Abstract
Encephalitis is a severe inflammatory disorder of the brain with many possible causes and a complex differential diagnosis. Advances in autoimmune encephalitis research in the past 10 years have led to the identification of new syndromes and biomarkers that have transformed the diagnostic approach to these disorders. However, existing criteria for autoimmune encephalitis are too reliant on antibody testing and response to immunotherapy, which might delay the diagnosis. We reviewed the literature and gathered the experience of a team of experts with the aims of developing a practical, syndrome-based diagnostic approach to autoimmune encephalitis and providing guidelines to navigate through the differential diagnosis. Because autoantibody test results and response to therapy are not available at disease onset, we based the initial diagnostic approach on neurological assessment and conventional tests that are accessible to most clinicians. Through logical differential diagnosis, levels of evidence for autoimmune encephalitis (possible, probable, or definite) are achieved, which can lead to prompt immunotherapy.
Introduction
Acute encephalitis is a debilitating neurological disorder that develops as a rapidly progressive encephalopathy (usually in less than 6 weeks) caused by brain inflammation.1 The estimated incidence of encephalitis in high-income countries is about 5–10 per 100 000 inhabitants per year; encephalitis affects patients of all ages and represents a significant burden to patients, families, and society.2,3
Because the most frequently recognised causes of encephalitis are infectious, existing diagnostic criteria and consensus guidelines for encephalitis assume an infectious origin.1,4–6 However, in the past 10 years an increasing number of non-infectious, mostly autoimmune, encephalitis cases have been identified and some of them do not meet existing criteria.7 These newly identified forms of autoimmune encephalitis might be associated with antibodies against neuronal cell-surface or synaptic proteins (table)8–23 and can develop with core symptoms resembling infectious encephalitis, and also with neurological and psychiatric manifestations without fever or CSF pleocytosis.7 To improve the recognition of these disorders, in this Position Paper, we aim to provide a practical clinical approach to diagnosis that should be accessible to most physicians.
Table 1.
Antibodies in the diagnosis of autoimmune encephalitis
| Syndrome | Diagnostic assay | Frequency of cancer | Main type of cancer | |
|---|---|---|---|---|
| Antibodies against intracellular antigens | ||||
|
| ||||
| Hu (ANNA1)8* | Limbic encephalitis | Western blot | >95% | Small-cell lung carcinoma |
| Ma29 | Limbic encephalitis† | Western blot | >95% | Testicular seminoma |
| GAD10 | Limbic encephalitis‡ | Radioimmunoassay | 25%§ | Thymoma, small-cell lung carcinoma |
|
| ||||
| Antibodies against synaptic receptors | ||||
|
| ||||
| NMDA receptor11 | Anti-NMDA receptor encephalitis | Cell-based assay | Varies with age and sex | Ovarian teratoma¶ |
| AMPA receptor12 | Limbic encephalitis | Cell-based assay | 65% | Thymoma, small-cell lung carcinoma |
| GABAB receptor13 | Limbic encephalitis | Cell-based assay | 50% | Small-cell lung carcinoma |
| GABAA receptor14 | Encephalitis | Cell-based assay | <5% | Thymoma |
| mGluR515 | Encephalitis | Cell-based assay | 70% | Hodgkin’s lymphoma |
| Dopamine 2 receptor16 | Basal ganglia encephalitis | Cell-based assay | 0% | .. |
|
| ||||
| Antibodies against ion channels and other cell-surface proteins | ||||
|
| ||||
| LGI117 | Limbic encephalitis | Cell-based assay | 5–10% | Thymoma |
| CASPR218 | Morvan’s syndrome|| or limbic encephalitis | Cell-based assay | 20–50% | Thymoma** |
| DPPX19 | Encephalitis†† | Cell-based assay | <10% | Lymphoma |
| MOG20‡‡ | Acute disseminated encephalomyelitis | Cell-based assay | 0% | .. |
| Aquaporin 421‡‡ | Encephalitis | Cell-based assay | 0% | .. |
| GQ1b22 | Bickerstaff’s brainstem encephalitis | ELISA | 0% | .. |
GAD=glutamic acid decarboxylase. LGI1=leucine-rich glioma inactivated 1. CASPR2=contactin associated protein 2. DPPX=dipeptidyl-peptidase-like protein-6. MOG=myelin oligodendrocyte glycoprotein.
Amphiphysin or CV2 (CRMP5) antibodies instead of Hu antibodies in a few patients with limbic encephalitis and small-cell lung carcinoma.
Limbic encephalitis frequently associated with hypothalamic and mesencephalic involvement.
GAD antibodies occur more frequently in patients with stiff person syndrome and cerebellar ataxia. The association with cancer preferentially occurs in patients with limbic encephalitis.
Tumours found more frequently in men older than 50 years.23
Ovarian teratoma usually found in young women aged 12–45 years.
Morvan’s syndrome usually has a more chronic clinical course, but might present with predominant cognitive and behavioural symptoms fulfilling criteria of possible autoimmune encephalitis.
Thymoma associated with Morvan’s syndrome rather than limbic encephalitis.
Encephalitis associated with diarrhoea and hyperekplexia.
Mostly restricted to children.
General scope and objectives
These guidelines focus on autoimmune encephalitis that presents with subacute onset of memory deficits or altered mental status, accompanied or not by other symptoms and manifestations, with the goal of helping to establish a prompt diagnosis. These guidelines do not address the clinical approach to other CNS autoimmune disorders (stiff person syndrome,24 progressive encephalomyelitis with rigidity and myoclonus,25 or autoimmune cerebellopathies26) that usually present with a clinical profile clearly different from autoimmune encephalitis.
Existing diagnostic criteria for autoimmune encephalitis are too reliant on antibody testing and response to immunotherapy.27 In our opinion, it is not realistic to include antibody status as part of the early diagnostic criteria in view of the fact that antibody testing is not readily accessible in many institutions and results can take several weeks to obtain. Furthermore, the absence of autoantibodies does not exclude the possibility that a disorder is immune mediated, and a positive test does not always imply an accurate diagnosis. Use of the response to immunotherapy as part of the diagnostic criteria is also not practical because this information is not available at the time of symptom onset or early clinical evaluation. Some patients with autoimmune encephalitis might not respond to immunotherapy or could need intensive and prolonged therapies that are not available in most health-care systems unless a firm diagnosis has been pre-established.28 Conversely, patients with other disorders might respond to immunotherapy (eg, primary CNS lymphoma).
The clinical facts and evidence suggesting that early immunotherapy improves outcome29–31 have been considered in the development of the guidelines presented here, in which conventional neurological evaluation and standard diagnostic tests (eg, MRI, CSF, or EEG studies) prevail in the initial assessment. This approach should allow the initiation of preliminary treatment while other studies and comprehensive antibody tests are processed and subsequently used to refine the diagnosis and treatment.
The above-mentioned focus of these guidelines and the initial approach based on conventional clinical assessment explain why some disorders are included in the main text and others are included in the appendix or excluded. As an example, we have included acute disseminated encephalomyelitis because the clinical presentation can be similar to that of other autoimmune encephalitis disorders.32 Another example is Hashimoto’s encephalopathy, the existence of which is under discussion, but in practice is frequently listed in the differential diagnosis of autoimmune encephalitis;33 thus, we believed it should be discussed, while emphasising the controversies and diagnostic limitations. By contrast, Morvan’s syndrome34 and Rasmussen’s encephalitis,35 which have a solid autoimmune basis, are not included in the main text because they usually follow a more chronic course and the initial or predominant symptoms (peripheral nerve hyperexcitability, or focal seizures and unilateral deficits) are different from those mentioned above. We recognise the overlap that can occur between these disorders and autoimmune encephalitis and for this reason they are discussed in the appendix.
Because children do not develop many of the autoimmune encephalitis disorders that affect adults, and the syndrome presentation might be different or less clinically recognisable, these guidelines should be applied with caution in children, particularly in children younger than 5 years.36,37
Methods
An initial draft of these guidelines was developed by two authors (FG and JD) and subsequently underwent three rounds of reviews and updates by a panel of investigators who have expertise in autoimmune encephalitis. In the first stage, we reviewed previously published guidelines and diagnostic criteria for encephalitis (of any cause or idiopathic). This review along with clinical experience with forms of autoimmune encephalitis described in the past 10 years (eg, some of them not necessarily causing alteration in consciousness, but changes in memory or personality) led us to a definition of so-called possible autoimmune encephalitis, which is not dependent on neuronal autoantibody status. We next reviewed the existing criteria for specific clinical syndromes (eg, limbic encephalitis or Bickerstaff’s brainstem encephalitis), identified other disorders for which criteria were unclear, and modified or developed new diagnostic criteria (eg, probable anti-NMDA receptor encephalitis), which focused on symptom assessment and standard paraclinical tests, and were not dependent on autoantibody status. This work resulted in the establishment of three levels of clinical evidence for autoimmune encephalitis: possible and probable for which the autoantibody status is not needed in most cases, and definite for which the autoantibody status is often needed. In parallel, we reviewed the literature and our experience in neuronal autoantibody studies and identified caveats for interpretation, which led to recommendations for the use and interpretation of findings of autoantibodies in autoimmune encephalitis.
Initial clinical assessment: possible autoimmune encephalitis
We regard a patient with new-onset encephalitis as having possible autoimmune encephalitis if the criteria shown in panel 1 are met. These criteria differ from those previously proposed for encephalitis (any cause or idiopathic) in which changes in the level of consciousness, fever, CSF pleocytosis, and EEG alterations are more often needed.1,4–6 These criteria needed to be adapted for autoimmune encephalitis because patients with autoimmune encephalitis could present with memory or behavioural deficits without fever or alteration in the level of consciousness, or with normal brain MRI or CSF results.7 In this context, memory deficits refer to the inability to form new, long-term memories owing to hippocampal dysfunction, or problems with working memory, which refers to structures and processes used for temporary storage and manipulation of information.
Panel 1. Diagnostic criteria for possible autoimmune encephalitis.
Diagnosis can be made when all three of the following criteria have been met:
Subacute onset (rapid progression of less than 3 months) of working memory deficits (short-term memory loss), altered mental status*, or psychiatric symptoms
-
At least one of the following:
New focal CNS findings
Seizures not explained by a previously known seizure disorder
CSF pleocytosis (white blood cell count of more than five cells per mm3)
MRI features suggestive of encephalitis†
Reasonable exclusion of alternative causes (appendix)
Most patients with encephalitis undergo brain MRI at early stages of the disease. The findings could be normal or non-specific, but sometimes they might suggest an autoimmune cause (see below). By contrast, alterations in EEG are rarely specific. We acknowledge the use of some EEG patterns in the diagnosis of specific forms of encephalitis (eg, extreme delta brush in anti-NMDA receptor encephalitis),38 in the differential diagnosis of other disorders (Creutzfeldt-Jakob disease), or to reveal subclinical seizures and non-convulsive status epilepticus.
In addition to the above criteria, patients should be carefully examined for other diseases that can mimic autoimmune encephalitis and cause rapidly progressive encephalopathy (appendix). These diseases should be excluded before immunotherapy begins and in most instances a detailed clinical history, complete general and neurological examination, routine blood and CSF analysis, and brain MRI including diffusion sequences will suffice to accomplish this goal. The most frequent differential diagnoses are herpes simplex virus encephalitis and other CNS infections. Importantly, CSF herpes simplex virus PCR can be negative if done too early (eg, within 24 h), and this test should be repeated if the clinical suspicion remains high.39 Previous reviews have addressed the differential diagnosis of infectious encephalitis.1,40
Approach to patients with clinically recognisable syndromes
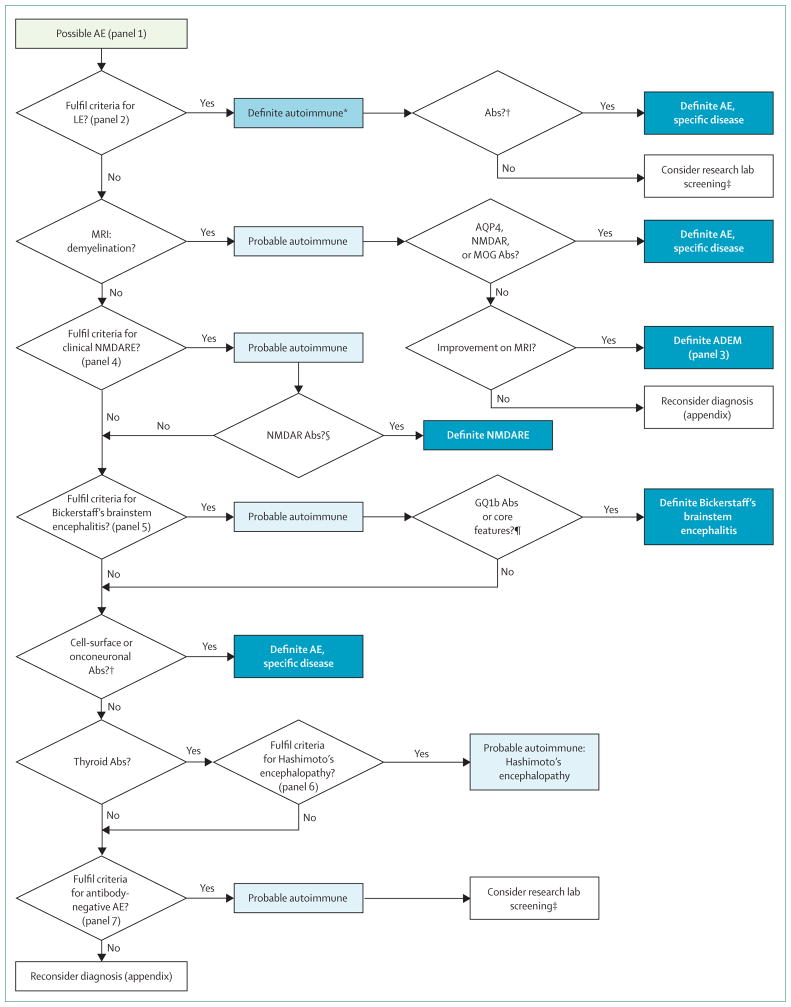
A substantial number of patients with autoimmune encephalitis do not present with a well defined syndrome. In some of these patients, demographic information and some comorbidities (eg, diarrhoea, ovarian teratoma, faciobrachial dystonic seizures) might initially suggest the underlying disorder (anti-dipeptidyl-peptidase-like protein-6 [DPPX], anti-NMDA receptor, anti-leucine-rich, glioma-inactivated 1 [LGI1] encephalitis), but these features are not pathognomonic and might be absent in some patients.11,41,42 In such cases, the diagnosis of definite autoimmune encephalitis greatly depends on the results of autoantibody tests. By contrast, disorders exist in which the clinical syndrome and MRI findings allow for classification as probable or definite autoimmune encephalitis before the autoantibody status is known. These include limbic encephalitis, acute disseminated encephalomyelitis and other syndromes with MRI features that predominantly involve white matter, anti-NMDA receptor encephalitis, and Bickerstaff’s brainstem encephalitis (Figure 1).43
Figure 1. Algorithm for the diagnosis of autoimmune encephalitis.
AE=autoimmune encephalitis. LE=limbic encephalitis. Abs=antibodies. AQP4=aquaporin 4. MOG=myelin oligodendrocyte glycoprotein. NMDARE=NMDA receptor encephalitis. ADEM=acute disseminated encephalomyelitis. *Although results of autoantibodies are not necessary for a definitive diagnosis of some types of autoimmune encephalitis, their determination is important to further characterise subtypes of limbic encephalitis that have different prognosis, type of treatment, and comorbidities. †See table. ‡Research laboratories can screen for new antibodies (eg, using live neurons). §IgG anti-GluN1 antibodies in the CSF; if only serum is used, confirmatory tests should be included (panel 4). ¶Definitive diagnosis of Bickerstaff’s brainstem encephalitis can be made in the presence of core clinical features (hypersomnolence, ophthalmoplegia, and ataxia)43 or positive GQ1b antibodies if core symptoms are incomplete.
Autoimmune limbic encephalitis
Diagnostic criteria for autoimmune limbic encephalitis are shown in panel 2.44,45 We have modified our previous criteria to include evidence of bilateral involvement of the medial temporal lobes on T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI studies (Figure 2; see below).46,47 In our proposed criteria, antibody status is not needed to consider limbic encephalitis as having a definite autoimmune origin because immune-mediated limbic encephalitis can occur without detectable autoantibodies (figure 2, appendix).48,49 Measurement of autoantibodies, however, remains important for two reasons: their presence clarifies the immunological subgroup of limbic encephalitis, with comorbidities, tumour association, and prognosis that might differ according to the autoantibody;8,10,50–53 and, in patients who do not satisfy the indicated criteria, detection of autoantibodies establishes the diagnosis of autoimmune limbic encephalitis (panel 2).
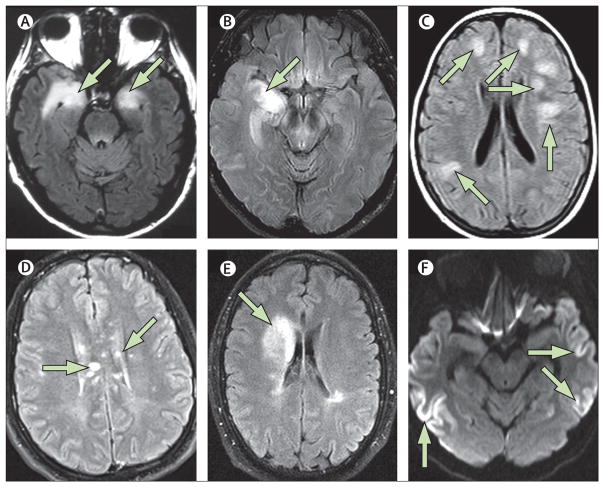
Figure 2. MRI patterns in autoimmune encephalitis and its mimics.
Typical MRI of limbic encephalitis (A) with bilateral abnormalities in the medial temporal lobe on T2-weighted fluid-attenuated inversion recovery imaging; this patient with autopsy-proven limbic encephalitis did not have serum or CSF antineuronal antibodies. Patient with final diagnosis of glioma (B) who presented with unilateral right hippocampal involvement mimicking limbic encephalitis. Typical MRI of acute disseminated encephalomyelitis (C) with bilateral large lesions in the white matter. Multiple lesions involving the corpus callosum in a patient with Susac’s syndrome (D). MRI of a patient with overlapping syndrome (NMDA receptor and myelin oligodendrocyte glycoprotein antibodies; E) showing a right frontal abnormality compatible with demyelination. Diffusion MRI sequence in a patient with AMPA receptor antibody-associated encephalitis (F) mimicking MRI changes seen in patients with Creutzfeldt-Jakob disease. Left side of images=right side of brain.
Panel 2. Diagnostic criteria for definite autoimmune limbic encephalitis.
Diagnosis can be made when all four* of the following criteria have been met:
Subacute onset (rapid progression of less than 3 months) of working memory deficits, seizures, or psychiatric symptoms suggesting involvement of the limbic system
Bilateral brain abnormalities on T2-weighted fluid-attenuated inversion recovery MRI highly restricted to the medial temporal lobes†
-
At least one of the following:
CSF pleocytosis (white blood cell count of more than five cells per mm3)
EEG with epileptic or slow-wave activity involving the temporal lobes
Reasonable exclusion of alternative causes (appendix)
The clinical picture of limbic encephalitis is characterised by rapid development of confusion, working memory deficit, mood changes, and often seizures. The subacute development of short-term memory loss is considered the hallmark of the disorder, but it can be overlooked because of the presence of other symptoms.46 CSF analysis shows mild-to-moderate lymphocytic pleocytosis (usually less than 100 white blood cells per mm3) in 60–80% of patients, and elevated IgG index or oligoclonal bands in approximately 50% of cases.46,51,52 Among all immunological subtypes of limbic encephalitis, patients with LGI1 antibodies present with a lower frequency of CSF pleocytosis (41%) or elevated CSF protein concentrations (47%) and rarely have intrathecal IgG synthesis.54 The absence of inflammatory changes in the CSF of these patients might initially suggest a non-inflammatory encephalopathy.
MRI often shows increased signal on T2-weighted FLAIR imaging in the medial aspect of the temporal lobes. Although limbic encephalitis can occur with MRI evidence of unilateral involvement (or be normal) we do not consider these cases as definite limbic encephalitis unless specific antibodies are subsequently detected. The reason for this is that several non-immune disorders could result in similar unilateral MRI abnormalities, including among others, seizures, herpes simplex virus encephalitis, or gliomas (appendix, figure 2).40,55–57 MRI findings of immune-compromised patients with human herpes virus 6-associated encephalitis can mimic precisely findings from patients with autoimmune limbic encephalitis, but the clinical setting is different and directs the diagnosis.58 By contrast, the findings in herpes simplex virus encephalitis are less confined to the limbic system, can occur with haemorrhagic features, and often show restricted diffusion abnormalities and contrast uptake.59
Some demographic and clinical clues could suggest the underlying immune response of limbic encephalitis (appendix), but the immunological subtypes can be established only by measurement of autoantibodies.7 Distinction among immunological subtypes is important because those associated with onconeuronal antibodies are much less responsive to immunotherapy than those associated with cell-surface antibodies. The onconeuronal antibodies that more frequently occur with limbic encephalitis are Hu and Ma2, and patients who have these antibodies almost always have an underlying cancer.8,9 By contrast, the neuronal cell-surface antibodies that are more frequently associated with limbic encephalitis are LGI1,18 GABAB receptor,51,60 and AMPA receptor52 antibodies (see appendix for less frequent antibodies). The frequency and type of tumours vary according to the antibody (table).7
Antibodies against the intracellular antigen glutamic acid decarboxylase (GAD) occur in a subgroup of patients with limbic encephalitis. These patients are mainly young women (median age 23 years) with predominant seizures and no evidence of cancer.10 The risk of cancer, usually small-cell lung carcinoma or thymoma, is higher, however, among patients with GAD antibodies and limbic encephalitis who are older than 50 years or have concomitant GABAB receptor antibodies.23
Acute disseminated encephalomyelitis and other syndromes with MRI features of demyelination
Acute disseminated encephalomyelitis is a monophasic, inflammatory disease of the CNS that mainly occurs in children and adults younger than 40 years.61 The disorder can be preceded by an acute systemic infection or vaccination.62,63 It is characterised by a variable extent of encephalopathy (a mandatory criterion for a definitive diagnosis; panel 3), and other neurological signs, such as cranial nerve palsies, ataxia, hemiparesis, myelopathy, or optic neuritis. CSF analysis typically shows mild pleocytosis (less than 50 lymphocytes per mm3), but CSF oligoclonal bands are uncommon (less than 7% of all cases).64 Brain MRI shows multiple, large (>2 cm) abnormalities on T2-weighted FLAIR imaging that can be present in the supratentorial white matter, basal ganglia, brainstem, cerebellum, and spinal cord, with or without contrast enhancement (figure 2).65 There are no specific biomarkers of acute disseminated encephalomyelitis, and a set of criteria has been proposed for children (panel 3).32 According to these criteria one of the requirements for definite acute disseminated encephalomyelitis is the absence of new clinical and MRI findings 3 months after symptom onset. Except for this criterion (which cannot be predicted at onset), we believe the rest of the criteria are robust enough to establish that patients who meet them have probable acute disseminated encephalomyelitis and can be started on immunotherapy.
Panel 3. Diagnostic criteria for definite acute disseminated encephalomyelitis32.
Diagnosis can be made when all five of the following criteria have been met:
A first multifocal, clinical CNS event of presumed inflammatory demyelinating cause
Encephalopathy that cannot be explained by fever
-
Abnormal brain MRI:
Diffuse, poorly demarcated, large (>1–2 cm) lesions predominantly involving the cerebral white matter
T1-hypointense lesions in the white matter in rare cases
Deep grey matter abnormalities (eg, thalamus or basal ganglia) can be present
No new clinical or MRI findings after 3 months of symptom onset
Reasonable exclusion of alternative causes
Evidence exists that myelin oligodendrocyte glycoprotein (MOG) antibodies can transiently occur in almost 50% of children with acute disseminated encephalomyelitis.20,66,67 At present, the inclusion of MOG antibodies in the diagnostic criteria for acute disseminated encephalomyelitis is not considered for two reasons: the antibodies can be present in demyelinating disorders with encephalopathy, but without MRI features of acute disseminated encephalomyelitis, or in patients with demyelinating disorders without encephalopathy;68 and antibody testing remains unavailable at many centres.
Susac’s syndrome is a rare, but important, differential diagnosis in patients who meet criteria for possible autoimmune encephalitis and have MRI features of demyelination. The syndrome is considered an autoimmune vasculopathy resulting in microvessel thromboses at three levels: the brain, retina, and inner ear.69 In a review of 304 cases of Susac’s syndrome, 230 (76%) patients presented with encephalopathy, but simultaneous involvement of the three levels at disease onset occurred in only 31 of 247 (13%) patients.70 The diagnosis is based on presence of branch retinal artery occlusions on fluorescein angiography, and MRI findings including snowball-like lesions or holes in the central portion of the corpus callosum and other periventricular white matter abnormalities on T2-weighted FLAIR imaging (figure 2). These MRI findings are different from those seen in acute disseminated encephalomyelitis and in the setting of encephalopathy are highly suggestive of Susac’s syndrome.70
Anti-NMDA receptor encephalitis
Anti-NMDA receptor encephalitis is frequently recognisable on clinical grounds and is associated with CSF IgG antibodies against the GluN1 subunit of the NMDA receptor.11 These antibodies are highly specific and their pathogenicity has been demonstrated in cultured neurons and in-vivo models.71,72 In a multicentre, observational study of 577 patients, the disease was shown to predominantly affect young individuals (549 [95%] younger than 45 years, and 211 [37%] younger than 18 years) with a female sex predominance of 4:1. This female predominance was less evident in children younger than 12 years and adults older than 45 years.28 The frequency of an underlying tumour varied with age and sex, ranging from 0–5% in children (male and female) younger than 12 years, to 58% in women older than 18 years (usually an ovarian teratoma).28 Adults older than 45 years have a lower frequency of tumours (23%), and these are usually carcinomas instead of teratomas.11
Teenagers and adults usually present with abnormal behaviour (psychosis, delusions, hallucinations, agitation, aggression, or catatonia) with irritability and insomnia, followed by speech dysfunction, dyskinesias, memory deficits, autonomic instability, and a decrease in the level of consciousness.11,73 Seizures can take place at any time during the disease, but tend to occur earlier in males.74 In the above-mentioned observational cohort study,28 compared with teenagers and adults, young children more frequently presented with abnormal movements or seizures. Regardless of the patient’s age and presentation, the clinical picture at 3–4 weeks after symptom onset was similar in most cases. By the end of the first month, 498 (87%) of 571 patients had four or more of the following categories of symptoms, including (from highest-to-lowest frequency) abnormal behaviour and cognition; memory deficit; speech disorder; seizures; abnormal movements (orofacial, limb, or trunk dyskinesias); loss of consciousness or autonomic dysfunction; central hypoventilation; and cerebellar ataxia or hemiparesis.28 Only six patients (1%) had one category of symptoms.
On the basis of these data, and while waiting for confirmatory IgG anti-GluN1 antibody results, we regard a patient with rapidly progressive encephalopathy as having probable anti-NMDA receptor encephalitis if they satisfy the criteria shown in panel 4. Memory deficit is common, but we have excluded it from the criteria because it is difficult to assess in patients with psychosis or agitation, or in young children. Hemiparesis and cerebellar ataxia are not included because these symptoms are less frequent and if they occur they predominantly affect children in combination with the other symptoms. In patients who meet these criteria, immunotherapy and the search for a neoplasm (according to sex and age) should be started. In a retrospective analysis of data from the observational cohort study,28 425 (80%) of 532 patients with anti-NMDA receptor encephalitis met these criteria within the first month of symptom onset, including 254 (74%) of 342 without teratoma and 171 (90%) of 189 with teratoma.
Panel 4. Diagnostic criteria for anti-NMDA receptor encephalitis.
Probable anti-NMDA receptor encephalitis*
Diagnosis can be made when all three of the following criteria have been met:
-
Rapid onset (less than 3 months) of at least four of the six following major groups of symptoms:
Abnormal (psychiatric) behaviour or cognitive dysfunction
Speech dysfunction (pressured speech, verbal reduction, mutism)
Seizures
Movement disorder, dyskinesias, or rigidity/abnormal postures
Decreased level of consciousness
Autonomic dysfunction or central hypoventilation
-
At least one of the following laboratory study results:
Abnormal EEG (focal or diffuse slow or disorganised activity, epileptic activity, or extreme delta brush)
CSF with pleocytosis or oligoclonal bands
Reasonable exclusion of other disorders (appendix)
Diagnosis can also be made in the presence of three of the above groups of symptoms accompanied by a systemic teratoma
Definite anti-NMDA receptor encephalitis*
Diagnosis can be made in the presence of one or more of the six major groups of symptoms and IgG anti-GluN1 antibodies,† after reasonable exclusion of other disorders (appendix)
Patients with partial symptoms who might be missed with these initial criteria will be identified with an antibody test (figure 1). Antibody studies should include CSF analysis; a risk of false-negative or false-positive diagnoses exists if only serum is used.75 Findings from three other studies have suggested that serum testing is less consistent, or showed antibodies in patients without anti-NMDA receptor encephalitis or immune-mediated disorders.74,76,77
Analysis of CSF for the presence of NMDA receptor antibodies is mandatory in patients with relapsing symptoms after herpes simplex encephalitis.78,79 This relapsing form of herpes simplex encephalitis is an autoimmune disorder that at times is indistinguishable from the full-blown syndrome of anti-NMDA receptor encephalitis, affects 20% of patients with herpes simplex encephalitis, and manifests with new-onset choreoathetosis (predominantly in children)79,80 or psychiatric symptoms (mainly in adults and teenagers) a few weeks or, rarely, months after the viral infection.81 In addition to NMDA receptor antibodies, a few patients develop GABAA receptor or dopamine receptor 2 antibodies.81,82
Bickerstaff’s brainstem encephalitis
Bickerstaff’s brainstem encephalitis is characterised by subacute onset, in less than 4 weeks, of progressive impairment of consciousness along with ataxia and bilateral, mostly symmetrical, ophthalmoparesis.83 The syndrome is usually preceded by an infectious event, runs a monophasic course, and has a good outcome. Additionally, patients frequently develop pupillary abnormalities, bilateral facial palsy, Babinski’s sign, and bulbar palsy. Generalised limb weakness can occur, which overlaps with features of Guillain-Barré syndrome.84 CSF pleocytosis occurs in 45% of patients. Brain MRI is usually normal, but brainstem abnormalities on T2- weighted FLAIR imaging are present in 23% of patients.83
Most of the proposed criteria for Bickerstaff’s brainstem encephalitis include the triad of abnormal mental status, bilateral external ophthalmoplegia, and ataxia (panel 5).83 IgG anti-GQ1b antibodies are highly specific for this disorder and the related Miller-Fisher syndrome, leading some clinicians to group these disorders under the term GQ1b antibody syndrome.22 We agree with the criteria proposed in 2014, which do not specify the need for GQ1b antibody testing for a definitive diagnosis of Bickerstaff’s brainstem encephalitis because up to 32% of patients do not have detectable antibodies.43 Measurement of these antibodies, however, allows confirmation of the diagnosis in patients with incomplete syndromes or atypical symptoms, or when the altered mental status prevents the assessment of ataxia. The occasional complexity in the differential diagnosis is exemplified by the third case in the original report by Bickerstaff and Cloake,85 in which a 24-year-old woman, who was admitted for ovarian cystectomy, in addition to brainstem symptoms, developed seizures, hyperthermia, psychosis, and episodes of maniacal excitement alternating with catatonia that lasted 2 months. Measurement of GQ1b and NMDA receptor antibodies (not available at that time) would probably have clarified the diagnosis.
Panel 5. Diagnostic criteria for Bickerstaff’s brainstem encephalitis.
Probable Bickerstaff’s brainstem encephalitis
Diagnosis can be made when both of the following criteria have been met:
-
Subacute onset (rapid progression of less than 4 weeks) of all the following symptoms:
Decreased level of consciousness
Bilateral external ophthalmoplegia
Ataxia
Reasonable exclusion of alternative causes
Definite Bickerstaff’s brainstem encephalitis
Diagnosis can be made in the presence of positive IgG anti-GQ1b antibodies even if bilateral external ophthalmoplegia is not complete or ataxia cannot be assessed, or if recovery has occurred within 12 weeks after onset
Disorders to consider in the differential diagnosis of Bickerstaff’s brainstem encephalitis include Listeria rhombencephalitis, EV71 encephalitis in children, paraneoplastic and postinfectious brainstem encephalitis, chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS), neurosarcoidosis, and primary CNS lymphoma.86–88
Antibody testing: clinical considerations and caveats
The detection of specific autoantibodies (table, figure 1) establishes a definitive diagnosis of autoimmune encephalitis, identifies immunological subtypes of limbic encephalitis, and assists in the differential diagnosis of atypical clinical cases. Therefore, measurement of antibodies is a crucial step in the definite diagnosis of many types of autoimmune encephalitis and clinicians must be aware of potential pitfalls in the interpretation of results.
Several concepts that apply to classic onconeuronal or GAD antibodies (discussed later) are not applicable to antibodies against neuronal cell-surface proteins. Onconeuronal and GAD antibodies target intracellular proteins and because they are present in the serum and CSF, and their epitopes are linear, they are detectable with many techniques including ELISA, immunoblotting, and immunohistochemistry. By contrast, antibodies against neuronal cell-surface proteins have different properties that should be considered for a better understanding of the most appropriate tests to use and interpretation of their results. Here, we discuss these issues and some more general caveats applicable to the detection of autoantibodies.
Conformational antigens
Most antibodies against neuronal cell-surface proteins recognise target epitopes only if they are expressed in their native conformation. Techniques that meet this requirement are cell-based assays (used by most clinical laboratories), immunohistochemistry of brain sections adapted to membrane proteins (commercially available; sometimes used as a confirmatory test), and immunocytochemistry of cultures of dissociated rodent live hippocampal neurons (only used in research laboratories).12
Molecular precision
The target antigens of autoantibodies can be composed of several subunits. Antibodies against each of the subunits can have different clinical significance and implications. For example, the NMDA receptor is a heterotetramer comprised of two GluN1 subunits and two GluN2/3 subunits. Detection of IgG antibodies against the GluN1 subunit is a signature of anti-NMDA receptor encephalitis.89 By contrast, antibodies against linear epitopes of GluN2 or GluR ε2 have been reported in many different disorders and their clinical significance is uncertain.90
Molecular precision is important for the voltage-gated potassium channel complex (VGKC) antibodies. This name was adopted by some investigators after they showed that the target antigen was not the VGKC itself, but the proteins LGI1 and contactin-associated protein-like 2 (CASPR2), complexed with the VGKC.17,18 Antibodies against LGI1 and CASPR2 have well defined syndrome associations. By contrast, radioimmunoassay studies have shown that antibodies directed against the VGKC complex that do not target LGI1 or CASPR2 are not syndrome specific and cannot be used as proof of an immune-mediated pathogenesis.91–93
Immunoglobulin class
The antibodies associated with autoimmune encephalitis in the table are IgG antibodies. Detection of IgA or IgM antibodies against any of these antigens has unclear significance. For example, whereas IgG antibodies against the GluN1 subunit of the NMDA receptor are specific for anti-NMDA receptor encephalitis, IgM or IgA antibodies have been reported in the serum of 10% of patients with different disorders and in a similar proportion of healthy people.94
CSF studies
Analysis of CSF plays a central part in all diagnostic criteria for encephalitis, including infectious encephalitis, and has a similar role in the detection of autoantibodies in suspected cases of autoimmune encephalitis. The investigation of CSF antibodies is important for four reasons: (1) most patients with autoimmune encephalitis have CSF antibodies and relevant antibodies might be found only in the CSF51,52—eg, in patients with anti-NMDA receptor encephalitis up to 14% have antibodies in the CSF, but not in the serum;75 (2) the repertoire of antibodies in the CSF and serum can be different in the same patient (eg, NMDA receptor in CSF and serum, and GABAA receptor only in serum), and in this setting, the types of antibodies in the CSF usually determine the clinical picture;14 (3) for some disorders, such as anti-NMDA receptor encephalitis, the concentration of CSF antibodies correlates better with the clinical course than antibody concentrations in the serum;75 and (4) neuronal antibody testing using serum and cell-based assays could lead to false-positive or false-negative results; this problem rarely occurs with CSF analysis. On the basis of these data and while we await larger studies with other autoantibodies, our recommendation is to include both CSF and serum for neuronal antibody testing in patients with suspected autoimmune encephalitis.
These concepts have implications for patient management. The approach of first testing the serum and proceeding with the CSF if negative could delay diagnosis. If serum testing is positive, but the CSF is negative, or if the clinical picture does not fit with the antibody identified, the possibilities of a laboratory result unrelated to the syndrome or a false-positive result should be considered;95 in such cases, the laboratory should be contacted regarding retesting of the samples or the use of confirmatory tests (eg, brain immunohistochemistry or cultured neurons). Finally, treatment decisions during the course of the disease should rely more on clinical assessment than on antibody titres. Although the titres might correlate with the clinical course, this correlation is imperfect, and antibodies often remain detectable after clinical recovery.75
Antibodies in demyelinating disorders that overlap with anti-NMDA receptor encephalitis
About 4% of patients with anti-NMDA receptor encephalitis develop two different syndromes that can occur separately or simultaneously. Each syndrome is related to a distinct pathogenic mechanism, such as anti-NMDA receptor encephalitis along with MOG-related or aquaporin 4 (AQP4)-related syndromes (figure 2).96 In practice, physicians should be aware that a demyelinating disorder can present as an autoimmune encephalitis disorder, and that overlapping syndromes can occur. Patients with a demyelinating disorder and atypical features (eg, dyskinesias or prominent psychiatric manifestations) or patients with anti-NMDA receptor encephalitis with atypical features (eg, optic neuritis or demyelination on MRI) should be comprehensively studied for coexisting disorders, rather than being classified as having an expansion of the spectrum of a single disease. These clinical situations imply the need for testing for AQP4 and MOG antibodies in the serum (because intrathecal production of these antibodies is rare),20,97 and for NMDA receptor antibodies in the serum and CSF.
GAD antibodies in limbic encephalitis and other syndromes
Serum antibodies against intracellular GAD can occur at low titres in 1% of healthy people and in 80% of people with type 1 diabetes mellitus.98 Only serum GAD antibodies at high titres are associated with autoimmune neurological disorders, such as limbic encephalitis and other syndromes.99 The definition of high titre depends on the technique used, but neurological symptoms usually occur with titres that are 100–1000 times higher than those seen in people with diabetes. When examining a patient with limbic encephalitis, clinicians should keep in mind that, albeit rare, high titres of serum GAD antibodies could suggest the presence of diabetes or other endocrine disorders. In this setting, specific intrathecal production of GAD antibodies or CSF oligoclonal bands support an association with the neurological syndrome.99
Approach to patients without recognisable syndromes or autoantibodies
After excluding all well characterised syndromes of autoimmune encephalitis (with or without autoantibodies) and other syndromes accompanied by well defined auto-antibodies, a group of patients who have possible autoimmune encephalitis will remain (panel 1). Patients in this group can be regarded as having probable autoimmune encephalitis if they satisfy criteria for Hashimoto’s encephalopathy (panel 6)101 or the criteria proposed in panel 7.
Panel 6. Diagnostic criteria for Hashimoto’s encephalopathy.
Diagnosis can be made when all six of the following criteria have been met:
Encephalopathy with seizures, myoclonus, hallucinations, or stroke-like episodes
Subclinical or mild overt thyroid disease (usually hypothyroidism)
Brain MRI normal or with non-specific abnormalities
Presence of serum thyroid (thyroid peroxidase, thyroglobulin) antibodies*
Absence of well characterised neuronal antibodies in serum and CSF
Reasonable exclusion of alternative causes
Panel 7. Criteria for autoantibody-negative but probable autoimmune encephalitis.
Diagnosis can be made when all four of the following criteria have been met:
Rapid progression (less than 3 months) of working memory deficits (short-term memory loss), altered mental status, or psychiatric symptoms
Exclusion of well defined syndromes of autoimmune encephalitis (eg, typical limbic encephalitis, Bickerstaff’s brainstem encephalitis, acute disseminated encephalomyelitis)
-
Absence of well characterised autoantibodies in serum and CSF, and at least two of the following criteria:
Reasonable exclusion of alternative causes
The definition of Hashimoto’s encephalopathy has been linked to a good response to steroids, and consequently the disorder is deemed immune mediated, despite the unclear physiopathology and the absence of response to prednisone in the patient in the original report.103 This disorder predominantly affects women in a wide age range, from the first to the eighth decade of life. Overt or subclinical thyroid disease, usually hypothyroidism, occurs in most cases (54 of 80 patients in a review of reported cases).104 By definition, patients develop encephalopathy, which can be associated with seizures (56 of 85 reviewed patients), myoclonus (32 patients), hallucinations (31 patients), and stroke-like episodes (23 patients) with normal or non-specific CSF and brain MRI abnormalities.33,104 Most reported patients (66 of 69 patients treated with corticosteroids with or without levothyroxine) improved;104 however, this outcome is expected in view of the definition of the disorder, which in 2006 was renamed as steroid-responsive encephalopathy with autoimmune thyroiditis.101
Patients who have a non-specific encephalopathy with subclinical or overt thyroid disease, anti-thyroid antibodies, and no better explanation for the symptoms should be considered for a trial of steroids. However, thyroid antibodies are not specific for Hashimoto’s encephalopathy because they are present in up to 13% of healthy individuals (27% in white women older than 60 years) and patients with other autoimmune encephalitis disorders.100 Similarly, α-enolase antibodies have been identified in up to 68% of patients with Hashimoto’s encephalopathy,105 but they cannot be used as biomarkers of the disease because they have been detected in healthy people and in patients with other autoimmune disorders.33,106
We propose use of the term Hashimoto’s encephalopathy only when rigorous clinical assessment and comprehensive testing for well characterised neuronal antibodies exclude other potential causes of encephalopathy (panel 6).100 Because the underlying pathogenic mechanism is unclear, diagnosis of Hashimoto’s encephalopathy should be classified as probable autoimmune encephalitis (figure 1).
Other poorly defined syndromes with no antibodies can be regarded as probable autoimmune encephalitis if they satisfy the criteria in panel 7. When considering these criteria the following should be kept in mind: (1) the absence of pleocytosis does not rule out autoimmune encephalitis (eg, 59% of patients with LGI1 antibody-associated encephalitis do not have CSF pleocytosis),54 normal routine CSF studies do not imply that there is no intrathecal IgG synthesis or an absence of CSF antibodies, and in fact, almost all antibody-associated autoimmune encephalitis disorders have detectable antibodies in the CSF; (2) autoimmune encephalitis can occur with normal or atypical MRI findings (figure 2); and (3) mainly applicable to children, several genetic disorders, mitochondrial diseases, or leukodystrophies can develop with MRI and CSF abnormalities (eg, symmetric brain involvement, pleocytosis) similar to those found in autoimmune encephalitis and might also respond to steroids.102
For patients who meet the criteria of probable autoimmune encephalitis, but do not have well characterised autoantibodies (panel 7), investigation of CSF and serum for new antibodies in reference laboratories is important. Detection of CSF antibodies that react with the cell surface of neurons (even when the antigens are unknown) strongly supports the diagnosis of autoimmune encephalitis; the clinical significance of the detection of antibodies in serum only is less clear (eg, serum GABAA receptor antibodies are associated with a wide variety of symptoms, some of unclear clinical relevance).14,107 The importance of these studies cannot be overemphasised and surpasses the clinical significance of inflammatory infiltrates in a brain biopsy, which suggest an inflammatory process, but cannot be used to establish the autoimmune cause.
For patients who do not satisfy criteria for probable autoimmune encephalitis and do not have any autoantibody (well characterised or against unknown neuronal cell-surface antigens), or who do not satisfy criteria for any of the aforementioned diseases and syndromes, the likelihood of an autoimmune cause becomes smaller and alternative diagnoses should be reconsidered.
There are several autoimmune CNS disorders (primary CNS angiitis [appendix],108 Rasmussen’s encephalitis,35 Morvan’s syndrome34) and other diseases of unclear cause (eg, febrile infection-related epilepsy syndrome [FIRES]109) that are often considered in the differential diagnosis of autoimmune encephalitis (panel 1). We have summarised these disorders (appendix) and emphasised the clinical features that lead to the differential diagnosis with autoimmune encephalitis.
Implications and directions for future research
We have shown that it is possible to proceed through a logical differential diagnosis of autoimmune encephalitis using criteria based on conventional clinical neurological assessment and standard diagnostic tests (MRI, EEG, and CSF studies). Through this approach, levels of evidence of probable and definite autoimmune encephalitis can be achieved early and therapies implemented quickly, with the possibility of fine-tuning the diagnosis and treatment when antibody results become available. Treatment recommendations for each type of autoimmune encephalitis are outside the scope of these guidelines; moreover, the evidence is limited for many of these disorders. The stepwise escalation of immunotherapy, which includes first-line therapy (steroids; IVIg, plasma exchange, or both) followed, if there is no clinical response, by second-line therapy (rituximab, cyclo phosphamide, or other), is often used in the treatment of anti-NMDA receptor and other autoimmune types of encephalitis, but rituximab is increasingly being considered as a first-line therapy.16 Not all autoimmune encephalitis syndromes, however, need a similar approach. For example, patients with limbic encephalitis and LGI1 antibodies appear to respond faster and better to steroids than patients with anti-NMDA receptor encephalitis, yet the long-term outcome seems to be better for those with anti-NMDA receptor encephalitis.28,53
We acknowledge the need for future research to drive improvements in the diagnosis of autoimmune encephalitis. The repertoire of autoimmune encephalitis in children is different from that of adults. The younger the child the more difficult it is to recognise specific autoimmune encephalitis syndromes, which suggests that guidelines for paediatric autoimmune encephalitis will be more dependent on antibody and other ancillary tests than the syndrome-based guidelines in this Position Paper. Conversely, clinical assessment of autoimmune encephalitis in elderly people (aged over 65 years) has another set of challenges imposed by the high frequency of brain changes in this group caused by systemic and non-immune-mediated disorders, or the coexistence of age-related disorders that can affect memory and cognition. Other areas of improvement will be dictated by cumulative clinical experience, better differential diagnoses with diseases that resemble autoimmune encephalitis, and increased accessibility to antibody tests with faster turnaround, while keeping in mind the caveats for interpretation of some of these tests.
Supplementary Material
Search strategy and selection criteria.
Relevant papers were identified through PubMed searches of articles published in English up to Nov 23, 2015, using the search terms (alone or in combination): “autoimmune encephalitis”, “limbic encephalitis”, “anti-NMDA receptor encephalitis”, “ acute disseminated encephalomyelitis”, “brainstem encephalitis”, “basal ganglia encephalitis”, “Hashimoto encephalopathy”, “Rasmussen encephalitis”, “primary CNS angiitis”, “primary CNS vaculitis”, “Susac syndrome”, “Morvan syndrome”, and “neuronal autoantibodies”. Additional studies were identified from the authors’ files. The final reference list was generated on the basis of relevance to the topics covered in this Position Paper.
Acknowledgments
We thank the Autoimmune Encephalitis Alliance (USA), the Encephalitis Society (UK), the Anti-NMDA Receptor Encephalitis Foundation Inc (Canada), and the Anti-NMDA Receptor Encephalitis Patient Initiative (Germany) for disseminating information, helping patients and families, and promoting research in autoimmune encephalitis. FG was supported in part by grant 20141830 Fundació la Marató TV3. MJT has been supported by an Erasmus fellowship, the Netherlands Organisation for Scientific Research (Veni-incentive), and a grant from the Dutch Epilepsy Foundations (NEF project 14–19). RCD has received research funding from the National Health and Medical Research Council, MS Research Australia, the Tourette Syndrome Association, the University of Sydney, and the Petre Foundation. MG receives grants from the National Institute on Aging; has received grants from CurePSP and the Tau Consortium; and has received speaker’s fees and research funding from Grand Round Lectures, and the Michael J Homer Family Fund. PW is supported by the National Health Service National Specialised Commissioning Group for Neuromyelitis Optica, UK, and the National Institute for Health Research Oxford Biomedical Research Centre, and has received travel grants from the Guthy-Jackson Charitable Foundation. JD was supported by the Instituto Carlos III (FIS 14/00203) grant, National Institutes of Health RO1NS077851 grant, and Fundació Cellex.
Footnotes
Altered mental status defined as decreased or altered level of consciousness, lethargy, or personality change.
Brain MRI hyperintense signal on T2-weighted fluid-attenuated inversion recovery sequences highly restricted to one or both medial temporal lobes (limbic encephalitis), or in multifocal areas involving grey matter, white matter, or both compatible with demyelination or inflammation.
If one of the first three criteria is not met, a diagnosis of definite limbic encephalitis can be made only with the detection of antibodies against cell-surface, synaptic, or onconeural proteins.
18 Fluorodeoxyglucose (18F-FDG) PET can be used to fulfil this criterion. Results from studies from the past 5 years suggest that 18F-FDG-PET imaging might be more sensitive than MRI to show an increase in FDG uptake in normal-appearing medial temporal lobes.44,45
Patients with a history of herpes simplex virus encephalitis in the previous weeks might have relapsing immune-mediated neurological symptoms (post-herpes simplex virus encephalitis).
Antibody testing should include testing of CSF. If only serum is available, confirmatory tests should be included (eg, live neurons or tissue immunohistochemistry, in addition to cell-based assay).
There is no disease-specific cutoff value for these antibodies (detectable in 13% of healthy individuals).100
Some inherited mitochondrial and metabolic disorders can present with symmetric or asymmetric MRI abnormalities and CSF inflammatory changes resembling an acquired autoimmune disorder.102
Contributors
FG and JD developed the idea for the Position Paper, chaired the project, wrote the initial draft of the manuscript, which was fully reviewed by MRR and MJT, and revised the manuscript. All other authors reviewed and commented on two subsequent drafts, and the complete manuscript was commented on, revised, and approved by all authors.
Declaration of interests
FG receives royalties from licensing fees to Euroimmun for the use of IgLON5 as a diagnostic test. MJT has received research funding for consultancy work for MedImmune, and a travel grant for Sun Pharma. CGB has given scientific advice to Eisai and UCB; undertaken industry-funded travel with support from Eisai, UCB, Desitin, and Grifols; obtained honoraria for speaking engagements from Eisai, UCB, Desitin, Diamed, Fresenius Medical Care; and received research support from Astellas Pharma, Octapharma, Diamed, and Fresenius Medical Care. CGB is an employee of Krankenhaus Mara, Bielefeld, Germany, which runs a laboratory for the detection of autoantibodies including those described in this paper; external senders are charged for antibody diagnostics. RCD has received research funding from the Star Scientific Foundation and Pfizer Neuroscience and speaker’s honoraria from Biogen Idec and Bristol-Myers Squibb. JMG has received compensation for medical legal consulting and for consulting on a scientific advisory board for Medimmune and Roche; he has received research funding through the University of California, San Francisco, USA, from Quest Diagnostic for work on a dementia care pathway. MG receives grants from Quest Diagnostics and has received personal fees for consultancy work from MedaCorp, Gerson-Lehman Group, Best Doctors, Advance Medical, Inc, and Optio LLC. JH receives royalties from licensing fees to Athena Diagnostics, Euroimmun, and ravo Diagnostika for a patent for the use of CV2/CRMP5 as diagnostic tests. SRI receives royalties from licensing fees to Euroimmun for patents for the use of LGI1, CASPR2, and contactin-2 as autoantibody tests. EL has received speaker’s honoraria and consultancy fees from Grifols, and consultancy fees from Medimmune. FL has received speaker’s honoraria from Grifols, Teva, and Biogen Idec and is employed by University Medical Center Schleswig-Holstein, Kiel, Germany, which offers commercial antibody testing without any personal reimbursements. MR reports that his employers, the University Hospital and Medical University of Innsbruck, Austria, receive payments for antibody assays (NMDA receptor, AQP4, and other autoantibodies) and for AQP4 antibody validation experiments organised by Euroimmun. MRR receives royalties from licensing fees to Euroimmun for a patent for the use of NMDA receptor as an autoantibody test, and from licensing fees to Athena Diagnostics for a patent for the use of Ma2. AS has received compensation for consulting services and speaker honoraria from Bayer-Schering, Merck-Serono, Biogen Idec, Sanofi-Aventis, Teva, and Novartis. AVe reports personal fees from Medimmune. AVi receives royalties from licensing fees to Euroimmun for the use of LGI1 and CASPR2 as diagnostic tests. PW receives royalties for the use of LGI1 and CASPR2 as autoantibody diagnostic tests; is a named inventor on a patent for the use of GABAA receptor as an autoantibody test; and has received speaker honoraria from Biogen Idec and Euroimmun. JD receives royalties from licensing fees to Athena Diagnostics for a patent for the use of Ma2 as an autoantibody test; licensing fees to Euroimmun for patents for the use of NMDA receptor and GABAB receptor as autoantibody tests; licensing fees for the use of DPPX, GABAA receptor, and IgLON5 antibodies as diagnostic tests; and has received a research grant from Euroimmun. RB, SB, TC, IC, CAG, RH, TI, HP, AR-G, KR, and K-PW declare no competing interests. None of the funding sources had any influence in the preparation of this Position Paper.
References
- 1.Venkatesan A, Tunkel AR, Bloch KC, et al. for the International Encephalitis Consortium. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–28. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jmor F, Emsley HC, Fischer M, Solomon T, Lewthwaite P. The incidence of acute encephalitis syndrome in Western industrialised and tropical countries. Virol J. 2008;5:134–46. doi: 10.1186/1743-422X-5-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology. 2014;82:443–51. doi: 10.1212/WNL.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 4.Ball R, Halsey N, Braun MM, et al. for the VAERS Working Group. Development of case definitions for acute encephalopathy, encephalitis, and multiple sclerosis reports to the vaccine: Adverse Event Reporting System. J Clin Epidemiol. 2002;55:819–24. doi: 10.1016/s0895-4356(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 5.Sejvar JJ, Kohl KS, Bilynsky R, et al. for the Brighton Collaboration Encephalitis Working Group. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5771–92. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 6.Britton PN, Eastwood K, Paterson B, et al. for the Australasian Society of Infectious Diseases (ASID), the Australasian College of Emergency Medicine (ACEM), the Australian and New Zealand Association of Neurologists (ANZAN), and the Public Health Association of Australia (PHAA) Consensus guidelines for the investigation and management of encephalitis in adults and children in Australia and New Zealand. Intern Med J. 2015;45:563–76. doi: 10.1111/imj.12749. [DOI] [PubMed] [Google Scholar]
- 7.Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94–114. doi: 10.1111/nyas.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alamowitch S, Graus F, Uchuya M, Reñé R, Bescansa E, Delattre JY. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120:923–28. doi: 10.1093/brain/120.6.923. [DOI] [PubMed] [Google Scholar]
- 9.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–44. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 10.Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67:470–78. doi: 10.1002/ana.21917. [DOI] [PubMed] [Google Scholar]
- 11.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–34. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–86. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698–701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale RC, Merheb V, Pillai S, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135:3453–68. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- 17.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–85. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–48. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4. 2 potassium channels. Ann Neurol. 2013;73:120–28. doi: 10.1002/ana.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brilot F, Dale RC, Selter RC, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol. 2009;66:833–42. doi: 10.1002/ana.21916. [DOI] [PubMed] [Google Scholar]
- 21.McKeon A, Lennon VA, Lotze T, et al. CNS aquaporin-4 autoimmunity in children. Neurology. 2008;71:93–100. doi: 10.1212/01.wnl.0000314832.24682.c6. [DOI] [PubMed] [Google Scholar]
- 22.Shahrizaila N, Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry. 2013;84:576–83. doi: 10.1136/jnnp-2012-302824. [DOI] [PubMed] [Google Scholar]
- 23.Ariño H, Höftberger R, Gresa-Arribas N, et al. Paraneoplastic neurological syndromes and glutamic acid decarboxylase antibodies. JAMA Neurol. 2015;72:874–81. doi: 10.1001/jamaneurol.2015.0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexopoulos H, Dalakas MC. Immunology of stiff person syndrome and other GAD-associated neurological disorders. Expert Rev Clin Immunol. 2013;9:1043–53. doi: 10.1586/1744666X.2013.845527. [DOI] [PubMed] [Google Scholar]
- 25.Carvajal-González A, Leite MI, Waters P, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137:2178–92. doi: 10.1093/brain/awu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demarquay G, Honnorat J. Clinical presentation of immune-mediated cerebellar ataxia. Rev Neurol (Paris) 2011;167:408–17. doi: 10.1016/j.neurol.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 27.Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry. 2012;83:638–45. doi: 10.1136/jnnp-2011-301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne S, Walsh C, Hacohen Y, et al. Earlier treatment of NMDAR antibody encephalitis in children results in a better outcome. Neurol Neuroimmunol Neuroinflamm. 2015;2:e130. doi: 10.1212/NXI.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- 32.Krupp LB, Tardieu M, Amato MP, et al. for the International Pediatric Multiple Sclerosis Study Group. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–67. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 33.Schiess N, Pardo CA. Hashimoto’s encephalopathy. Ann N Y Acad Sci. 2008;1142:254–65. doi: 10.1196/annals.1444.018. [DOI] [PubMed] [Google Scholar]
- 34.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72:241–55. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- 35.Bien CG, Granata T, Antozzi C, et al. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain. 2005;128:454–71. doi: 10.1093/brain/awh415. [DOI] [PubMed] [Google Scholar]
- 36.Armangue T, Petit-Pedrol M, Dalmau J. Autoimmune encephalitis in children. J Child Neurol. 2012;27:1460–69. doi: 10.1177/0883073812448838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillai SC, Hacohen Y, Tantsis E, et al. Infectious and autoantibody-associated encephalitis: clinical features and long-term outcome. Pediatrics. 2015;135:e974–84. doi: 10.1542/peds.2014-2702. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79:1094–100. doi: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weil AA, Glaser CA, Amad Z, Forghani B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis. 2002;34:1154–57. doi: 10.1086/339550. [DOI] [PubMed] [Google Scholar]
- 40.Solomon T, Michael BD, Smith PE, et al. for the National Encephalitis Guidelines Development and Stakeholder Groups. Management of suspected viral encephalitis in adults—association of British Neurologists and British Infection Association National Guidelines. J Infect. 2012;64:347–73. doi: 10.1016/j.jinf.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Tobin WO, Lennon VA, Komorowski L, et al. DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology. 2014;83:1797–803. doi: 10.1212/WNL.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69:892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 43.Wakerley BR, Uncini A, Yuki N for the GBS Classification Group, and the GBS Classification Group. Guillain-Barré and Miller Fisher syndromes—new diagnostic classification. Nat Rev Neurol. 2014;10:537–44. doi: 10.1038/nrneurol.2014.138. [DOI] [PubMed] [Google Scholar]
- 44.Baumgartner A, Rauer S, Mader I, Meyer PT. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260:2744–53. doi: 10.1007/s00415-013-7048-2. [DOI] [PubMed] [Google Scholar]
- 45.Heine J, Prüss H, Bartsch T, Ploner CJ, Paul F, Finke C. Imaging of autoimmune encephalitis - Relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68–83. doi: 10.1016/j.neuroscience.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 46.Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123:1481–94. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- 47.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–40. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graus F, Saiz A, Lai M, et al. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology. 2008;71:930–36. doi: 10.1212/01.wnl.0000325917.48466.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Najjar S, Pearlman D, Zagzag D, Devinsky O. Spontaneously resolving seronegative autoimmune limbic encephalitis. Cogn Behav Neurol. 2011;24:99–105. doi: 10.1097/WNN.0b013e3182248193. [DOI] [PubMed] [Google Scholar]
- 50.Voltz R, Gultekin SH, Rosenfeld MR, et al. A serologic marker of paraneoplastic limbic and brain-stem encephalitis in patients with testicular cancer. N Engl J Med. 1999;340:1788–95. doi: 10.1056/NEJM199906103402303. [DOI] [PubMed] [Google Scholar]
- 51.Höftberger R, Titulaer MJ, Sabater L, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. 2013;81:1500–06. doi: 10.1212/WNL.0b013e3182a9585f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Höftberger R, van Sonderen A, Leypoldt F, et al. Encephalitis and AMPA receptor antibodies: novel findings in a case series of 22 patients. Neurology. 2015;84:2403–12. doi: 10.1212/WNL.0000000000001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malter MP, Frisch C, Schoene-Bake JC, et al. Outcome of limbic encephalitis with VGKC-complex antibodies: relation to antigenic specificity. J Neurol. 2014;261:1695–705. doi: 10.1007/s00415-014-7408-6. [DOI] [PubMed] [Google Scholar]
- 54.Jarius S, Hoffmann L, Clover L, Vincent A, Voltz R. CSF findings in patients with voltage gated potassium channel antibody associated limbic encephalitis. J Neurol Sci. 2008;268:74–77. doi: 10.1016/j.jns.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Athauda D, Delamont RS, Pablo-Fernandez ED. High grade glioma mimicking voltage gated potassium channel complex associated antibody limbic encephalitis. Case Rep Neurol Med. 2014;2014:458790. doi: 10.1155/2014/458790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chevret L, Husson B, Nguefack S, Nehlig A, Bouilleret V. Prolonged refractory status epilepticus with early and persistent restricted hippocampal signal MRI abnormality. J Neurol. 2008;255:112–16. doi: 10.1007/s00415-008-0713-1. [DOI] [PubMed] [Google Scholar]
- 57.Chow FC, Glaser CA, Sheriff H, et al. Use of clinical and neuroimaging characteristics to distinguish temporal lobe herpes simplex encephalitis from its mimics. Clin Infect Dis. 2015;60:1377–83. doi: 10.1093/cid/civ051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69:156–65. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- 59.Renard D, Nerrant E, Lechiche C. DWI and FLAIR imaging in herpes simplex encephalitis: a comparative and topographical analysis. J Neurol. 2015;262:2101–05. doi: 10.1007/s00415-015-7818-0. [DOI] [PubMed] [Google Scholar]
- 60.Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A. GABAB receptor autoantibody frequency in service serologic evaluation. Neurology. 2013;81:882–87. doi: 10.1212/WNL.0b013e3182a35271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wingerchuk DM. The clinical course of acute disseminated encephalomyelitis. Neurol Res. 2006;28:341–47. doi: 10.1179/016164106X98251. [DOI] [PubMed] [Google Scholar]
- 62.Wingerchuk DM. Postinfectious encephalomyelitis. Curr Neurol Neurosci Rep. 2003;3:256–64. doi: 10.1007/s11910-003-0086-x. [DOI] [PubMed] [Google Scholar]
- 63.Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13:215–24. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Wingerchuk DM, Weinshenker BG. Acute disseminated encephalomyelitis, transverse myelitis, and neuromyelitis optica. Continuum (Minneap Minn) 2013;19:944–67. doi: 10.1212/01.CON.0000433289.38339.a2. [DOI] [PubMed] [Google Scholar]
- 65.Kesselring J, Miller DH, Robb SA, et al. Acute disseminated encephalomyelitis. MRI findings and the distinction from multiple sclerosis. Brain. 1990;113:291–302. doi: 10.1093/brain/113.2.291. [DOI] [PubMed] [Google Scholar]
- 66.Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86:265–72. doi: 10.1136/jnnp-2014-308346. [DOI] [PubMed] [Google Scholar]
- 67.Huppke P, Rostasy K, Karenfort M, et al. Acute disseminated encephalomyelitis followed by recurrent or monophasic optic neuritis in pediatric patients. Mult Scler. 2013;19:941–46. doi: 10.1177/1352458512466317. [DOI] [PubMed] [Google Scholar]
- 68.Reindl M, Di Pauli F, Rostásy K, Berger T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol. 2013;9:455–61. doi: 10.1038/nrneurol.2013.118. [DOI] [PubMed] [Google Scholar]
- 69.Kleffner I, Duning T, Lohmann H, et al. A brief review of Susac syndrome. J Neurol Sci. 2012;322:35–40. doi: 10.1016/j.jns.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 70.Dörr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. 2013;9:307–16. doi: 10.1038/nrneurol.2013.82. [DOI] [PubMed] [Google Scholar]
- 71.Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;76:108–19. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Planagumà J, Leypoldt F, Mannara F, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–98. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Viaccoz A, Desestret V, Ducray F, et al. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. 2014;82:556–63. doi: 10.1212/WNL.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 75.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167–77. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zandi MS, Paterson RW, Ellul MA, et al. Clinical relevance of serum antibodies to extracellular N-methyl-D-aspartate receptor epitopes. J Neurol Neurosurg Psychiatry. 2015;86:708–13. doi: 10.1136/jnnp-2014-308736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang R, Guan HZ, Ren HT, Wang W, Hong Z, Zhou D. CSF findings in patients with anti-N-methyl-D-aspartate receptor-encephalitis. Seizure. 2015;29:137–42. doi: 10.1016/j.seizure.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Prüss H, Finke C, Höltje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72:902–11. doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Armangue T, Leypoldt F, Málaga I, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol. 2014;75:317–23. doi: 10.1002/ana.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hacohen Y, Deiva K, Pettingill P, et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord. 2014;29:90–96. doi: 10.1002/mds.25626. [DOI] [PubMed] [Google Scholar]
- 81.Armangue T, Moris G, Cantarín-Extremera V, et al. for the Spanish Prospective Multicentric Study of Autoimmunity in Herpes Simplex Encephalitis. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology. 2015;85:1736–43. doi: 10.1212/WNL.0000000000002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohammad SS, Sinclair K, Pillai S, et al. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-Methyl-D-aspartate receptor or dopamine-2 receptor. Mov Disord. 2014;29:117–22. doi: 10.1002/mds.25623. [DOI] [PubMed] [Google Scholar]
- 83.Koga M, Kusunoki S, Kaida K, et al. Nationwide survey of patients in Japan with Bickerstaff brainstem encephalitis: epidemiological and clinical characteristics. J Neurol Neurosurg Psychiatry. 2012;83:1210–15. doi: 10.1136/jnnp-2012-303060. [DOI] [PubMed] [Google Scholar]
- 84.Odaka M, Yuki N, Yamada M, et al. Bickerstaff’s brainstem encephalitis: clinical features of 62 cases and a subgroup associated with Guillain-Barré syndrome. Brain. 2003;126:2279–90. doi: 10.1093/brain/awg233. [DOI] [PubMed] [Google Scholar]
- 85.Merwick A, Dalmau J, Delanty N. Insights into antibody-associated encephalitis—Bickerstaff’s 1950’s papers revisited. J Neurol Sci. 2013;334:167–68. doi: 10.1016/j.jns.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 86.Moragas M, Martínez-Yélamos S, Majós C, Fernández-Viladrich P, Rubio F, Arbizu T. Rhombencephalitis: a series of 97 patients. Medicine (Baltimore) 2011;90:256–61. doi: 10.1097/MD.0b013e318224b5af. [DOI] [PubMed] [Google Scholar]
- 87.Pittock SJ, Debruyne J, Krecke KN, et al. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) Brain. 2010;133:2626–34. doi: 10.1093/brain/awq164. [DOI] [PubMed] [Google Scholar]
- 88.Wang SM, Liu CC. Enterovirus 71: epidemiology, pathogenesis and management. Expert Rev Anti Infect Ther. 2009;7:735–42. doi: 10.1586/eri.09.45. [DOI] [PubMed] [Google Scholar]
- 89.Gleichman AJ, Spruce LA, Dalmau J, Seeholzer SH, Lynch DR. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci. 2012;32:11082–94. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takahashi Y, Mori H, Mishina M, et al. Autoantibodies and cell-mediated autoimmunity to NMDA-type GluRepsilon2 in patients with Rasmussen’s encephalitis and chronic progressive epilepsia partialis continua. Epilepsia. 2005;46(suppl 5):152–58. doi: 10.1111/j.1528-1167.2005.01024.x. [DOI] [PubMed] [Google Scholar]
- 91.Huda S, Wong SH, Pettingill P, O’Connell D, Vincent A, Steiger M. An 11-year retrospective experience of antibodies against the voltage-gated potassium channel (VGKC) complex from a tertiary neurological centre. J Neurol. 2015;262:418–24. doi: 10.1007/s00415-014-7588-0. [DOI] [PubMed] [Google Scholar]
- 92.Paterson RW, Zandi MS, Armstrong R, Vincent A, Schott JM. Clinical relevance of positive voltage-gated potassium channel (VGKC)-complex antibodies: experience from a tertiary referral centre. J Neurol Neurosurg Psychiatry. 2014;85:625–30. doi: 10.1136/jnnp-2013-305218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klein CJ, Lennon VA, Aston PA, et al. Insights from LGI1 and CASPR2 potassium channel complex autoantibody subtyping. JAMA Neurol. 2013;70:229–34. doi: 10.1001/jamaneurol.2013.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dahm L, Ott C, Steiner J, et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol. 2014;76:82–94. doi: 10.1002/ana.24189. [DOI] [PubMed] [Google Scholar]
- 95.Armangue T, Santamaria J, Dalmau J. When a serum test overrides the clinical assessment. Neurology. 2015;84:1379–81. doi: 10.1212/WNL.0000000000001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75:411–28. doi: 10.1002/ana.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jarius S, Paul F, Franciotta D, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci. 2011;306:82–90. doi: 10.1016/j.jns.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 98.Meinck HM, Faber L, Morgenthaler N, et al. Antibodies against glutamic acid decarboxylase: prevalence in neurological diseases. J Neurol Neurosurg Psychiatry. 2001;71:100–03. doi: 10.1136/jnnp.71.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–63. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 100.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 101.Castillo P, Woodruff B, Caselli R, et al. Steroid-responsive encephalopathy associated with autoimmune thyroiditis. Arch Neurol. 2006;63:197–202. doi: 10.1001/archneur.63.2.197. [DOI] [PubMed] [Google Scholar]
- 102.Tardieu M, Deiva K. Rare inflammatory diseases of the white matter and mimics of multiple sclerosis and related disorders. Neuropediatrics. 2013;44:302–08. doi: 10.1055/s-0033-1358599. [DOI] [PubMed] [Google Scholar]
- 103.Brain L, Jellinek EH, Ball K. Hashimoto’s disease and encephalopathy. Lancet. 1966;2:512–14. doi: 10.1016/s0140-6736(66)92876-5. [DOI] [PubMed] [Google Scholar]
- 104.Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol. 2003;60:164–71. doi: 10.1001/archneur.60.2.164. [DOI] [PubMed] [Google Scholar]
- 105.Yoneda M, Fujii A, Ito A, Yokoyama H, Nakagawa H, Kuriyama M. High prevalence of serum autoantibodies against the amino terminal of alpha-enolase in Hashimoto’s encephalopathy. J Neuroimmunol. 2007;185:195–200. doi: 10.1016/j.jneuroim.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 106.Servettaz A, Guilpain P, Tamas N, Kaveri SV, Camoin L, Mouthon L. Natural anti-endothelial cell antibodies. Autoimmun Rev. 2008;7:426–30. doi: 10.1016/j.autrev.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 107.Pettingill P, Kramer HB, Coebergh JA, et al. Antibodies to GABAA receptor α1 and γ2 subunits: clinical and serologic characterization. Neurology. 2015;84:1233–41. doi: 10.1212/WNL.0000000000001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hajj-Ali RA, Singhal AB, Benseler S, Molloy E, Calabrese LH. Primary angiitis of the CNS. Lancet Neurol. 2011;10:561–72. doi: 10.1016/S1474-4422(11)70081-3. [DOI] [PubMed] [Google Scholar]
- 109.Nabbout R. FIRES and IHHE: delineation of the syndromes. Epilepsia. 2013;54(suppl 6):54–56. doi: 10.1111/epi.12278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.