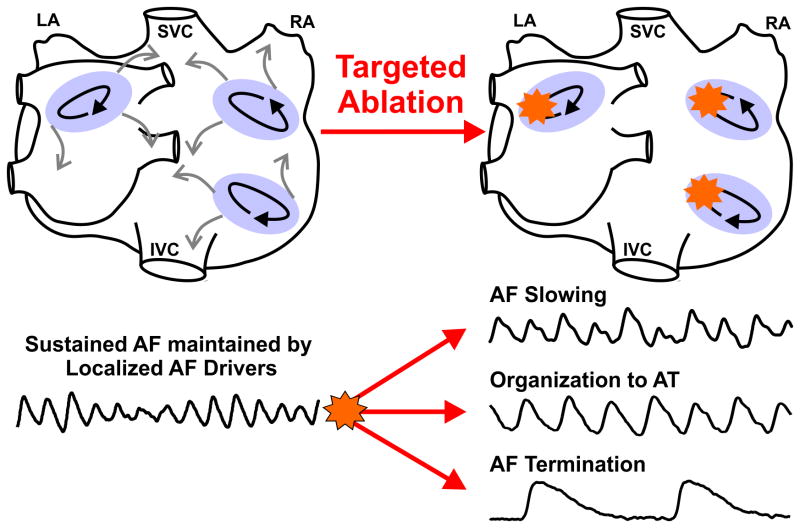
Presented in the following review are the insights on atrial fibrillation (AF) gained from our recent simultaneous endo-epicardial transmural and panoramic optical mapping studies of the human atria that may begin to resolve the controversy regarding AF maintenance mechanisms1-3. It is still hotly debated whether the sustaining mechanism of AF involves multi-wavelets self-replicating across the entire atrial myocardium4, or if fibrillatory conduction in the atria is maintained by localized AF drivers1. Even the term “AF driver”, which is widely used in the current literature,1-3, 5-15 is not well defined. In this review, AF drivers are defined as one or several patient-specific localized sources of fast, repetitive activity from which activation propagates and breaks down into fibrillatory conduction in the rest of the atria, targeted ablation of drivers would slow AF, organize AF to atrial tachycardia, or terminate AF (Figure 1).
Figure 1.
Defining AF drivers. Atrial fibrillation (AF) drivers are defined as localized sources of fast, repetitive activity from which activation propagates and breaks down into fibrillatory conduction in the rest of the atria. One or few patient-specific AF drivers may exist simultaneously or sequentially, and targeted ablation (orange stars) of AF drivers would slow, organize to atrial tachycardia (AT), or terminate AF. Light purple ovals, black arrows, and grey arrows represent the AF driver region, path of reentry, and fibrillatory conduction, respectively. Abbreviations: AF-atrial fibrillation; AT-atrial tachycardia, IVC/SVC-inferior/superior vena cava; LA/RA-left and right atria.
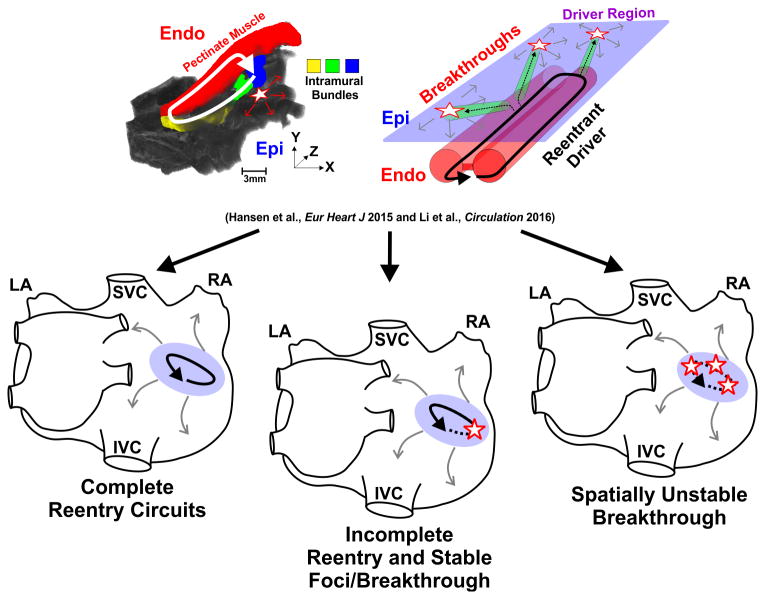
Even if one is convinced that AF drivers exist, there is no clear consensus on the specific electrophysiologic mechanism of AF drivers. AF drivers have been hypothesized to represent reentry circuits, rotors, focal sources, and/or a complex mixture of all these mechanisms. We hypothesize that human AF is maintained by a limited number of spatially stable but temporally competing, patient-specific intramural microanatomic reentries, the true nature of which may remain hidden to clinical surface electrode mapping, which instead visualizes the activity as stable/unstable rotors, focal activity, or breakthroughs due to the transmural complexity of the atrial wall (Figure 2). We propose a theory for AF maintenance that incorporates nearly all observations made by different clinical mapping studies based upon direct evidence from transmural and panoramic optical mapping of the human heart.
Figure 2.
Different types of intramural reentrant AF driver visualization by single-surface mapping. Ex-vivo contrast-enhanced MRI and schematic of the 3D atrial wall show the possible transmurality of microanatomic reentrant AF drivers. The transmurality of a microanatomic reentrant AF driver can cause three different visualizations: 1) complete reentry circuits (solid arrow), 2) incomplete reentry circuits (half-dotted arrow) and spatially stable breakthrough (single star), and 3) spatially unstable breakthroughs (multiple stars). Light purple oval and grey arrows represents AF driver region and fibrillatory conduction, respectively. Abbreviations as in Figure 1. Endo-endocardium; Epi-epicardium. From Hansen et al.1 and Li et al.3 with permission.
Intramural Microanatomic Reentry as AF Driver Mechanism
Almost 100 years ago, Lewis16 hypothesized that reentry, where electrical circus movement can occur around a site of anatomical or functional unexcitable core/block, could drive AF with fibrillatory conduction propagating from it. This hypothesis was supported by surface electrode mapping by Schuessler's group17 and optical mapping studies by Jalife's group5 ex-vivo in animal models of acetylcholine-induced sustained AF and later by simultaneous endo-epicardial (dual-sided) mapping18. However, the above studies were performed in animal models and the spatiotemporal stability and electrophysiologic characteristics of these reentrant drivers may differ in the diseased human heart.
Early studies of the aged, diseased human heart, such as the microelectrode study by Spach et al19, showed that myofiber orientations and microfibrosis in right atrial pectinate bundles act as substrates for microanatomic reentry, which represents a possible driver of AF. Indeed, the complex human atrial anatomy, consisting of a web of small fibrotically-insulated myobundle tunnels, creates an ideal substrate for intramural reentry1, which would require transmural mapping to determine activation patterns within the 3D atrial wall (∼1-7mm). To accomplish this, we recently studied diseased human right atria (RA) with simultaneous endo-epicardial optical mapping (330μm spatial resolution)1. The utilization of non-toxic near-infrared voltage-sensitive dye20, 21 allowed us to record optical action potentials up to 4mm deep simultaneously from sub-endocardial and sub-epicardial layers (Figure 3A). Rapid pacing led to endo-epicardial activation dissociation due to transmural conduction blocks along fibrotically-insulted pectinate bundles (Figure 3B). These conduction blocks allowed induction of sustained intramural reentry that appeared to drive AF1. These reentrant AF drivers were spatially and temporally stable (>90 min) sources of electrical activity correlating with the highest dominant frequency (DF) during fibrillatory conduction. The AF drivers were confirmed by targeted radiofrequency ablation that eventually terminated AF.
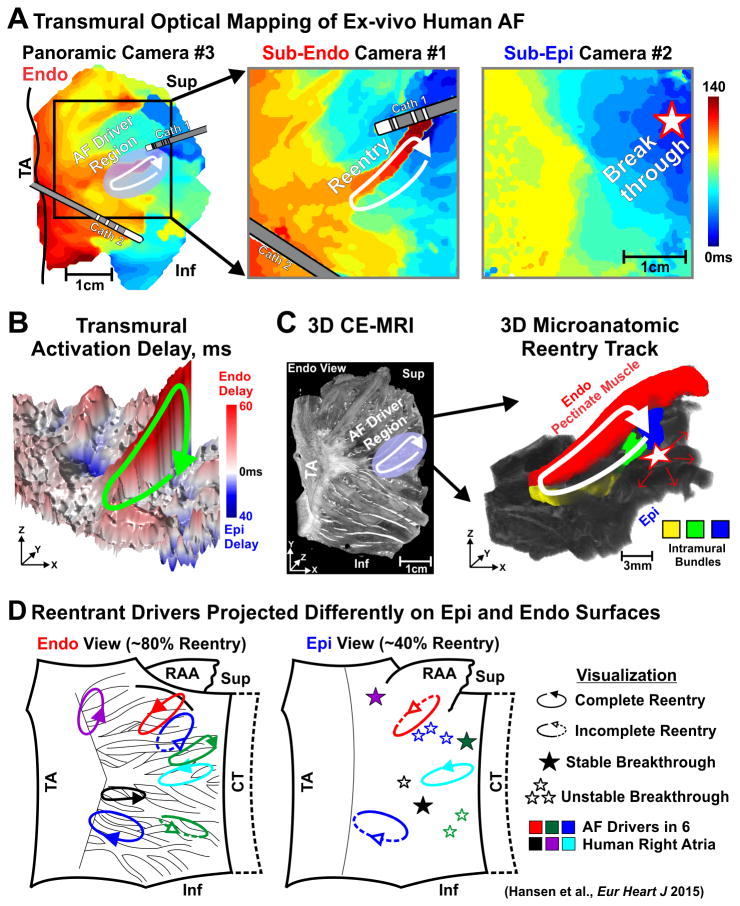
Figure 3.
Microanatomic tracks of reentrant AF drivers resolved by dual-sided optical mapping and CE-MRI. (A) Ex-vivo activation maps from panoramic (left), sub-Endo (middle) and sub-Epi (right) cameras. Pink oval, solid arrow, and star represent the AF driver region, path of reentry, and breakthrough, respectively. (B) Transmural activation delay map with reentrant pathway shown by green arrow. (C) Left: CE-MRI of the LRA showing fibrosis (white) distribution and location of reentrant AF driver (white arrow). Right: 3D view of colored reentry track. (D) Summary of intramural microanatomic reentries visualized from the Endo and Epi. Abbreviations as in Figures 1 and 2; CE-MRI- contrast-enhanced magnetic resonance imaging; CT-crista terminalis; Inf-inferior; LAA-left atrial appendage; RAA-right atrial appendage; Sup-superior; TA-tricuspid annulus. From Hansen et al.1 with permission.
Importantly, integration of transmural optical mapping with contrast-enhanced MRI (CE-MRI, 80μm3) allowed us to study region-specific structural substrates of reentrant AF drivers1 (Figure 3C). This revealed that the combination of increased intramural fibrotic strands (∼200μm thick), greater endo-epicardial myofiber misalignment, and atrial thickness variation created microanatomic tracks for stable reentrant AF drivers. Specifically, the 3D microanatomic tracks consisted of two limbs of either sub-endocardial and/or sub-epicardial pectinate muscle bundles, which were connected by small myobundles that created conduction pivot points (Figure 3C).
How Intramural Microanatomic Reentry can be Visualized from Atrial Surfaces
Here, we discuss how the visualization of microanatomic reentrant AF drivers becomes much more complex when intramural conduction and the 3D atrial structure is considered. The 3D size of microanatomic reentrant tracks observed in our integrated optical mapping and 3D contrast-enhanced MRI study1, and more recently with 3D micro-CT2, 3, were on average ∼15 × 6 mm, with a 3 mm depth, supporting microanatomic reentry as opposed to macroanatomic reentry around anatomic obstacles such as the tricuspid valve or the intercaval region. The size of microanatomic substrates suggests that these AF drivers could be seen not only by optical mapping but by contact electrode mapping with adequate resolution when the track is located only in the sub-epicardial or sub-endocardial surface, which is usually not the case.
Importantly, sub-endocardial optical mapping visualized ∼80% of the intramural reentry circuit vs ∼40% by simultaneous sub-epicardial mapping, since the complex sub-endocardial myobundle network is more suitable for reentry than the smoother sub-epicardial myocardial layer1. Interestingly, the stable breakthrough visualized by sub-epicardial mapping frequently represented a transmural pivot point of the reentry circuit seen from the sub-endocardium (Figure 3A). In general, transmural optical mapping revealed four distinct patterns of intramural driver visualization from a single atrial surface: (1) complete reentry circuits; (2) incomplete reentry circuits; (3) stable breakthrough; and (4) unstable, variable breakthroughs. Thus, transmural optical mapping1 demonstrated that alternative mechanisms of AF maintenance could be hypothesized based upon the visualization by single surface electrode mapping (Figure 3D).
One of the visualization patterns of intramural reentrant AF drivers may appear as rotors10, which are functional reentries with an excitable but unexcited core. A continuum may exist with a variety of mechanistic explanations of reentrant AF drivers having varying degrees of experimental support. However, we observed, with our high-resolution transmural optical mapping and 3D contrast-enhanced MRI, activation conducting unidirectionally through microanatomic reentry tracks which are composed of distinct fibrotically-insulated myobundles (tunnels) 0.5-2mm thick1. Therefore, reentrant AF drivers observed directly in the human heart1 and discussed in this review refer only to microanatomic reentry19 that lack in their structural track/tunnels a functional rotor core seen in animals and computer models, but create similar rotational activation patterns observed in clinical AF mapping.
The Number of Reentrant AF Drivers Present in the Heart may Affect Visualization and Treatment
Temporal Stability
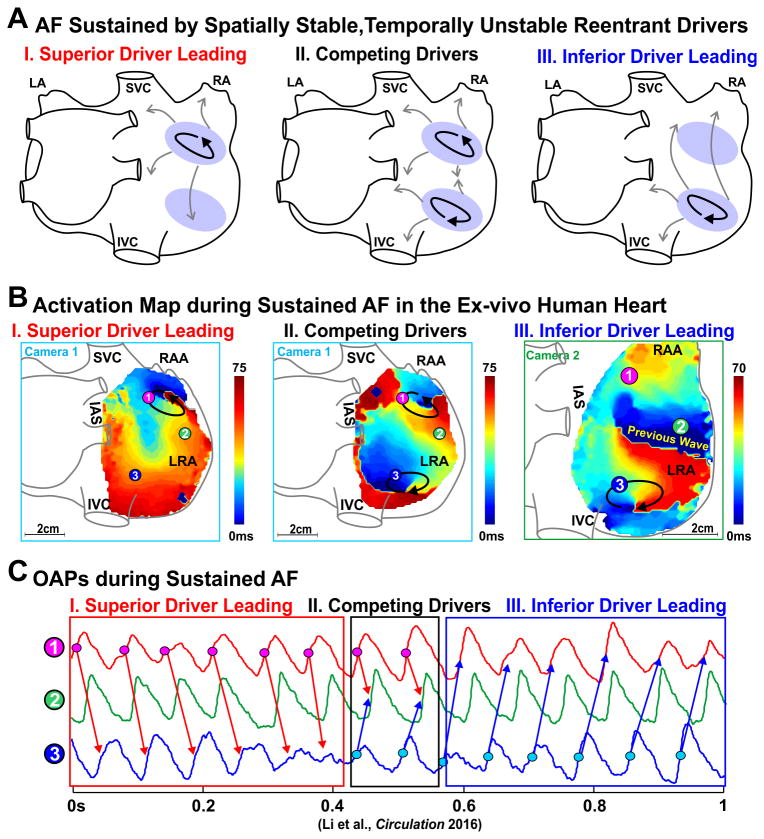
The temporal stability of an AF driver is defined as the percent of AF cycle lengths that are generated by the AF driver. Our transmural optical mapping study on the isolated human lateral RA showed a single driver that was 100% temporally stable. However, recent panoramic biatrial optical mapping studies2, 3 revealed that sustained AF in explanted human hearts may be maintained by two or more spatially stable, but temporally competing, reentrant drivers in both left and right atria (Figure 4). In cases when two or more localized AF drivers are present, the temporal stability of each driver may be less than 100%. In our experience, the presence of two or more spatially stable reentrant drivers within the human atria may allow these drivers to be temporally unstable or intermittent while permitting AF to be constantly maintained by at least one active driver. We consider these drivers to be spatially stable since activation returns to the same location with the same pattern when the driver is not 100% temporally stable. A localized AF driver lacking complete temporal stability directly correlates with some clinical mapping observations8, 9 that may only show driver activity at one location for a limited number of beats.
Figure 4.
AF maintained by competing drivers during sustained AF in the ex-vivo human heart. (A) Schematic of two spatially stable AF drivers competing in the RA. Pink oval, black arrows, and grey arrows represent the AF driver region, path of reentry, and fibrillatory conduction, respectively. (B) Activation maps showing AF driven first by a superior and then an inferior spatially stable AF driver. Arrows indicate the location and direction of AF reentrant drivers. (C) Optical action potentials (OAPs) from superior driver, inferior driver, and the middle right atrial free wall. Abbreviations as in Figure 3. From Li et al.3 with permission.
Driver Hierarchy
Furthermore, during our ex-vivo experiments where radiofrequency ablation successfully disrupted the microanatomic reentry track of the sole AF driver, a secondary reentrant AF driver with a longer cycle length was unmasked in some hearts1. These secondary reentrant drivers were in unique locations and not present when the original primary driver was active. The presence of several temporally competing drivers and secondary drivers may have an impact on the lack of acute AF termination seen clinically after ablation treatments6-8. These findings suggest repeated mapping of AF may be necessary to identify all patient-specific drivers.
Translational Application of Ex-vivo Optical Mapping of Human Atria
As we have discussed in our recent review22, optical mapping, while it provides the opportunity to detect intramural activation within the 3D atrial wall, has inherent limitations. Previous concerns with optical mapping such as tissue absorbing and scattering light as well as the phototoxicity of fluorescent dye have been mitigated by new near-infrared voltage sensitive dye, di-4-ANBDQBS20, 21. Importantly, our ex-vivo electrophysiologic parameters, including action potential duration, conduction velocity, and AF dominant frequency are within clinical ranges for patients with AF22, even after the addition of autonomic2, 3 or metabolic pharmacologic stimulation1. Therefore, with functional parameters within clinical ranges, it is highly likely that the mechanism we have observed in the diseased human atrial structure is representative of clinical phenomena. Finally, and most importantly, optical mapping cannot be performed in patients. Thus, this review looks to clinical studies that use surface electrodes, with their own sets of limitations, for evidence that may suggest the presence of intramural microanatomic reentrant AF drivers in patients. Our optical mapping observations of intramural microanatomic reentrant AF drivers corroborate, as well as bridge, the results from all major clinical epicardial and/or endocardial mapping studies.
Clinical Surface Electrode AF Mapping: Two Sides of the Same Coin
Endocardial Contact Electrode Mapping
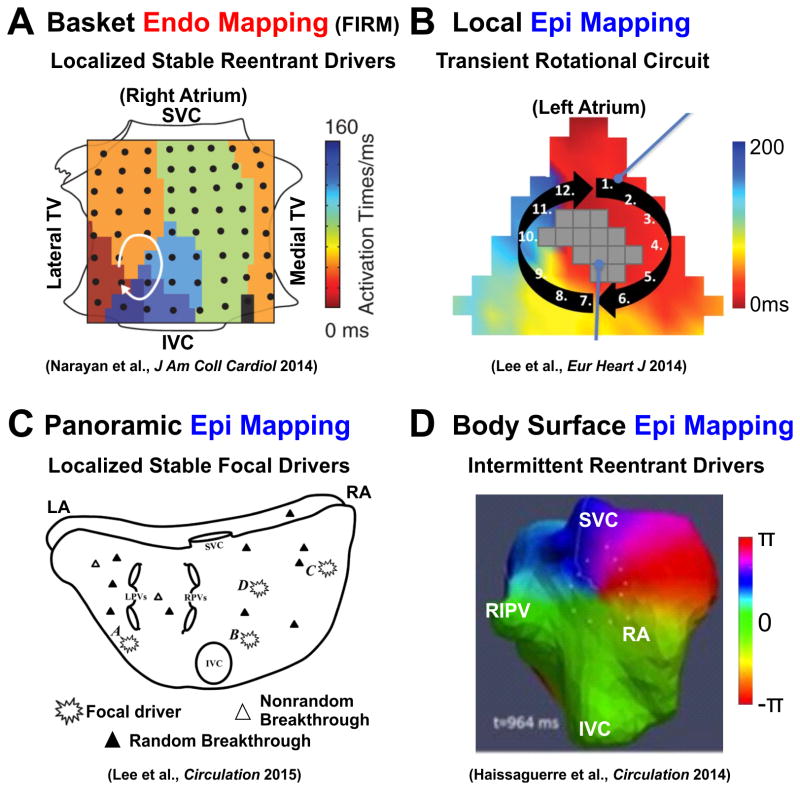
In concordance with our optical mapping studies, clinical studies that mapped AF from the endocardial surface were more likely to visualize a reentry circuit, which may be visualized as rotor activity by phase mapping10 (Figure 5A). Local endocardial mapping using a PentaRay catheter (35mm diameter, 20 electrodes, 4mm inter-electrode distance)11 found reentry circuits as well as discrete centrifugal activation. Recently, several clinical groups6, 7 have identified both localized reentrant drivers/rotors and focal impulses in paroxysmal and persistent AF patients by using panoramic endocardial basket (FIRM) catheters (64 electrodes, 9mm inter-electrode distance). In general, FIRM mapping6, 7, 23, 25 found a limited number (∼2-3/patient) of spatially and temporally stable (>10 min), primarily reentrant drivers in both atria that become more prevalent with progression/duration of AF. Furthermore, these studies demonstrated that targeted ablation could successfully alter or terminate AF in the majority of patients, which supports a localized driving mechanism for AF maintenance, rather than multiple random wavelets.
Figure 5.
Diverse clinical approaches and results for electrode mapping of human AF drivers. (A) Panoramic Endo mapping by Focal Impulse and Rotor Mapping (FIRM) finds stable, localized reentrant AF driver in the RA. (B) Local Epi mapping finds reentry in the left atria. (C) Panoramic Epi mapping finds some stable focal AF drivers as well as random and nonrandom breakthroughs. (D) Non-invasive body surface, panoramic Epi mapping finds intermittent reentrant AF drivers that return to the same regions. Abbreviations as in Figure 3. From Narayan et al.23, Lee et al.24, Lee et al.9, and Haissaguerre et al.8 with permission.
However, two other studies26, including the recent OASIS trial27, show relatively poor outcomes for ablations relying on FIRM maps alone. The OASIS trial compared FIRM only ablation to FIRM plus pulmonary vein isolation (PVI) or PVI plus trigger elimination. Of the three available studies that have investigated FIRM only ablation, two report 14%27 and 22%26 success rates in a total of 56 patients, while one study28 in 31 patients reports 81% success. With contradicting results from FIRM only ablation, some attribute the lack of success as evidence against the presence of AF drivers; however, it may be due to the fact that FIRM did not identify all AF drivers, especially drivers at the ostium of a PV that are hidden by poor basket catheter contact with these regions. Other possible reasons why some studies only achieved marginal success with FIRM only ablation procedures could be operator learning curve, map interpretation, and choice of comparator group, emphasizing the opportunity for improvement/validation of FIRM10.
It is worth mentioning that two studies27, 29 in roughly 100 patients that report only a 37-52% success rate of FIRM guided ablation plus PVI may represent a minor proportion of the field. In contrast, 8 studies6, 7, 25, 30-34 from multiple groups with a total of roughly 500 patients show a collective success rate of 75-88%, which suggest localized AF drivers may exist in the majority of paroxysmal and persistent AF patients, and appropriate ablation of these drivers could treat AF. Overall, the findings from the majority of studies attest to the ability of endocardial mapping to locate and guide ablation of localized AF drivers.
Epicardial Contact Electrode Mapping
Epicardial mapping during open-chest surgery also revealed reentry circuits in several clinical studies. However, these studies had a lower probability of visualizing reentrant drivers, and the reentrant drivers that were identified had lower stability, compared to endocardial mapping, which may be explained by the differences in structural organization between the endocardium and the epicardium of the human atria (Figure 3C). Cox et al.35 epicardially mapped both atria during acute AF and showed a single stable reentrant circuit in the RA could maintain AF in 6 patients with WPW syndrome. Moreover, local RA epicardial mapping (36mm diameter, 244 electrodes, 2.25mm inter-electrode distance) by Allessie's group36 showed random reentry and a shifting leading circle in patients with acute AF. Another local epicardial mapping (6.75cm2 or ∼10% of the atria, 128 electrodes, 2.5mm inter-electrode distance) study by Lee et al24 reported mostly multiple wavefronts, disorganized activation, and focal activation but also saw reentrant circuits in 3 out of 18 patients (Figure 5B). Biatrial epicardial mapping (250 electrodes) by Schuessler et al37 identified a single discrete region of high frequency in the majority of persistent and paroxysmal AF patients, yet they were only able to visualize a reentrant circuit within this region in some patients.
However, other recent contact epicardial mapping studies9, 24, 38 observed only stable and unstable breakthroughs or focal discharges in patients with persistent AF that match how intramural reentrant drivers appear when recorded from the epicardial surface in our optical mapping studies. A study by de Groot et al 38 employed high-resolution local epicardial mapping (64–244 unipolar electrodes, 2.25-2.5mm distance) which visualized a large proportion of fibrillatory waves originating from a ‘focal’ origin, suggesting breakthroughs from endocardial waves.
Lee et al's9 biatrial epicardial mapping study (92.85cm2, 512 bipolar electrodes, 5.2-7mm inter-electrode distance) mapped atrial activation of persistent AF and concluded that AF could be maintained by epicardial focal activity but lacked confirmation by intervention (Figure 5C). Notably, this study provided the longest whole atrial activation analysis (32 seconds). These focal drivers, present across both atria, were found to be temporally stable for 32 seconds in 6/12 patients, with most focal sources having intermittent activity but a recurring location. Our study1 showed intramural reentry circuits often travel between the sub-endocardium and sub-epicardium through small intramural bundles, which causes both “focal” and “breakthrough” phenomenon if mapped from a single surface (Figure 3).
Non-Invasive Body Surface Epicardial Mapping
Cuculich et al's39 non-invasive body surface mapping (256 electrodes) visualized the most common patterns of AF were multiple wavelets with focal sites across both atria but rotor/reentry activity was rarely seen. Later, Haissaguerre et al8 using a similar non-invasive body surface mapping approach but with phase processing8, found temporally unstable reentrant drivers (∼2.6 rotations) (80%) and focal breakthroughs (20%) that often localized in specific anatomical regions, ablation of which could terminate or organize AF (Figure 5D). Both studies saw that AF complexity (number of drivers or wavelets) increased with longer clinical history of AF. Further analysis by the Berenfeld group40 showed that reentrant activity seen on the body surface is significantly more stable when a narrow band-pass filter at the highest dominant frequency is applied. They suggest that the instability of AF drivers observed may stem from interference by atrial activation elsewhere and does not represent an inherent instability of the drivers themselves. Although the body surface mapping benefits from being non-invasive, the inverse approach has relatively low spatial resolution, and it is inherently sensitive to noise and motion, which may also increase the instability of AF drivers.
Dual-sided Endocardial/Epicardial Contact Mapping
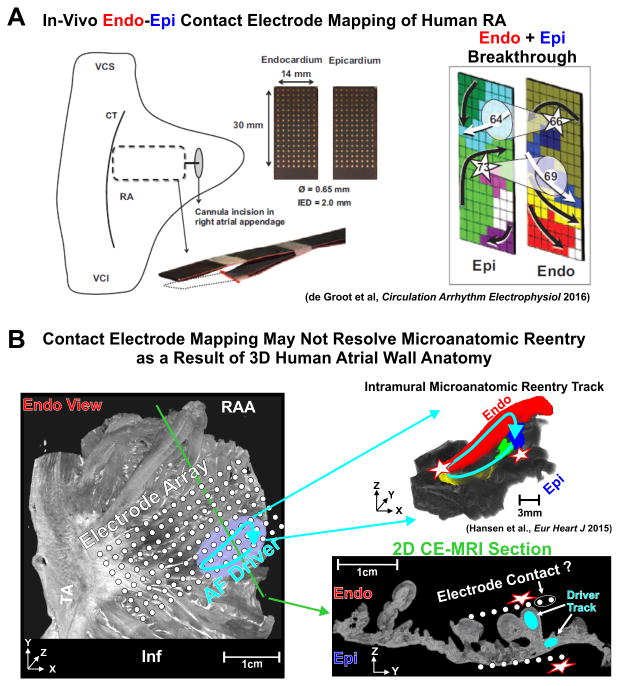
Finally, the recent surgical study by de-Groot et al13 utilized local endo-epicardial electrode mapping of the lateral RA by dissecting the atrial appendage and inserting a clamp of two electrode plaques (1.5×3cm2, 128 electrodes, 2mm inter-electrode distance) during acute, paroxysmal, and persistent AF (Figure 6A). They demonstrated endo-epicardial dissociation and focal waves/breakthroughs in all patients, but they did not report reentrant activity. The majority (65%) of focal waves could be attributed to transmural excitation. In general, this in-vivo study directly supports our ex-vivo panoramic endo-epicardial optical mapping study1 of the same region where we demonstrated that intramural microanatomic reentry causes endo-epicardial dissociation and focal/breakthrough patterns (Figure 3). Specifically, the >50ms endo-epicardial dissociation reported in the de Groot et al study13 is comparable to the 67±31ms endo-epicardial dissociation seen during AF in our transmural optical mapping of the human heart1.
Figure 6.
Contact electrode mapping is limited by the human 3D atrial wall in the detection of intramural microanatomic reentrant AF drivers. (A) Left: Endo-epicardial mapping clamp for simultaneous dual-sided mapping of AF drivers in-vivo. Right: Breakthroughs visualized on Epi and Endo surfaces. (B) Left: Endo view of diseased human RA by contrast-enhanced MRI (CE-MRI) with a hypothetical electrode array projected onto the image. Reentrant AF driver is represented by blue solid arrow, and green line shows the location of 2D CE-MRI section. Right: 3D microanatomic intramural reentry track shown by color coded bundles on CE-MRI with overlaying blue arrow. 2D CE-MRI section showing the variability of electrode contact across the pectinate muscles of the right atrial wall and resulting breakthroughs. Abbreviations as in Figure 3. From de Groot et al.13 and Hansen et al.1 with permission.
This study13 represents a significant step towards measuring transmural activation in clinical settings, but it is no surprise that intramural reentries remained hidden due to limited mapping resolution, tissue coverage, and surface only electrode recordings. Moreover, 3D structural analysis of the mapped human atrial region was missing; consequently, structural knowledge is needed to visualize what exact tissues/pectinate bundles that each electrode mapped. Specifically, as the mapping approach used in the study suffered from a lack of panoramic coverage of the atria, concluding no driver is present in a limited 1.5 × 3 mm2 section of the RA does not exclude the possibility of drivers elsewhere in the heart. Furthermore, the clamp of flat electrode plaques placed in the RA does not conform to the topography of the atrial wall (Figure 6B), and electrode contact is limited to the thickest portions even when disruptive pressure is applied as the clamp squeezes down on the atrium. Activation within smaller intramural bundles being obscured by far-field influence from larger, thicker bundles with good contact or a lack of contact with intramural bundles would lose some continuity of activation within a reentry circuit. Thus, only endocardial and epicardial breakthrough would be visualized.
Clinical Electrode Mapping Limitations
Electrogram Analysis and Interpretation
Importantly, for clinical electrode studies, no consensus has been reached on not only the resolution and coverage needed to resolve AF mechanisms, but there is no unifying approach to how electrograms should be analyzed and interpreted10. Both unipolar and bipolar electrodes record extracellular activation with numerous problems, such as incomplete contact, mechanical movement, and far-field influence from surrounding tissue18. Moreover, fibrotic layers and epicardial fat can diminish the already low amplitude, multi-component electrogram signals. Phase signal processing may present another technical limitation as it is biased for rotor visualization10. At the same time, it may provide an opportunity to visualize reentrant AF drivers as a core of rotational activity in the clinical setting limited by electrode resolution.
Intramurality
The specific path of intramural reentry through the atrial wall can be relatively tight, even a few millimeters when the circuit is viewed at an angle, as revealed in our study with 330μm resolution1. Thus, even dual-sided electrode mapping with >1mm resolution may show only the AF driver location but not the mechanism. Therefore, clinical studies using surface electrograms may not see the whole picture and are destined to draw conclusions based on one of the four surface visualizations of intramural reentrant AF drivers. Surface electrograms may be complex and/or fractionated at AF driver locations, and studies of electrogram morphologies that define human AF drivers would benefit greatly from validation with transmural optical mapping that could distinguish electrogram deflections resulting from far-field vs local activation41.
Temporal stability
As discussed above, the temporal stability of an AF driver may be affected by the total number of AF drivers in the heart. Local mapping that may only see a driver for a fraction of the mapped arrhythmia might conclude that the AF episode did not rely on an AF driver for maintenance. Notably, panoramic clinical mapping studies have shown the presence of intermittent drivers that return to or stabilize in specific locations within the atria8, 9. Signal processing and far-field influence may further complicate the detection of AF drivers. Moreover, fibrillatory conduction outside the driver or through a surface layer overlaying the intramural driver may mask the small signal moving through intramural bundles. This may inappropriately cause the reentrant AF driver to appear and disappear from the surface maps, while in fact the reentry circuit may continuously drive the arrhythmia throughout this time.
Integration of CE-MRI to Overcome Clinical Electrode Mapping Limitations
More importantly, a definitive answer on intramural AF driver mapping may not come from electrogram recordings alone, and instead may require integration with 3D structural imaging. The role of human atrial 3D myofiber anatomy is often overlooked in the functional studies that hunt for AF's sustaining mechanisms10, 15, 18, 19, 42. We showed in our ex-vivo human heart study1 how the complexities of the 3D human atria, such as intramural fibrosis, transmural myofiber misalignment, and variations in atrial thickness affect atrial conduction, which is made worse by the fast activation rate during sustained AF. CE-MRI may be able to help bridge the gap between ex-vivo and in-vivo studies, and it may aid ablation strategies by placing in-vivo functional, electrode-based maps in the context of the 3D human atrial anatomy. For now, clinical CE-MRI is limited by its resolution and some atrial structural features identified by CE-MRI based on signal intensity differences, like fibrosis, have yet to be definitively validated in-vivo; therefore, the need for ex-vivo studies directly on the human heart that integrate clinically available functional and structural mapping are still necessary, as we recently reviewed22.
Future Studies of AF Maintenance Mechanisms Ex-vivo and In-vivo
We propose that the focal, breakthrough, and rotor activity seen by the studies mentioned above represent different visualizations of intramural microanatomic reentrant AF drivers. Our theory of intramural microanatomic reentry appears to unify all clinical electrode mapping observations with this single unifying mechanism. However, we cannot rule out the possibility of other mechanisms responsible for sustaining AF present in a large, diverse cohort of AF patients. We suggest that the human heart experiments in which AF is mapped with high resolution transmural optical mapping integrated with structural 3D CE-MRI are one of the needed steps to provide direct evidence of other specific AF driving mechanisms (e.g. multiwavelets with unstable breakthrough, focal activity, or rotors).
Thus, further integration of 3D CE-MRI imaging and electrode mapping studies both ex-vivo and in-vivo are needed to provide more support to our and all other theories of AF maintenance. Specifically, studies that combine current contact electrode mapping with transmural optical mapping could accurately identify intramural drivers by optical mapping and then validate the electrogram by analyzing the contact electrogram morphologies from the AF driver region. These validated electrogram patterns, or intramural microanatomic reentrant AF driver “fingerprints”, could then be used as references when analyzing contact electrode mapping signals from in-vivo clinical studies. Furthermore, a better understanding of the microanatomic reentry mechanism may be gained from detailed studies that use atrial pacing or local entrainment pacing near an AF driver, similar to the studies that have characterized reentry circuits in the human ventricle43, 44.
Based on our integrated functional, structural, and molecular studies1-3, we suggest further study is needed on heterogeneous cellular refractoriness, complex myofiber twists, tissue dimension variations, and fibrotic remodeling as these pathologic features may predispose some locations to serve as ideal substrates for microanatomic reentrant AF drivers in a patient-specific manner. This underlying structural substrate and its heart-specific progression may explain variations in driver locations, sizes, and plurality as patients progress from paroxysmal to long-standing persistent AF7, 8. Thus, it is important to study the diverse clinical histories and comorbidities of AF patients. Differences may exist in the type and extent of structural and molecular remodeling brought about by the various comorbidities such as heart failure, myocardial infarction, hypertension, and even between paroxysmal and persistent AF. Additional attention should also be paid to how comorbidities may affect the right and left atria independently, as AF drivers have been shown to exist in both atria7, 9. Thus, structural information gained through CE-MRI could become as indispensable as electrode mapping in the identification of patient-specific AF drivers.
One unique avenue of research would be the development of computational models based on functional and structural results from integrated high-resolution optical and 3D CE-MRI mapping of human hearts during AF, which allowed us to reconstruct true “fingerprints” of AF driver characteristics that can be applied to AF driver identification in-vivo. These heart-specific computer models would present the opportunity to test multiple targeted ablation strategies for the same AF driver, as in silico ablation is uniquely reversible compared to ex-vivo and in-vivo studies45.
Conclusion
The interpretation of surface mapping techniques, especially without considering intramural conduction within the complex 3D human atria, will continue to fuel the debate of AF mechanisms. Based on our ex-vivo experimental results and multiple clinical studies, we hypothesize that the primary mechanism of AF maintenance in diseased human hearts is localized intramural reentry anchored to patient-specific microanatomic tracks of varying number, size, and distribution. The intramural microanatomic reentry mechanism may unify the findings from many clinical electrode mapping studies. We are optimistic that better understanding of microanatomic reentrant AF driver “fingerprints” could lead to more reliable identification of these drivers in large cohort of patients and targeted ablation strategies that will effectively treat AF.
Acknowledgments
Sources of Funding: This work was supported by NIH HL115580 and American Heart Association Grant in Aid #16GRNT31010036 (VVF).
Footnotes
Disclosures: Research Grant from Abbott Laboratories (VVF)
References
- 1.Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36:2390–2401. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao J, Hansen BJ, Csepe TA, Lim P, Wang Y, Williams M, Mohler PJ, Janssen PML, Weiss R, Hummel JD, Fedorov VV. Intergration of High-Resolution Optical Mapping and 3-Dimensional Micro-Computed Tomographic Imaging to Resolve the Structure Basis of Atrial Conduction in the Human Heart. Circ Arrhythm Electrophysiol. 2015;8:1514–1517. doi: 10.1161/CIRCEP.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Csepe TA, Hansen BJ, Sul LV, Kalyanasundaram A, Zakharkin SO, Zhao J, Guha A, Van Wagoner DR, Kilic A, Mohler PJ, Janssen PM, Biesiadecki BJ, Hummel JD, Weiss R, Fedorov VV. Adenosine-Induced Atrial Fibrillation: Localized Reentrant Drivers in Lateral Right Atria due to Heterogeneous Expression of Adenosine A1 Receptors and GIRK4 Subunits in the Human Heart. Circulation. 2016;134:486–498. doi: 10.1161/CIRCULATIONAHA.115.021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moe GK, Abildskov JA. Atrial fibrillation as a self-sustained arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 5.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 6.Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–929. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL. Simultaneous Biatrial High-Density (510-512 Electrodes) Epicardial Mapping of Persistent and Long-Standing Persistent Atrial Fibrillation in Patients: New Insights Into the Mechanism of Its Maintenance. Circulation. 2015;132:2108–2117. doi: 10.1161/CIRCULATIONAHA.115.017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintanilla JG, Perez-Villacastin J, Perez-Castellano N, Pandit SV, Berenfeld O, Jalife J, Filgueiras-Rama D. Mechanistic Approaches to Detect, Target, and Ablate the Drivers of Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2016;9:e002481. doi: 10.1161/CIRCEP.115.002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haissaguerre M, Hocini M, Sanders P, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Jonsson A, O'Neill MD, Bordachar P, Reuter S, Roudaut R, Clementy J, Jais P. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006;113:616–625. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 12.Atienza F, Almendral J, Ormaetxe JM, Moya A, Martinez-Alday JD, Hernandez-Madrid A, Castellanos E, Arribas F, Arias MA, Tercedor L, Peinado R, Arcocha MF, Ortiz M, Martinez-Alzamora N, Arenal A, Fernandez-Aviles F, Jalife J. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol. 2014;64:2455–2467. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 13.de Groot N, van der Does L, Yaksh A, Lanters E, Teuwen C, Knops P, van de Woestijne P, Bekkers J, Kik C, Bogers A, Allessie M. Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans. Circ Arrhythm Electrophysiol. 2016;9:e003648. doi: 10.1161/CIRCEP.115.003648. [DOI] [PubMed] [Google Scholar]
- 14.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 15.Zahid S, Cochet H, Boyle PM, Schwarz EL, Whyte KN, Vigmond EJ, Dubois R, Hocini M, Haissaguerre M, Jais P, Trayanova NA. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res. 2016;110:443–454. doi: 10.1093/cvr/cvw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis T, Drury AN, Iliescu CC. Further observations upon the state of rapid re-excitation of the auricles. Heart. 1921;8:311–340. [Google Scholar]
- 17.Schuessler RB, Grayson TM, Bromberg BI, Cox JL, Boineau JP. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res. 1992;71:1254–1267. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- 18.Schuessler RB, Kawamoto T, Hand DE, Mitsuno M, Bromberg BI, Cox JL, Boineau JP. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation. 1993;88:250–263. doi: 10.1161/01.cir.88.1.250. [DOI] [PubMed] [Google Scholar]
- 19.Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988;62:811–832. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- 20.Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei MD, Watras J, Loew LM. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm. 2007;4:1441–1451. doi: 10.1016/j.hrthm.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorov VV, Ambrosi CM, Kostecki G, Hucker WJ, Glukhov AV, Wuskell JP, Loew LM, Moazami N, Efimov IR. Anatomic Localization and Autonomic Modulation of AV Junctional Rhythm in Failing Human Hearts. Circ Arrhythm Electrophysiol. 2011;4:515–525. doi: 10.1161/CIRCEP.111.962258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Csepe TA, Hansen BJ, Fedorov VV. Atrial fibrillation driver mechanisms: Insight from the isolated human heart. Trends Cardiovasc Med. 2016 doi: 10.1016/j.tcm.2016.05.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K, Miller JM. Ablation of Rotor and Focal Sources Reduces Late Recurrence of Atrial Fibrillation Compared With Trigger Ablation Alone: Extended Follow-Up of the CONFIRM Trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) J Am Coll Cardiol. 2014;63:1761–1768. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM, Kalman JM. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- 25.Sommer P, Kircher S, Rolf S, John S, Arya A, Dinov B, Richter S, Bollmann A, Hindricks G. Successful Repeat Catheter Ablation of Recurrent Longstanding Persistent Atrial Fibrillation With Rotor Elimination as the Procedural Endpoint: A Case Series. J Cardiovasc Electrophysiol. 2016;27:274–280. doi: 10.1111/jce.12874. [DOI] [PubMed] [Google Scholar]
- 26.Berntsen RF, Haland TF, Skardal R, Holm T. Focal impulse and rotor modulation as a stand-alone procedure for the treatment of paroxysmal atrial fibrillation: A within-patient controlled study with implanted cardiac monitoring. Heart Rhythm. 2016;13:1768–1774. doi: 10.1016/j.hrthm.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Mohanty S, Gianni C, Mohanty P, Halbfass P, Metz T, Trivedi C, Deneke T, Tomassoni G, Bai R, Al-Ahmad A, Bailey S, Burkhardt JD, Gallinghouse GJ, Horton R, Hranitzky PM, Sanchez JE, Di BL, Natale A. Impact of Rotor Ablation in Non-Paroxysmal AF Patients: Results from a Randomized Trial (OASIS) J Am Coll Cardiol. 2016;68:274–282. doi: 10.1016/j.jacc.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Narayan SM, Krummen D, Donsk A, Swarup V, Miller J. Long-term Freedom From Paroxysmal Atrial Fibrillation by Eliminating Localized Sources without Pulmonary Vein Isolation. Heart Rhythm. 2015;12:S107. Abstract. [Google Scholar]
- 29.Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J, Mandapati R, Ellenbogen KA, Shivkumar K. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: A multicenter experience. Heart Rhythm. 2016;13:636–641. doi: 10.1016/j.hrthm.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller JM, Das MK, Dandamudi G, Jain R, Garlie J, Brewster J. Single-Center Experience with Rotor Mapping and Ablation for Treatment of Atrial Fibrillation in 170 Patients. Heart Rhythm. 2016;13:S116–117. Abstract. [Google Scholar]
- 31.Tomassoni G, Duggal S, Muir M, Hutchins L, Turner K, McLoney AM, Hesselson A. Long-Term Follow-Up of FIRM-Guided Ablation of Atrial Fibrillation: A Single-Center Experience. The Journal of Innovations in Cardiac Rhythm Management. 2015;6:2145–2151. [Google Scholar]
- 32.Rashid H, Sweeney A. Approaches for Focal Impulse and Rotor Mapping in Complex Patients: A US Private Practice Perspective. The Journal of Innovations in Cardiac Rhythm Management. 2015;6:2193–2198. [Google Scholar]
- 33.Tilz RR, Lin T, Rillig A, Scholz L, Heeger CH, Metzner A, Mathew S, Wissner E, Ouyang F, Kuck KH. Nine Months Outcomes Following Focal Impulse and Rotor Modulation for the Treatment of Atrial Fibrillation using the Novel 64-Electrode Basket Catheter. Europace. 2015;17:P192. Abstract. [Google Scholar]
- 34.Yoakum SH, Greer E, Cooper D, Nowaczyk J, Hildreth J, Jaynes C, Moyeda A, Hopper K, Krummen DE, Narayan SM. Patient-Tailored Rotor Ablation For Persistent Atrial Fibrillation Reduces Recurrence In The Blanking Period Compared To Trigger-Based Ablation Alone. Heart Rhythm. 2014;11:S107. Abstract. [Google Scholar]
- 35.Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone C, Smith PK, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:406–426. [PubMed] [Google Scholar]
- 36.Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 37.Schuessler RB, Kay MW, Melby SJ, Branham BH, Boineau JP, Damiano RJ., Jr Spatial and temporal stability of the dominant frequency of activation in human atrial fibrillation. J Electrocardiol. 2006;39:S7–12. doi: 10.1016/j.jelectrocard.2006.04.009. Abstract. [DOI] [PubMed] [Google Scholar]
- 38.de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 39.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr, Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigo M, Guillem MS, Climent AM, Pedron-Torrecilla J, Liberos A, Millet J, Fernandez-Aviles F, Atienza F, Berenfeld O. Body surface localization of left and right atrial high-frequency rotors in atrial fibrillation patients: a clinical-computational study. Heart Rhythm. 2014;11:1584–1591. doi: 10.1016/j.hrthm.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayan SM, Zaman JA. Mechanistically based mapping of human cardiac fibrillation. J Physiol. 2016;594:2399–2415. doi: 10.1113/JP270513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verheule S, Eckstein J, Linz D, Maesen B, Bidar E, Gharaviri A, Schotten U. Role of endo-epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog Biophys Mol Biol. 2014;115:173–185. doi: 10.1016/j.pbiomolbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Waldo AL, Henthorn RW. Use of transient entrainment during ventricular tachycardia to localize a critical area in the reentry circuit for ablation. Pacing Clin Electrophysiol. 1989;12:231–244. doi: 10.1111/j.1540-8159.1989.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 44.Asirvatham SJ, Stevenson WG. Editor's perspective: Reentry, pseudo-reentry, and pseudo-pseudo-reentry. Circ Arrhythm Electrophysiol. 2014;7:557–558. doi: 10.1161/CIRCEP.114.001675. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J, Kharche SR, Hansen BJ, Csepe TA, Wang Y, Stiles MK, Fedorov VV. Optimization of catheter ablation of atrial fibrillation: insights gained from clinically-derived computer models. Int J Mol Sci. 2015;16:10834–10854. doi: 10.3390/ijms160510834. [DOI] [PMC free article] [PubMed] [Google Scholar]