 0.029 | 0.216 | −7.5 | 0.264 | Keratin contamination |
| 384 | 7.1 | 12,035 | 0.713 | 1.325 | −1.9 | 0.036 | N.D. |
4. DISCUSSION
DFB was developed as a commercial insecticide in the early 1970s by Philips-Duphar B.V. (Netherlands) and many other benzoylphenyl urea derivatives have been successfully established as even more potent insecticides, several of which are still in wide use in agriculture and forestry. Different effects of DFB and other benzoylphenyl urea compounds have been examined in single insect species from various orders including Diptera, Lepidoptera, Coleoptera, Acari, and Hemiptera (Grosscurt, 1978a). Because the important stored product pest T. castaneum has been developed as a genomic model for studies of development and pest biology (Brown et al., 2009), we aimed to use this beetle model for thoroughly investigating the effects of DFB and related insecticides in a single insect species.
4.1 DFB effects on growth and development of T. castaneum
By feeding T. castaneum larvae a diet with DFB for three days, we were able to confirm the toxic effects described previously for this species (Gazit et al., 1989). The LC50 values for DFB were reported previously to be approximately 13 ppm for fourth instar larvae until pupation and 11 ppm until emergence. Our data are generally in good agreement with those data as DFB exhibited LC50 values of 22.6 ppm (R2=0.81) for 1.5 mg larvae until pupation and 12.8 ppm (R2=0.85) until emergence (data not shown). We also found that the toxicity of DFB was significantly higher in younger than in older larvae, a finding that is in line with a previous report (Ishaaya and Ascher, 1977). Albeit oviposition is not affected, DFB treatment has been reported to have ovicidal effects in T. castaneum as hatchability of the eggs laid by DFB treated females was significantly decreased at relatively low concentrations (Carter, 1975). We also observed in our experiments that the number of eggs that were laid by female beetles fed a diet containing 100 ppm DFB was nearly the same as for females fed a control diet without DFB. Most of the embryos developed normally inside the egg shell but they failed to hatch, indicative of a weakened larval cuticle that lacked the mechanical strength to break through the egg shell. This phenotype is similar to the one we have reported for eggs derived from females subjected to RNAi for the CHS-A gene (Arakane et al., 2008). Our direct chitin measurements on the developing embryos indeed confirmed that DFB substantially inhibited the increase in chitin that occurs during larval cuticle deposition during embryonic development.
DFB has been reported to interfere with normal endocuticle deposition during larval development (Mulder and Gijswijk, 1973). More recently, Gangishetti et al. (2009) have shown that DFB treatment of adult females results in embryos that have lower chitin staining and lack the chitin laminae in the developing larval cuticle as shown by transmission electron microscopy. In our studies with T. castaneum penultimate instar larvae, we found that compared to control larvae, the procuticle of larvae treated with 10 ppm DFB showed a reduction in the number of laminae that were also less compact than in the control. After treatment with 100 ppm DFB, the newly synthesized larval cuticle was completely devoid of laminae and appeared to be amorphous.
The latter findings were similar to observations made in Manduca sexta and Lucilia cuprina (Binnington, 1985; Hassan and Charnley, 1987). Furthermore, in line with studies reporting a decrease in cuticular chitin amounts in response to DFB treatment (Hajjar and Casida, 1978; Post et al., 1974; van Eck, 1979), we determined that the decrease of the total chitin content was about 50% in T. castaneum larvae consistent with the observed reduction in CFW fluorescence in the cryosections of larval cuticles. The TEM data provide further evidence for the loss of chitin-containing laminae in the larval cuticle in a manner that is dependent on the dose of DFB. These results suggest that the steady-state level of chitin in the integument is significantly inhibited by DFB. According to our measurements of total and midgut chitin amounts, cuticular chitin may account for more than 90% of total chitin content, while PM-associated chitin represents less than 10%. Although generally low, we found that the chitin content in the PMs of isolated midguts from DFB treated T. castaneum larvae, which were freed of fore- and hindguts, was reduced by about 40%. This observation in turn is in line with the reduction in fluorescence intensity of midguts stained with FITC-CBD in larvae fed a DFB-containing diet and with previous reports from several insect species in which DFB appeared to block chitin synthesis during the production of the PM (Clarke et al., 1977; Cohen and Casida, 1980; Soltani, 1984).
To examine potential changes in the developmental rate, which could contribute to a reduction in chitin content at a particular stage in development, we monitored the onset of the developmental marker 3xP3-EGFP in the transgenic T. castaneum line pu11 during the relevant observation time of three days. Until pupation we could not detect significant changes in the onset of the developmental marker, and those beetles that progressed to the pupal stage continued to express 3xP3-EGFP. The only study that previously analyzed the effects of DFB on developmental rate in T. castaneum reported a small but significant effect for very young larvae (0–3 h old) that were continuously exposed to DFB until emergence. In this case the developmental period to emergence was reduced by only about 15% (Ishaaya and Ascher, 1977).
4.2 DFB-effects on global gene expression
Although many scientists have attempted to elucidate DFB’s mode of action, the question of how it interferes with insect molting and egg hatching is still a matter of speculation (Gangishetti et al., 2009; Muthukrishnan et al., 2012). Therefore, we decided to adopt an unbiased genome-wide approach profiling changes in gene expression levels in response to DFB treatment of larvae. Since conventional microarrays usually only use a few probes to interrogate a gene, and do not provide data on unknown transcriptionally active regions, we probed Roche NimbleGen HD2 whole genome DNA tiling array chips covering two million features of the T. castaneum genome. We developed a statistically evaluated procedure for background subtraction based on “Random-GC oligonucleotides” that were randomly placed on each chip, and an evaluation method to obtain gene expression levels based on averaged intensity scores obtained from numerous features interrogating all exons of each GLEAN-model. Hence, we for the most part obtained expression levels from a high number of individual hybridization events per gene. For instance, GLEAN_09580 (NM_001168449.1) encoding PM protein 2C (PMP2C; Jasrapuria et al., 2010) comprises three predicted exons represented by 48 individual tiles (see Figure S3). After background subtraction, the mean intensity values for these tiles averaged for three biological replicates were 13.09 ±1.24 for the control and 13.47±0.75 for the DFB treated animals. The corresponding rE-value of 0.97 indicates no significant changes in the expression levels of this gene in response to DFB treatment. Our study revealed that only comparatively few T. castaneum genes exhibit significant changes in steady-state transcript levels in response to DFB treatment. They included genes involved in protein, lipid, nucleotide and sugar metabolism, replication, transcription and translation, development, signal transduction, cuticle formation and degradation, and genes encoding channels, transporters and receptors. Of particular interest are those functional categories that include more genes that are up-rather than down-regulated, as is the case for genes involved in the metabolism and modification of proteins, lipids, nucleotides and sugars, as well as replication, transcription and translation. While up-regulation of the latter genes may reflect a general stress response to DFB, other genes that are up-regulated may be involved in specific metabolic pathways involved in detoxification of this compound or in cuticle repair processes that compensate for malformations of the cuticle.
4.3 Induction of genes potentially involved in the elimination of xenobiotics
Among the genes that were up-regulated in response to DFB treatment were several that are known to be associated with insecticide detoxification by phase I and II reactions. Phase I reactions are commonly mediated by carboxy-esterases and cytochrome P450 enzymes (CYPs), while phase II reactions involve sulfotransferases, glucosyl/glucuronosyl-transferases and glutathione S-transferase (Ahmad and Forgash, 1976; Ffrench-Constant et al., 2004; Ishaaya, 1993). However, it should be pointed out that the up-regulation of genes known to be involved in detoxification may reflect a general response to xenobiotic stress, without necessarily playing a specific role in the detoxification of DFB.
Experiments with T. castaneum using esterase inhibitors suggested that the major metabolic pathway in the larva for DFB is by hydrolytic cleavage of the amide bond at the benzoyl carbon to yield 4-chlorophenylurea and 2,6-difluorobenzoic acid, which may be further converted into 4-chloroaniline, CO2, and 2,6-difluorobenzamide (Gazit et al., 1989). The DFB hydrolase sensitive to the esterase inhibitors has not yet been identified, and there is no obvious hydrolase-encoding gene in T. castaneum, which is up-regulated in response to DFB treatment.
The tiling array data indicate furthermore that the transcript levels of genes encoding sulfotransferase, glucosyl/glucuronosyl-transferase, glutathione S-transferase and one particular cytochrome P450 isoform (TcCyp4C3) are up-regulated, suggesting that aromatic metabolites formed after cleavage may require hydroxylation and conjugation to small hydrophilic molecules in order to get eliminated. This is, however, in contrast to the house fly Musca domestica and the boll weevil Anthonomus grandis, in which direct oxidation appears to be the major route for DFB detoxification, because the major metabolites in these two species are hydroxylated forms of DFB (Chang, 1978; Chang and Stokes, 1979).
In addition to the detoxifying enzymes, ATP binding cassette (ABC) transporters of subfamilies B, C and G have been implicated in the insect defense response against xenobiotics including insecticides (Labbe et al., 2011). Experiments performed with Aedes caspius revealed synergistic effects between DFB and the ABC transporter inhibitor, verapamil, increasing DFB toxicity significantly when both compounds are co-administered (Porretta et al., 2008). The latter finding suggests that certain ABC transporters could act as efflux pumps for DFB or its metabolites. Indeed, the tiling array data revealed several genes encoding ABC transporters whose expression was altered in DFB-treated T. castaneum larvae. However, among the 200 most up-regulated genes was only one encoding an ABC multidrug transporter of subfamily C, TcABC-C5K. Hence, TcABC-C5K might have a function during the course of DFB elimination. In contrast, the expression of TcABC-G9B (TcWhite) as well as TcABC-C7A was found to be down-regulated. However, many of the ABC transporter-encoding genes such as TcABC-G8A were unaltered in their expression levels. Particularly, we could not detect changes in expression levels of TcABC-C9A and TcABC-C4A, which encode two homologues of the D. melanogaster sulfonylurea receptor, DmSUR, a ABC transporter of the C subfamily, which has been suggested to act as a receptor for DFB based on the ability of DFB to compete with glibenclamide binding (Abo-Elghar et al., 2004). By studying Ca2+ uptake into vesicles prepared from abdominal integuments of Blattella germanica nymphs and the effects of DFB in this process, it has been proposed that DFB binding to SUR inhibits a GTP- or ATP-dependent Ca2+ and/or K+ transport system (Nakagawa and Matsumura, 1994). The mere absence of transcriptional responsiveness of SUR homologs from T. castaneum does not exclude the possibility that they are involved in DFB toxicity.
4.4 Genes involved in cuticulogenesis and PM formation
It is well established that the addition of DFB neither inhibits chitin synthesis in cell-free membrane preparations that are actively making chitin nor does it inhibit the activity of the partially purified chitin synthase preparations (Cohen, 2001; Merzendorfer, 2006). These results are inconsistent with the well-known effect of DFB and other structurally related “chitin inhibitors” to bring about drastic reductions in chitin levels in cuticles and PMs of insects exposed to these compounds. We therefore examined genes involved in chitin metabolism with the expectation that there will be alterations in their expression at the transcriptional level. Down-regulation of these genes could directly affect the enzyme levels available for chitin synthesis and turnover and up-regulation could reflect transcriptional mechanisms to compensate for the loss in chitin production. Increased AqCHS1 mRNA levels associated with decreased chitin synthesis have been reported, for example, in Anopheles quadrimaculatus exposed to DFB (Zhang and Zhu, 2006). In contrast to our expectation, however, the expression levels of genes encoding trehalases, various enzymes of the Leloir pathway including UDP-GlcNAc pyrophosphorylases, chitin synthases, chitin deacetylases and chitinolytic enzymes were not significantly altered in response to DFB. Our findings are in line with previous observations obtained for embryos of two different Drosophila strains, where the expression of neither mmy that encodes UDP-GlcNAc pyrophosphorylase nor kkv that encodes chitin synthase 1 (CHS1) was changed in response to treatment with either 100 ppm DFB or lufenuron as measured by qRT-PCR (Gangishetti et al., 2009). Similarly, another study performed utilizing several different insect species also reported no significant alterations in the expression levels of CHS genes due to the treatment with benzoylphenyl urea compounds (Ashfaq et al., 2007).
Although the expression of genes involved in chitin metabolism was unaffected, many other genes encoding cuticle proteins (CPs) were significantly up- or down-regulated in response to DFB treatment, a finding that to our knowledge has not been described so far. Therefore, it is likely that in addition to the loss of chitin microfibrils, altered CP composition may contribute to the major structural changes in the procuticle induced by benzoylphenyl urea compounds, as observed in this and many other studies (Binnington, 1985; Gangishetti et al., 2009; Ker, 1977). Altered mechanical properties of the cuticle may be a major problem in successfully shedding the old cuticle and hence one of the contributing causes of abortive molting.
4.5 Changes in the midgut proteome
Another remarkable outcome of the tiling array analysis is that the expression levels of genes encoding PM proteins (PMPs) are widely unchanged, while many CP-encoding genes were altered significantly. Although structurally different from CPs, PMPs are believed to play similar roles, as they bind and cross-link chitin microfibrils via chitin-binding (peritrophin A) domains and finally help to organize the PM (Hegedus et al., 2009; Jasrapuria et al., 2010; Wang et al., 2004). The possibility remains that PMP levels could be changed at a post-transcriptional level. To examine DFB-induced changes in the midgut proteome, we performed 2D gel electrophoresis, densitometric quantification of protein spots and mass spectrometric identification of proteins that significantly changed in their amounts. Similar to the tiling array data, we found that about 7% of the detected midgut proteins changed significantly in their levels. Again, no PMP was among the proteins, whose steady-state levels were altered by more than 2.5-fold. However, the extraction conditions that we have used may have been too mild to extract tightly bound PMPs. Interestingly, UDP-GlcNAc pyrophosphorylase I (TcUAP1) was increased more than 5-fold in DFB treated larvae. There are two genes encoding UDP-GlcNAc pyrophosphorylases in T. castaneum, but only TcUAP1 has been shown to be required for chitin synthesis in the cuticle and PM (Arakane et al., 2011). As the TcUAP1 gene expression level is unaltered following DFB treatment, increased protein levels in the midgut appear rather to be due to increased translation or to a decrease in the turnover of this protein. We currently do not know whether TcUAP1 protein levels are also increased in the cuticle. An increase in TcUAP1 may reflect a compensatory mechanism to elevate cytosolic levels of UDP-GlcNAc for chitin synthesis. However, at least in the cuticle it would also be necessary to accommodate the greater need for this substrate arising from increased protein glycosylation, as many genes encoding CPs are up-regulated at this stage. At least some CPs from different insect species including T. castaneum are likely to be glycoproteins (Lemoine et al., 1990; Missios et al., 2000; Silvert et al., 1984; Stiles, 1991). Another protein that was significantly increased in amount was glutathione synthetase, whose gene was also found to be slightly up-regulated in the tiling array. The increase in glutathione synthetase levels may provide additional reduced form of glutathione in midgut cells, which is utilized for the elimination of radical oxygen species and for glutathionylation of DFB metabolites (Forcella et al., 2007). It is possible that the up-regulation of several other proteins in the midgut may be in response to metabolic stress induced by the insecticide. As a consequence of increased levels of the mitochondrial NADH-ubiquinone oxidoreductase additional oxidative stress and mitochondrial dysfunction may arise, which can trigger apoptosis (Jezek and Hlavata, 2005). Likewise, paramyosin and actin-2 have been frequently observed to be affected in response to metabolic stress resulting in the formation of stress fibers to remodel both tissues and extracellular matrix (Pellegrin and Mellor, 2007). Finally, we found that a 12 kDa hemolymph protein to be up-regulated in the midgut in response to DFB. This protein belongs to a family of proteins known to function as carriers that bind hydrophobic compounds such as odorants and pheromones, and transport them through aqueous solutions (Vogt and Riddiford, 1981). Some of these have direct roles in sensual perception (Pelosi and Maida, 1995), but for many of them a clear function has not been ascribed (Graham et al., 2003). The finding of increased amounts for the 12 kDa hemolymph protein in the midgut of DFB-treated T. castaneum larvae suggests that it may be involved in transport and inactivation of DFB, which is a highly hydrophobic compound as long it is not conjugated with a hydrophilic agent.
5. Conclusions
Our study on DFB-mediated changes revealed expected effects on chitin content in cuticle and midgut, cuticle structure and egg hatching in a single insect model species, which have been previously found in several different insect species. However, we were unable to document significant changes in expression of several genes of chitin metabolism, indicating that net changes in chitin content following DFB-treatment must be due to events that are downstream of transcription of these genes. The negligible changes in developmental rate after DFB-treatment in surviving animals and the availability of genomic resources along with the amenability to systemic RNAi make the red flour beetle a good model organism for investigating the mode of action of DFB and other benzoylphenyl urea-based IGRs as well as other kinds of insecticides.
Supplementary Material
The intensity values of the random probes for each array were scattered against the x-axis and y-axis. All intensity values except the few outliers fall around approximately the same mean with approximately the same standard deviation along the x-axis and y-axis.
By using UNIVARIATE procedure in SAS, the fitted normal curves were described on the histograms of the intensity values (x) for each array and the quantiles for each of the curves were estimated.
As replicate #1 (and replicate #3, not shown) has a higher standard deviation, the 95% cut-off appears to be better for global background subtraction (A). Replicate #2 has a lower standard deviation. Hence, the 99% cut-off was used for background subtraction (B).
In total, 100 larvae (1.5 mg) were fed a control diet or a diet containing 100 ppm DFB and observed for 20 d. The numbers of live insects at the larval, pupal and adult stages were counted together with the number of dead insects, and displayed as percent of the total number of insects in this study.
Larvae (1.5 mg) of the strain pu11 were reared on a control diet (A) or on a diet containing 100 ppm DFB (B). Reporter gene expression in the imaginal discs under the control of the PAX6 promoter was monitored by fluorescence microscopy at the last instar (left) and at the pupal stage (day 3) for those insects that survived the larval-pupal molt.
Averaged fluorescence intensity levels obtained from the tiling array chip hybridization were compared between control larvae and DFB treated larvae. The frequencies of the obtained relative expression values (rE-values) were plotted. The expression of most genes was not affected or only marginally affected by DFB treatment.
T. castaneum genes were divided into fifteen broad categories based on their putative functions. The inner ring displays the percentages of genes in the corresponding category, which were up-regulated, and the outer ring the ones down-regulated. They were compared to the total number of genes that were affected by DFB (middle ring). Genes involved in protein, lipid, nucleotide and sugar metabolism, and replication, transcription and translation were mostly up-regulated. While genes involved in chitin metabolism were unaffected, genes encoding cuticle proteins were up-regulated.
Larvae (1.5 mg) were fed a control diet or a diet containing 100 ppm DFB until a mortality of 30% was achieved. Total RNA was extracted from the surviving larvae and used as a template for RT-PCR using specific pairs of primers for the indicated genes. Primers corresponding to the T. castaneum ribosomal protein S6 (TcRPS6) transcript were used as an internal loading control. The reactions were carried out for 25 and 30 as needed cycles to avoid saturation.
Magnified pictures of individual protein spots are shown, which were identified by MALDI-TOF analysis (see Table S3), and changed more than 2-fold in response to DFB treatment (Table 3).
HIGHLIGHTS.
We examine the insecticidal effects of diflubenzuron (DFB) in Tribolium castaneum.
DFB treatment leads to abortive molting, hatching defects and reduced chitin levels.
We observe a loss of lamellate structure in the larval procuticle.
A genomic tilling array reveals changes in the expression levels for numerous genes.
Genes associated with chitin metabolism are unaffected, but genes encoding cuticle proteins and detoxifying enzymes are.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 431 and GRK 612) and National Science Foundation (IOS-0615818). The authors are grateful to Barb van Slyke for her help in preparing cDNA from T. castaneum. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Abbreviations: Chitin synthase, CHS; Diflubenzuron, DFB; Calcofluor white, CFW; Insect growth regulator, IGR; N-acetylglucosamine, GlcNAc; Peritrophic matrix, PM; Sulfonylurea, SU; Sulfonylurea receptor, SUR.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abo-Elghar GE, Fujiyoshi P, Matsumura F. Significance of the sulfonylurea receptor (SUR) as the target of diflubenzuron in chitin synthesis inhibition in Drosophila melanogaster and Blattella germanica. Insect Biochem Mol Biol. 2004;34:743–752. doi: 10.1016/j.ibmb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Forgash AJ. Nonoxidative enzymes in the metabolism of insecticides. Drug Metab Rev. 1976;5:141–164. [PubMed] [Google Scholar]
- Akasaka T, Klinedinst S, Ocorr K, Bustamante EL, Kim SK, Bodmer R. The ATP-sensitive potassium (KATP) channel-encoded dSUR gene is required for Drosophila heart function and is regulated by tinman. Proc Natl Acad Sci USA. 2006;103:11999–12004. doi: 10.1073/pnas.0603098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakane Y, Baguinon M, Jasrapuria S, Chaudhari S, Doyungan A, Kramer KJ, Muthukrishnan S, Beeman RW. Two uridine-diphosphate N-acetylglucosamine pyrophosphorylases are critical for Tribolium castaneum molting, survival and fecundity. Insect Biochem Mol Biol. 2011;41:42–50. doi: 10.1016/j.ibmb.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Arakane Y, Muthukrishnan S, Kramer KJ, Specht CA, Tomoyasu Y, Lorenzen MD, Kanost M, Beeman RW. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol Biol. 2005;14:453–463. doi: 10.1111/j.1365-2583.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- Arakane Y, Specht CA, Kramer KJ, Muthukrishnan S, Beeman RW. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2008;38:959–962. doi: 10.1016/j.ibmb.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashfaq M, Sonoda S, Tsumuki H. Developmental and tissuespecific expression of CHS1 from Plutella xylostella and its response to chlorfluazuron. Pest Biochem Physiol. 2007;89:20–30. [Google Scholar]
- Becker B. Effects of 20-hydroxy-ecdysone, juvenile hormone, Dimilin, and Captan on in vitro synthesis of peritrophic membranes in Calliphora erythrocephala. J Insect Physiol. 1978;24:529–533. [Google Scholar]
- Binnington KC. Ultrastructural changes in the cuticle of the sheep blowfly, Lucilia, induced by certain insecticides and biological inhibitors. Tissue Cell. 1985;17:131–140. doi: 10.1016/0040-8166(85)90021-7. [DOI] [PubMed] [Google Scholar]
- Broehan G, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S, Merzendorfer H. Chymotrypsin-like peptidases from Tribolium castaneum: a role in molting revealed by RNA interference. Insect Biochem Mol Biol. 2010;40:274–283. doi: 10.1016/j.ibmb.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Denell RE, Beeman RW. Beetling around the genome. Genet Res. 2003;82:155–161. doi: 10.1017/s0016672303006451. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Shippy TD, Miller S, Bolognesi R, Beeman RW, Lorenzen MD, Bucher G, Wimmer EA, Klingler M. The red flour beetle, Tribolium castaneum (Coleoptera): a model for studies of development and pest biology. Cold Spring Harb Protoc. 2009 doi: 10.1101/pdb.emo126. pdb emo126. [DOI] [PubMed] [Google Scholar]
- Carter SW. Laboratory evaluation of three novel insecticides inhibiting cuticle formation against some susceptible and resistant stored products beetles. J Stored Prod Res. 1975;11:187–193. [Google Scholar]
- Chang SC. Conjugation: the major metabolic pathway of C-diflubenzuron in the house fly. J Econ Entomol. 1978;71:31–39. doi: 10.1093/jee/71.1.31. [DOI] [PubMed] [Google Scholar]
- Chang SC, Stokes JB. Conjugation: the major metabolic pathway of 14C-diflubenzuron in the boll weevil. J Econ Entomol. 1979;72:15. doi: 10.1093/jee/71.1.31. [DOI] [PubMed] [Google Scholar]
- Chaudhari SS, Arakane Y, Specht CA, Moussian B, Boyle DL, Park Y, Kramer KJ, Beeman RW, Muthukrishnan S. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc Natl Acad Sci USA. 2011;108:17028–17033. doi: 10.1073/pnas.1112288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BS, Jewess PJ. The inhibition of chitin synthesis in Spodoptera littoralis larvae by flufenoxuron, teflubenzuron and diflubenzuron. Pestic Sci. 1990;28:377–388. [Google Scholar]
- Clarke L, Temple GH, Vincent JF. The effects of a chitin inhibitor-dimilin- on the production of peritrophic membrane in the locust, Locusta migratoria. J Insect Physiol. 1977;23:241–246. doi: 10.1016/0022-1910(77)90037-3. [DOI] [PubMed] [Google Scholar]
- Cohen E. Chitin biochemistry: Synthesis and inhibition. Annu Rev Entomol. 1987;32:71–93. [Google Scholar]
- Cohen E. Chitin synthesis and inhibition: a revisit. Pest Manag Sci. 2001;57:946–950. doi: 10.1002/ps.363. [DOI] [PubMed] [Google Scholar]
- Cohen E, Casida JE. Inhibition of Tribolium gut chitin synthetase. Pestic Biochem Physiol. 1980;13:129–136. [Google Scholar]
- DeLoach JR, Meola SM, Mayer RT, Thompson JM. Inhibition of DNA synthesis by diflubenzuron in pupae of the stable fly Stomoxys calcitrans (L.) Pestic Biochem Physiol. 1981;15:172–180. [Google Scholar]
- Dhadialla TS, Carlson GR, Le DP. New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol. 1998;43:545–569. doi: 10.1146/annurev.ento.43.1.545. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant RH, Daborn PJ, Le Goff G. The genetics and genomics of insecticide resistance. Trends Genet. 2004;20:163–170. doi: 10.1016/j.tig.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Forcella M, Berra E, Giacchini R, Parenti P. Antioxidant defenses preserve membrane transport activity in Chironomus riparius larvae exposed to anoxia. Arch Insect Biochem Physiol. 2007;65:181–194. doi: 10.1002/arch.20197. [DOI] [PubMed] [Google Scholar]
- Gangishetti U, Breitenbach S, Zander M, Saheb SK, Muller U, Schwarz H, Moussian B. Effects of benzoylphenylurea on chitin synthesis and orientation in the cuticle of the Drosophila larva. Eur J Cell Biol. 2009;88:167–180. doi: 10.1016/j.ejcb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Gazit A, Ishaaya I, Perry SA. Detoxification and synergism of diflubenzuron and chlorfluazuron in the red flour beetle Tribolium castaneum. Pest Biochem Physiol. 1989;34:103–110. [Google Scholar]
- Gijwijt MJ, Deul DH, deJong BJ. Inhibition of chitin synthesis by benzoyl-phenylurea insecticides. III Similarity in action in Pieris brassicae (L.) with polyoxin-D. Pestic Biochem Physiol. 1979;12:87–94. [Google Scholar]
- Graham LA, Brewer D, Lajoie G, Davies PL. Characterization of a subfamily of beetle odorant-binding proteins found in hemolymph. Mol Cell Proteom. 2003;2:541–549. doi: 10.1074/mcp.M300018-MCP200. [DOI] [PubMed] [Google Scholar]
- Grosscurt AC. Diflubenzuron: some aspects of its ovicidal and larvicidal mode of action and an evaluation of its practical possibilities. Pestic Sci. 1978a;9:373–386. [Google Scholar]
- Grosscurt AC. Effect of diflubenzuron on mechanical penetrability, chitin formation, and structure of the eyltra of Leptinotarsa decemlineata. J Insect Physiol. 1978b;24:827–831. [Google Scholar]
- Hajjar NP, Casida JE. Insecticidal benzoylphenyl ureas: Structure-activity relationships as chitin synthesis inhibitors. Science. 1978;200:1499–1500. doi: 10.1126/science.200.4349.1499. [DOI] [PubMed] [Google Scholar]
- Haliscak JP, Beeman RW. Status of malathion resistance in five genera of beetles infesting farm-stored corn, wheat and oats in the United States. J Econ Entomol. 1983;76:717–722. [Google Scholar]
- Hassan AEM, Charnley AK. The effect of Dimilin on the ultrastructure of the integument of Manduca sexta. J Insect Physiol. 1987;33:669–676. [Google Scholar]
- Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- Ishaaya I. Insect detoxifying enzymes: their importance in pesticide synergism and resistance. Arch Insect Biochem Physiol. 1993;22:263–276. doi: 10.1002/arch.940220119. [DOI] [PubMed] [Google Scholar]
- Ishaaya I, Ascher K. Effect of diflubenzuron on growth and carbohydrate hydrolases of Tribolium castaneum. Phytoparasitica. 1977;5:149–158. [Google Scholar]
- Ishaaya I, ECJ Dietary TH-6040 alters cuticle composition and enzyme activity of housefly larval cuticle. Pestic Biochem Physiol. 1974;4:484–490. [Google Scholar]
- Jasrapuria S, Arakane Y, Osman G, Kramer KJ, Beeman RW, Muthukrishnan S. Genes encoding proteins with peritrophin A-type chitin-binding domains in Tribolium castaneum are grouped into three distinct families based on phylogeny, expression and function. Insect Biochem Mol Biol. 2010;40:214–227. doi: 10.1016/j.ibmb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Ker RF. Investigation of locust cuticle using the insecticide diflubenzuron. J Insect Physiol. 1977;23:39–48. doi: 10.1016/0022-1910(77)90107-x. [DOI] [PubMed] [Google Scholar]
- Kitahara K, Nakagawa Y, Nishioka T, Fujita T. Cultured integument of Chilo suppressalis as a bioassay system of insect growth regulators. Agric Biol Chem. 1983;47:1583–1589. [Google Scholar]
- Labbe R, Caveney S, Donly C. Genetic analysis of the xenobiotic resistance-associated ABC gene subfamilies of the Lepidoptera. Insect Mol Biol. 2011;20:243–256. doi: 10.1111/j.1365-2583.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- Lemoine A, Millot C, Curie G, Delachambre J. Spatial and temporal variations in cuticle proteins as revealed by monoclonal antibodies. Immunoblotting analysis and ultrastructural immunolocalization in a beetle, Tenebrio molitor. Tissue Cell. 1990;22:177–189. doi: 10.1016/0040-8166(90)90020-a. [DOI] [PubMed] [Google Scholar]
- Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SJ, Lee SS. The toxicity of diflubenzuron on Oxya japonica (Willemse) and its effect on moulting. Pestic Sci. 1982;13:537–544. [Google Scholar]
- Lorenzen MD, Berghammer AJ, Brown SJ, Denell RE, Klingler M, Beeman RW. piggyBac-mediated germline transformation in the beetle Tribolium castaneum. Insect Mol Biol. 2003;12:433–440. doi: 10.1046/j.1365-2583.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- Mayer RT, Chen AC, DeLoach JR. Characterization of a chitin synthase from the stable fly, Stomoxys calcitrans (L.) Insect Biochem. 1980;10:549–556. doi: 10.1016/0003-9861(82)90216-8. [DOI] [PubMed] [Google Scholar]
- Mayer RT, Meola SM, DeLoach JR. Chitin synthesis inhibiting insect growth regulators do not inhibit chitin synthetase. Exs. 1981;37:337–338. [Google Scholar]
- Meissner D, Odman-Naresh J, Vogelpohl I, Merzendorfer H. A novel role of the yeast CaaX protease Ste24 in chitin synthesis. Mol Biol Cell. 2010;21:2425–2433. doi: 10.1091/mbc.E10-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzendorfer H. Insect chitin synthases: a review. J Comp Physiol B. 2006;176:1–15. doi: 10.1007/s00360-005-0005-3. [DOI] [PubMed] [Google Scholar]
- Missios S, Davidson HC, Linder D, Mortimer L, Okobi AO, Doctor JS. Characterization of cuticular proteins in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2000;30:47–56. doi: 10.1016/s0965-1748(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Mitlin N, Wiygul G, Haynes JW. Inhibition of DNA synthesis in boll weevils (Anthonomus grandis boheman) sterilized by dimilin. Pestic Biochem Physiol. 1977;7:559–563. [Google Scholar]
- Mulder R, Gijswijk MT. The laboratory evaluation of two promising new insecticides which interefere with cuticle formation. Petic Sci. 1973;4:737–745. [Google Scholar]
- Muthukrishnan S, Merzendorfer H, Arakane Y, Kramer KJ. Chitin Metabolism in Insects. In: Gilbert LI, editor. Comprehensive Molecular Insect Science. Academic Press; London: 2012. pp. 193–235. [Google Scholar]
- Nakagawa Y, Matsumura F. Diflubenzuron affects gamma-thioGTP stimulated Ca2+ transport in vitro in intracellular vesicles from the integument of the newly molted American cockroach, Periplaneta americana L. Insect Biochem. Mol Biol. 1994;24:1009–1015. doi: 10.1016/0965-1748(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Nasonkin I, Alikasifoglu A, Ambrose C, Cahill P, Cheng M, Sarniak A, Egan M, Thomas PM. A novel sulfonylurea receptor family member expressed in the embryonic Drosophila dorsal vessel and tracheal system. J Biol Chem. 1999;274:29420–29425. doi: 10.1074/jbc.274.41.29420. [DOI] [PubMed] [Google Scholar]
- O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Maida R. Odorant-binding proteins in insects. Comp Biochem Physiol B. 1995;111:503–514. doi: 10.1016/0305-0491(95)00019-5. [DOI] [PubMed] [Google Scholar]
- Porretta D, Gargani M, Bellini R, Medici A, Punelli F, Urbanelli S. Defence mechanisms against insecticides temephos and diflubenzuron in the mosquito Aedes caspius: the P-glycoprotein efflux pumps. Med Vet Entomol. 2008;22:48–54. doi: 10.1111/j.1365-2915.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- Post LC, de Jong BJ, Vincent WR. 1-(2,6-Disubstituted benzoyl)-3-phenylurea insecticides: inhibitors of chitin synthesis. Pestic Biochem Physiol. 1974;4:473–483. [Google Scholar]
- Post LC, Vincent WR. A new insecticide inhibits chitin synthesis. Naturwissenschaften. 1973;60:431–432. doi: 10.1007/BF00623561. [DOI] [PubMed] [Google Scholar]
- Reissig JL, Storminger JL, Leloir LF. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955;217:959–966. [PubMed] [Google Scholar]
- Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, Gibbs R, Bucher G, Friedrich M, Grimmelikhuijzen CJ, Klingler M, Lorenzen M, Roth S, Schroder R, Tautz D, Zdobnov EM, Muzny D, Attaway T, Bell S, Buhay CJ, Chandrabose MN, Chavez D, Clerk-Blankenburg KP, Cree A, Dao M, Davis C, Chacko J, Dinh H, Dugan-Rocha S, Fowler G, Garner TT, Garnes J, Gnirke A, Hawes A, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Jackson L, Kovar C, Kowis A, Lee S, Lewis LR, Margolis J, Morgan M, Nazareth LV, Nguyen N, Okwuonu G, Parker D, Ruiz SJ, Santibanez J, Savard J, Scherer SE, Schneider B, Sodergren E, Vattahil S, Villasana D, White CS, Wright R, Park Y, Lord J, Oppert B, Brown S, Wang L, Weinstock G, Liu Y, Worley K, Elsik CG, Reese JT, Elhaik E, Landan G, Graur D, Arensburger P, Atkinson P, Beidler J, Demuth JP, Drury DW, Du YZ, Fujiwara H, Maselli V, Osanai M, Robertson HM, Tu Z, Wang JJ, Wang S, Song H, Zhang L, Werner D, Stanke M, Morgenstern B, Solovyev V, Kosarev P, Brown G, Chen HC, Ermolaeva O, Hlavina W, Kapustin Y, Kiryutin B, Kitts P, Maglott D, Pruitt K, Sapojnikov V, Souvorov A, Mackey AJ, Waterhouse RM, Wyder S, Kriventseva EV, Kadowaki T, Bork P, Aranda M, Bao R, Beermann A, Berns N, Bolognesi R, Bonneton F, Bopp D, Butts T, Chaumot A, Denell RE, Ferrier DE, Gordon CM, Jindra M, Lan Q, Lattorff HM, Laudet V, von Levetsow C, Liu Z, Lutz R, Lynch JA, da Fonseca RN, Posnien N, Reuter R, Schinko JB, Schmitt C, Schoppmeier M, Shippy TD, Simonnet F, Marques-Souza H, Tomoyasu Y, Trauner J, Van der Zee M, Vervoort M, Wittkopp N, Wimmer EA, Yang X, Jones AK, Sattelle DB, Ebert PR, Nelson D, Scott JG, Muthukrishnan S, Kramer KJ, Arakane Y, Zhu Q, Hogenkamp D, Dixit R, Jiang H, Zou Z, Marshall J, Elpidina E, Vinokurov K, Oppert C, Evans J, Lu Z, Zhao P, Sumathipala N, Altincicek B, Vilcinskas A, Williams M, Hultmark D, Hetru C, Hauser F, Cazzamali G, Williamson M, Li B, Tanaka Y, Predel R, Neupert S, Schachtner J, Verleyen P, Raible F, Walden KK, Angeli S, Foret S, Schuetz S, Maleszka R, Miller SC, Grossmann D. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–955. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- Shippy TD, Ronshaugen M, Cande J, He J, Beeman RW, Levine M, Brown SJ, Denell RE. Analysis of the Tribolium homeotic complex: insights into mechanisms constraining insect Hox clusters. Dev Genes Evol. 2008;218:127–139. doi: 10.1007/s00427-008-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvert DJ, Doctor J, Quesada L, Fristrom JW. Pupal and larval cuticle proteins of Drosophila melanogaster. Biochemistry. 1984;23:5767–5774. doi: 10.1021/bi00319a015. [DOI] [PubMed] [Google Scholar]
- Soltani N. Effects of ingested diflubenzuron on the longevity and the peritrophic membrane of adult mealworms (Tenebrio molitor L.) Pestic Sci. 1984;15:221–225. [Google Scholar]
- Soltani N, Besson MT, Delachambre J. Effects of diflubenzuron on the pupal-adult development of Tenebrio molitor L. (Coleoptera, Tenebrionidae): Growth and development, cuticle secretion, epidermal cell density, and DNA synthesis. Pest Biochem Physiol. 1984;21:256–264. [Google Scholar]
- Squazzo SL, O’Geen H, Komashko VM, Krig SR, Jin VX, Jang SW, Margueron R, Reinberg D, Green R, Farnham PJ. Suz12 binds to silenced regions of the genome in a cell-type-specific manner. Genome Res. 2006;16:890–900. doi: 10.1101/gr.5306606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles B. Cuticle proteins of the boll weevil, Anthonomus grandis, abdomen: Structural similarities and glycosylation. Insect Biochem. 1991;21:249–258. [Google Scholar]
- Tomoyasu Y, Denell RE. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol. 2004;214:575–578. doi: 10.1007/s00427-004-0434-0. [DOI] [PubMed] [Google Scholar]
- van Eck WH. Mode of action of two bezoylphenyl ureas as inhibitors of chitin synthesis in insects. Insect Biochem. 1979;9:295–300. [Google Scholar]
- Verloop A, Ferrell CD. Benzoylphenyl Ureas - A new group of larvicides interfering with chitin deposition. In: Plimmer JR, editor. Pesticide Chemistry in the 20th Century ACS Symp Ser Am Chem Soc. Washington, DC: 1977. pp. 237–270. [Google Scholar]
- Vogt RG, Riddiford LM. Pheromone binding and inactivation by moth antennae. Nature. 1981;293:161–163. doi: 10.1038/293161a0. [DOI] [PubMed] [Google Scholar]
- Wang P, Li G, Granados RR. Identification of two new peritrophic membrane proteins from larval Trichoplusia ni: structural characteristics and their functions in the protease rich insect gut. Insect Biochem Mol Biol. 2004;34:215–227. doi: 10.1016/j.ibmb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Yu SJ. The Toxicology and Biochemistry of Insecticides. CRC Press, Llc; London, England: 2008. [Google Scholar]
- Zhang J, Zhu KY. Characterization of a chitin synthase cDNA and its increased mRNA level associated with decreased chitin synthesis in Anopheles quadrimaculatus exposed to diflubenzuron. Insect Biochem Mol Biol. 2006;36:712–725. doi: 10.1016/j.ibmb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc Natl Acad Sci USA. 2008;105:6650–6655. doi: 10.1073/pnas.0800739105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimoch L, Hogenkamp DG, Kramer KJ, Muthukrishnan S, Merzendorfer H. Regulation of chitin synthesis in the larval midgut of Manduca sexta. Insect Biochem Mol Biol. 2005;35:515–527. doi: 10.1016/j.ibmb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Zimoch L, Merzendorfer H. Immunolocalization of chitin synthase in the tobacco hornworm. Cell Tissue Res. 2002;308:287–297. doi: 10.1007/s00441-002-0546-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The intensity values of the random probes for each array were scattered against the x-axis and y-axis. All intensity values except the few outliers fall around approximately the same mean with approximately the same standard deviation along the x-axis and y-axis.
By using UNIVARIATE procedure in SAS, the fitted normal curves were described on the histograms of the intensity values (x) for each array and the quantiles for each of the curves were estimated.
As replicate #1 (and replicate #3, not shown) has a higher standard deviation, the 95% cut-off appears to be better for global background subtraction (A). Replicate #2 has a lower standard deviation. Hence, the 99% cut-off was used for background subtraction (B).
In total, 100 larvae (1.5 mg) were fed a control diet or a diet containing 100 ppm DFB and observed for 20 d. The numbers of live insects at the larval, pupal and adult stages were counted together with the number of dead insects, and displayed as percent of the total number of insects in this study.
Larvae (1.5 mg) of the strain pu11 were reared on a control diet (A) or on a diet containing 100 ppm DFB (B). Reporter gene expression in the imaginal discs under the control of the PAX6 promoter was monitored by fluorescence microscopy at the last instar (left) and at the pupal stage (day 3) for those insects that survived the larval-pupal molt.
Averaged fluorescence intensity levels obtained from the tiling array chip hybridization were compared between control larvae and DFB treated larvae. The frequencies of the obtained relative expression values (rE-values) were plotted. The expression of most genes was not affected or only marginally affected by DFB treatment.
T. castaneum genes were divided into fifteen broad categories based on their putative functions. The inner ring displays the percentages of genes in the corresponding category, which were up-regulated, and the outer ring the ones down-regulated. They were compared to the total number of genes that were affected by DFB (middle ring). Genes involved in protein, lipid, nucleotide and sugar metabolism, and replication, transcription and translation were mostly up-regulated. While genes involved in chitin metabolism were unaffected, genes encoding cuticle proteins were up-regulated.
Larvae (1.5 mg) were fed a control diet or a diet containing 100 ppm DFB until a mortality of 30% was achieved. Total RNA was extracted from the surviving larvae and used as a template for RT-PCR using specific pairs of primers for the indicated genes. Primers corresponding to the T. castaneum ribosomal protein S6 (TcRPS6) transcript were used as an internal loading control. The reactions were carried out for 25 and 30 as needed cycles to avoid saturation.
Magnified pictures of individual protein spots are shown, which were identified by MALDI-TOF analysis (see Table S3), and changed more than 2-fold in response to DFB treatment (Table 3).


