Abstract
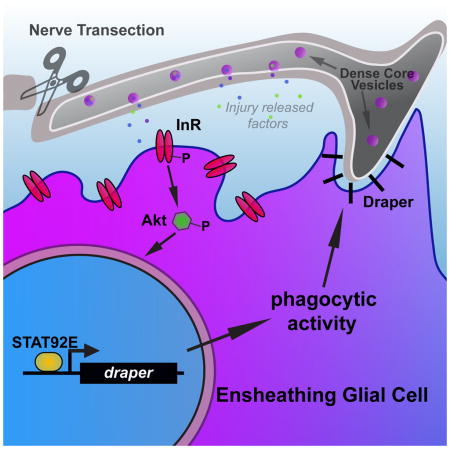
Neuronal injury triggers robust responses from glial cells, including altered gene expression and enhanced phagocytic activity to ensure prompt removal of damaged neurons. The molecular underpinnings of glial responses to trauma remain unclear. Here, we find that the evolutionarily conserved Insulin-like signaling (ILS) pathway promotes glial phagocytic clearance of degenerating axons in adult Drosophila. We find that the Insulin-like Receptor (InR) and downstream effector Akt1 are acutely activated in local ensheathing glia after axotomy, and are required for proper clearance of axonal debris. InR/Akt1 activity is also essential for injury-induced activation of STAT92E and its transcriptional target draper, which encodes a conserved receptor essential for glial engulfment of degenerating axons. Increasing Draper levels in adult glia partially rescues delayed clearance of severed axons in glial InR-inhibited flies. We propose that ILS functions as a key post-injury communication relay to activate glial responses, including phagocytic activity.
eTOC
Musashe et al. find that the Insulin-like signaling (ILS) pathway is stimulated in local glia after nerve axotomy thus upregulating expression of the engulfment receptor gene draper in a STAT92E-dependent manner. Activation of the ILS/Draper pathway is required for proper glial clearance of degenerating axonal debris.
INTRODUCTION
Glial cells are highly sensitive to changes in neuronal health and respond swiftly to diverse forms of neural trauma. Acute injury triggers robust responses in glia, including altered gene expression, glial infiltration of damaged areas and glial clearance of degenerating neurons through phagocytic engulfment (Logan and Freeman, 2007; MacDonald et al., 2006; Napoli and Neumann, 2009). Many of these reactive glial responses are neuroprotective. Impaired glial clearance of damaged axons and myelin can inhibit axonal regeneration after neural injury and provoke inflammation (Akiyama et al., 2000; Bamberger and Landreth, 2002; Glezer et al., 2007). Finally, there is growing evidence that defects in glial immunity are coupled to the onset and progression of numerous neurodegenerative diseases (Giunti et al., 2014; Hamby and Sofroniew, 2010; Scuderi et al., 2013). As a result, there is intense interest in elucidating the molecular and cellular features of glial immunity that govern how glia sense and respond to neuronal stress and damage, including the extrinsic injury cues that rapidly activate robust intrinsic immune response programs in glia.
Drosophila is a powerful model for investigating fundamental aspects of neurodegeneration and glial responses to neural damage (Ayaz et al., 2008; Cantera and Barrio, 2015; Etchegaray et al., 2016; Fang and Bonini, 2012; Freeman et al., 2003; Kato et al., 2011; Lee and Sun, 2015; Logan and Freeman, 2007; Liu et al., 2015; Mishra et al., 2013; Rooney and Freeman, 2014; Ugur et al., 2016). Axotomy in adult flies elicits glial reactions that share many cellular hallmarks with mammalian glia responding to neurodegeneration, including altered morphology and increased phagocytic function (Kurant 2011; Logan and Freeman, 2007). Notably, conserved molecules essential for glial engulfment activity exist are conserved, including the Draper/MEGF10 receptor, which signals through a tyrosine based-activation motif (ITAM)/Src tyrosine kinase signaling cascade to regulate phagocytic removal of degenerating axons and apoptotic neurons (Chung et al., 2013; Logan et al., 2012; MacDonald et al., 2006; Scheib et al., 2012; Wu et al., 2009). In Drosophila, olfactory nerve axotomy triggers upregulation of Draper in local ensheathing glial cells, which is essential for efficient glial clearance of axonal debris (Doherty et al., 2014; Logan et al., 2012; MacDonald et al., 2006), but little is known about the upstream signals that trigger upregulation of Draper or other innate immune genes in responding glia.
The insulin-like signaling (ILS) pathway is a critical regulator of energy homeostasis, cellular growth and cell survival during embryonic and postnatal development (Barbieri et al., 2003; Broughton and Partridge, 2009; Puig and Mattila, 2011). This highly conserved pathway is initiated by activation of the insulin receptor (IR) or insulin-like growth factor (IGF) receptors in vertebrates and the homologous Insulin-like Receptor (InR) in Drosophila to trigger downstream signaling cascades that modulate a range of molecular and cellular events, including transcription, translation and autophagy. ILS is essential for development, but there is growing evidence that ILS also plays important roles in the mature brain. Boosting serum IGF-1 levels protects against various insults, including kainic acid injection (Maltiadous et al., 2010) and hypoxic-ischemic injury (Guan et al., 1993). Conversely, pharmacological inhibition of IGF-1 results in greater neuronal death after trauma (Carro et al., 2003; Guan et al., 2003), as well as faster cognitive decline in neurodegenerative disease models (Carro et al., 2006; Torres-Aleman, 2007). ILS may support functional integrity and survival of neurons in diverse contexts, but the mechanisms of ILS-mediated neuroprotection are not well understood.
Here we show that activity of the insulin-like receptor (InR) and the downstream effector Akt1 are upregulated in ensheathing glia following olfactory nerve axotomy in Drosophila and are required for proper glial clearance of degenerating axonal debris. Inhibition of glial InR inhibits STAT92E-dependent transcriptional upregulation of draper after axon injury and also prevents recruitment of the Draper receptor to degenerating axons. We also show that dense core vesicle release from severed axons may be required for proper InR activation in local ensheathing glia. Our results implicate ILS as key signaling relay that promotes glial engulfment activity in response to axotomy through activation of STAT92E/Draper.
RESULTS
Glial InR signaling is required for glial phagocytic clearance of severed axons in the adult Drosophila brain
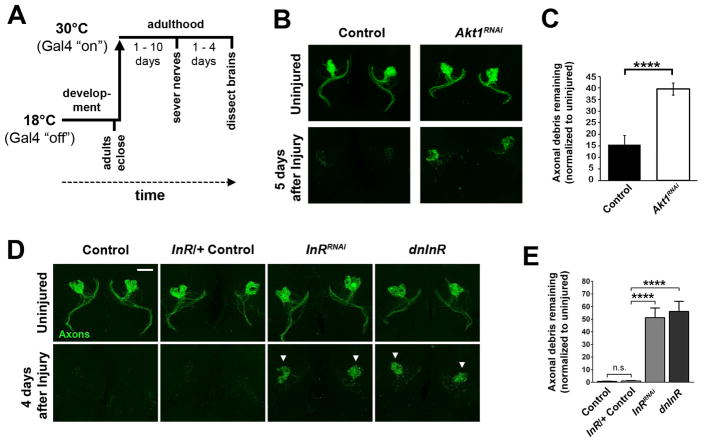
In an ongoing in vivo screen to identify genes required for glial engulfment of degenerating axons (Table S1), we found that RNA interference (RNAi) against the serine/threonine kinase Akt1 (UAS-Akt1RNAi) in adult glia inhibited glial clearance of severed olfactory receptor neuron axons. We used flies that expressed membrane-tethered GFP in a subset of maxillary palp olfactory receptor neurons (ORNs) (OR85e-mCD8::GFP), as well as the pan-glial driver repo-Gal4. We also temporally restricted activity of Gal4 to adult post-mitotic glia by expressing Gal80ts under the control of tubulin (tubulin-Gal80ts) (McGuire et al., 2003) so as not to perturb glial development (see Figure 1A). Maxillary nerve axotomy triggers Wallerian degeneration of maxillary nerve ORN axons. Local ensheathing glia clear degenerating OR85e maxillary axons within ~48 hours (MacDonald et al., 2006). Expression of AktRNAi in adult glia significantly inhibited glial engulfment of OR85e axonal debris (Figure 1B, C). We screened candidate RNAi lines against receptors known to signal via Akt-dependent cascades and discovered that knockdown of the Insulin-like Receptor (InR) with UAS-InRRNAi phenocopied the Akt1RNAi clearance phenotype. Significantly more GFP+ axonal debris was present in glial InRRNAi-expressing brains 4 days after axotomy (white arrowheads in Figure 1D and 1E). To complement our RNAi analysis, we independently inhibited InR activity by expressing a dominant negative version (UAS-dnInR) (Demontis and Perrimon, 2009) and similarly observed impaired glial clearance of GFP+ debris post-injury (Figure 1D and 1E). These manipulations were performed in a InR heterozygous mutant background (InRex15) (Song et al., 2003), since it is well established that numerous compensatory mechanisms exist to strengthen ILS activity when inhibited (Ferguson et al., 2012; Kannan et al., 2013; Marr et al., 2007). Efficacy of Gal80ts/Gal4 temporal regulation was confirmed by testing each genotype maintained at 18°C (Figure S1A–D). Importantly, we also confirmed that delayed glial clearance of OR85e axonal debris was rescued in glial Akt1RNAi and glial InRRNAi animals with expression of UAS-Akt1 (Wang et al., 2011) or UAS-InR (Martin-Pena et al., 2006) (Figure S1E–H). Together, these results reveal that InR/Akt1 activity is required in adult glia for proper phagocytic clearance of degenerating axons after injury.
Figure 1. InR/Akt1 is required in adult glia for proper glial clearance of degenerating axons.
(A) Schematic of experimental method to temporally control UAS-driven gene expression. (B) Maximum intensity projections of the antennal lobe region show axons of OR85e ORNs expressing membrane-tethered GFP (green). (C) Quantification of GFP+ axonal material shown in B. N ≥ 18 for each genotype and condition. (D) Confocal projections of OR85e axons in control, InRRNAi or dominant negative InR (dnInR)-expressing flies. White arrowheads point to persistent axonal debris post-injury. (E) Quantification of GFP shown in B. N ≥ 22 for each genotype and condition. ****p < 0.0001. Pooled data plotted as mean ± SEM. All image scale bars represent 20 μm. Genotypes: Control (OR85e-mCD8::GFP, tub-Gal80ts/+; repo-Gal4/+), dnInR (OR85e-mCD8::GFP, tub-Gal80ts/+; repo-Gal4, InRex15/UAS-Dominant Negative InR), InRRNAi (OR85e-mCD8::GFP, tub-Gal80ts/+; repo-Gal4, InRex15/UAS-InRRNAi), AktRNAi (OR85e-mCD8::GFP, tub-Gal80ts/+; repo-Gal4, InRex15/UAS-AktRNAi). See Figure S1.
InR and Akt1 activity is increased in local glia after ORN axotomy
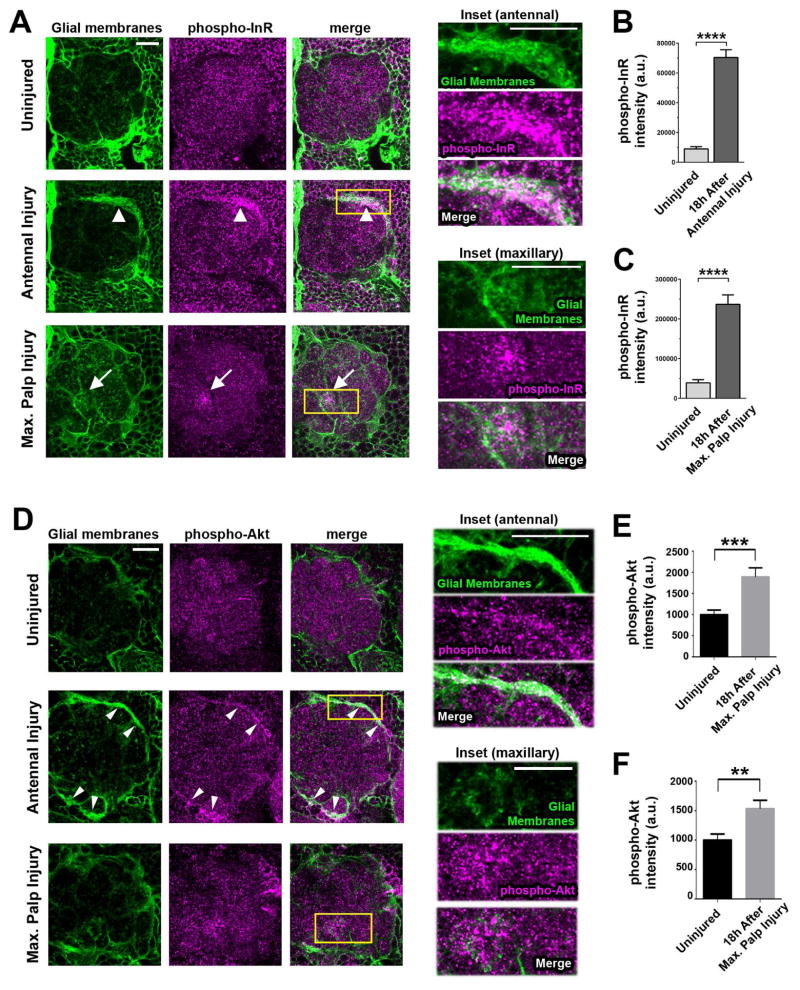
Basal changes in ILS can alter the metabolic state of cells but activation of ILS cascades also serve as local signaling relays to intrinsically alter events such as transcription, translation and/or or cell motility (Fernandez and Torees-Aleman, 2012; Gu et al., 2014; Hua et al., 2009; Luckhart and Riehle, 2007). To determine if InR activity is altered in local glia following olfactory nerve axotomy, we took advantage of a well-characterized phospho-specific InR antibody that recognizes the activated form of Drosophila InR (Root et al., 2008). We used flies that transgenically expressed membrane-tethered GFP in glia, performed bilateral antennal nerve axotomy and then stained brains with anti-phospho-InR and anti-GFP at various time points after axotomy. Within 24 hours, glial cells surrounding the antennal lobes expand their membranes as they invade the antennal neuropil (MacDonald et al., 2006) (Figure 2A). We also observed a striking increase in phospho-InR signal on these glial membranes (Figure 2A and 2B). Maxillary nerve axotomy severs a smaller cohort (~20%) of ORNS that project into the antennal lobes, but this still elicited a significant increase in phospho-InR signal at sites where glial membranes were accumulating on degenerating maxillary axons (Figure 2A and 2C). Similarly, we detected a significant increase in phosphorylated Akt1 (phospho-Akt1) levels in ensheathing glia responding to antennal or maxillary nerve axotomy (Figure 2D–F). Our findings suggest that InR/Akt1 activity increases in activated antennal lobe glia within one day after axon injury, supporting the notion that the ILS pathway functions as a local injury communication cascade required for proper innate glial immune responses to axon degeneration.
Figure 2. InR and Akt1 are acutely activated in local glia responding to axon injury.
(A) Single confocal slice images of the antennal lobe region in uninjured, antennal nerve axotomized and maxillary nerve axotomized animals. Phospho-InR signal (magenta) overlaps with GFP-labeled glial membranes (green) expanding after antennal nerve axotomy (white arrowheads) or accumulating on severed maxillary glomeruli post-maxillary nerve axotomy (white arrowheads). Insets: magnified view of outlined area (yellow rectangle). (B) Phospho-InR signal in the dorsal half of antennal lobes was quantified after computationally segmenting to GFP (antennal injury in A). N ≥ 20 for each condition. (C) Quantification of phospho-InR signal in OR85e-innervated glomeruli in A (maxillary injury). N ≥ 16 for each condition. (D) Single confocal slice images of antennal lobes. Phospho-Akt (magenta) intensity is increased in ensheathing glia (green) responding to antennal (white arrowheads, middle panels) or maxillary nerve injury (lower panels). Insets: magnification of yellow boxed areas. (E) Quantification of phospho-Akt signal in dorsal antennal lobes after computationally segmenting to GFP. N ≥ 18 for each condition. (F) Quantification of phospho-Akt signal in OR85e-innervated glomeruli in D (bottom panels). N ≥ 20 for each condition. **p<0.01, ***p<0.001, ****p < 0.0001. Pooled data plotted as mean ± SEM. All image scale bars represent 20 μm. Genotypes: UAS-mCD8::GFP, repo-Gal4/+. See Figure S2.
InR signaling is required for axotomy-induced upregulation and recruitment of glial Draper to injury sites
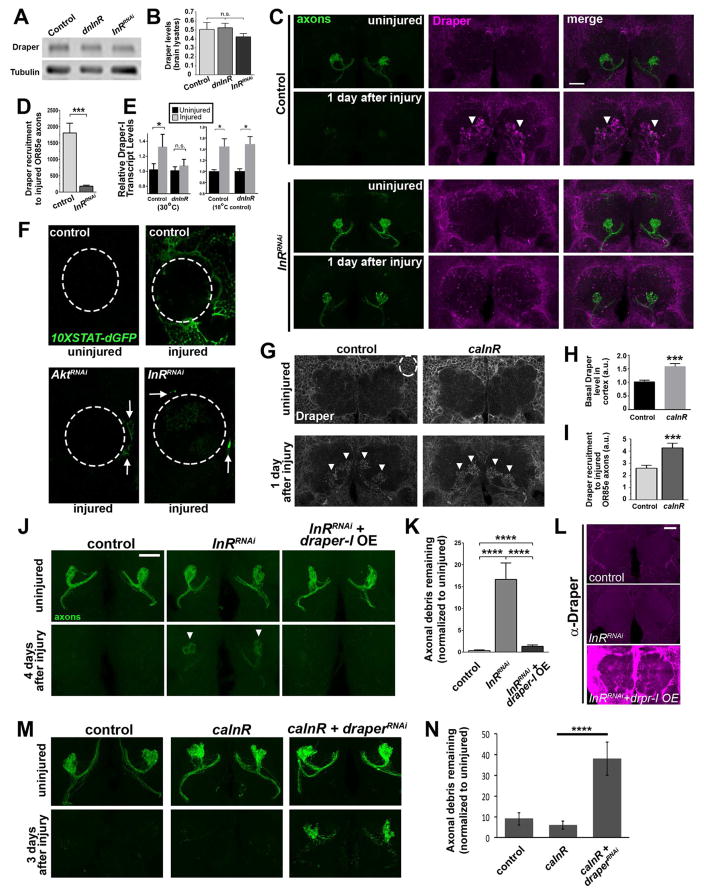
The Draper receptor is essential for glial clearance of severed ORN axons (Logan et al., 2012; MacDonald et al., 2006). draper is transcriptionally upregulated in local glia within hours after injury, and ~24 hours later, robust accumulation of Draper protein is visible on olfactory glomeruli that contain degenerating axons (Doherty et al. 2014, Logan et al., 2012; MacDonald et al., 2006). We wondered if InR signaling might regulate phagocytic activity of glia by influencing Draper levels. First, we compared basal levels of Draper in central brain lysates of control flies compared to dnInR or InRRNAi-expressing flies and found no difference in basal Draper levels (Figure 3A and 3B). Instead, we found that Draper accumulation on degenerating OR85e axons was dramatically reduced one day after maxillary nerve injury when adult glia were depleted of InR (arrowheads in Figure 3C and 3D). This phenotype did not result from glial cell death as we detected no change in the number of Repo+ nuclei in the central brain region following 10 days of InRRNAi expression (Figure S3A, B), and gross glial morphology appeared normal when InR activity was inhibited in adult glia for 10 days (Figure S3C). Finally, to confirm specificity of our RNAi constructs, we confirmed that reduced Draper recruitment was reversed in glial InRRNAi and Akt1RNAi following expression of UAS-InR or UAS-Akt1, respectively (Figure S4B–E).
Figure 3. Glial InR signaling positively regulates Draper expression and Draper recruitment to severed axons.
(A) Western blot of fly brain lysates. (B) Quantification of normalized Draper in A. (C) Representative images of adult antennal lobes. Brains stained with Draper (magenta) and GFP to visualize OR85e axons (green). Robust Draper accumulation typically observed post-injury (white arrowheads) is attenuated following glial InRRNAi expression. (D) Volumetric quantification of Draper fluorescence intensity on injured OR85e axons (arrowheads) in C. (E) Q-PCR analysis of draper-I transcript in central brains. 2−ΔΔCt ± SEM values are plotted. N= 6 independent samples of pooled animals for each time point and genotype. (F) Images of single antennal lobes (dotted line) show activation of of 10XSTAT92E-dGFP (destabilized GFP) reporter 18 hours after antennal nerve axotomy in control animals and glial AktRNAi or InRRNAi -expressing flies. (G) Antennal lobe region of uninjured brains (single confocal slice) and 1 day post axotomy (maximum intensity projection) immunostained for Draper. (H) Volumetric quantification of basal Draper levels in the cortex region of uninjured flies (ROI shown with white dotted circle in panel G). (I) Volumetric quantification of Draper accumulation on injured maxillary palp glomeruli (see arrowheads in G). (J) Glial overexpression (OE) of Draper-I reverses glial clearance defects in InR-depleted animals. Representative Z-stack projections of OR85e-innervated glomeruli show GFP+ OR85e axons. (K) Quantification of GFP in J. (L) Images of each experimental genotype shown in J immunostained for Draper. (M) OR85e axons (green) before and after axotomy. (N) Quantification of GFP+ OR85e axonal debris in M. Genotypes in A–E: control (OR85e-mCD8::GFP, tub-Gal80ts/+; repo-Gal4/+), dnInR (OR85e-mCD8::GFP, tub-Gal80ts/+; repo-Gal4, InRex15/UAS-Dominant Negative InR), InRRNAi (OR85e-mCD8::GFP, tub-Gal80ts/+; repo-Gal4, InRex15/UAS-InRRNAi). F: control (10XSTAT92E-dGFP/+; repo-Gal4/+), AktRNAi (10XSTAT92E-dGFP/+; repo-Gal4/UAS-AktRNAi), InRRNAi (10XSTAT92E-dGFP/+; repo-Gal4/UAS-InRRNAi). G–I: control (+/tub-Gal80ts/+; repo-Gal4), caInR (UAS-caInR/ tub-Gal80ts/+; repo-Gal4). J–L: control, InRRNAi (OR85e-mCD8::GFP, tub-Gal80ts/UAS-LacZ; repo-Gal4, InRex15/UAS- InRRNAi), InRRNAi + draper-I OE (OR85e-mCD8::GFP, UAS-Draper-I/tub-Gal80ts; repo-Gal4, InRex15/UAS- InRRNAi). M, N: control (OR85e-mCD8::GFP/+; repo-Gal4/+). caInR (OR85e-mCD8::GFP/UAS-LacZ::NLS, UAS-caInR; repo-Gal4/+). caInR+draperRNAi (OR85e-mCD8::GFP/UAS-draperRNAi, UAS-caInR; repo-Gal4/+). *p < 0.05; ***p < 0.001. ****p< 0.0001. All pooled data are represented as mean ± SEM. All scale bars represent 20 μm. N ≥ 18 for each condition. See Figure S3 and S4.
Next, we performed quantitative PCR for draper-I transcript on dissected central brains from uninjured flies and 3 hours post- axotomy. There are 3 predicted splice variants of draper, Draper-I is the activating form essential for glial phagocytic activity in adults (Logan et al., 2012). As previously reported (Logan et al. 2012), draper-I was significantly increased after antennal nerve axotomy in control animals; however, draper-I was not upregulated in dnInR-expressing flies (Figure 3E). Surprisingly, basal levels of draper-I transcript were not altered by inhibition of glial InR (2^−(dCt) values in uninjured control and uninjured dnInR were 0.01908 +− 0.00155 and 0.01614 +− 0.000859, respectively; p=0.22). The Draper promoter contains several binding sites for the STAT92E transcription factor, which are required for injury-induced upregulation of draper (Doherty et al 2014). Activation of a 10XSTAT-dGFP in vivo reporter, which contains 10 tandem STAT92E binding sites driving expression of destabilized GFP (dGFP), mirrors Draper upregulation in local ensheathing glia following olfactory nerve axotomy (Doherty et al 2014). Consistent with our finding that ILS is required to upregulate draper in glia after axon injury, we found that activation of the 10XSTAT-dGFP reporter was largely inhibited 24 hours post-axotomy in adult glia depleted of InR or Akt1 (Figure 3F). Together, these results suggest that basal InR signaling does not appreciably influence glial Draper levels at the transcriptional or protein level, but instead is essential for activation of a STAT92E-dependent transcriptional program to upregulate the phagocytic receptor gene draper at times when glial phagocytic loads are high.
Activation of glial InR is sufficient to raise Draper levels
To determine if activation of the ILS could alter Draper expression levels in adult glia, we used tubulin-Gal80ts combined with the repo-Gal4 driver to express a constitutively active form of the InR (UAS-InR.del, referred to below as caInR) (Werz et al., 2009) specifically in adult glia for 24 hours prior to and after maxillary nerve axotomy (by shifting flies to 30°C). We found that, even in the absence of injury, expression of caInR resulted in significantly higher levels of basal Draper in the central brain region (p<0.001) (Figure 3G and 3H) and significantly higher levels of Draper on maxillary palp glomeruli that contained degenerating axons one day after axotomy (p<0.001) (Figure 3G and 3I), further supporting InR signaling as a positive regulator of Draper in adult glial cells.
Forced expression of draper reverses glial clearance defects in InR knockdown animals
Upregulation of innate immunity factors, including engulfment receptors, occurs in many activated phagocytes; this provides a positive feedback loop that ensures an adequate number of receptors are available to recognize engulfment targets and efficiently shuttle them through phagolysosomal destruction pathways (Kingsolver et al., 2013; A-Gonzalez & Hidalgo, 2014; Doherty et al., 2014). Since our findings indicated that InR is required for glial cells to upregulate Draper as phagocytic demands increase in response to local axon degeneration, we wondered if boosting glial Draper levels might reverse axon clearance defects in InR/Akt1-inhibited flies. We expressed UAS-InRRNAi and UAS-draper-I in adult glia under the control of repo-Gal4 and tubulin-Gal80ts in flies that also carried OR85e-mCD8::GFP. Notably, overexpression of Draper-I in a glial InRRNAi background partially restored clearance of degenerating axons 4 days post-axotomy (p<0.0001) (Figure 3J–L). Conversely, we also used a well-characterized RNAi transgenic line to knockdown Draper (UAS-DraperRNAi) in glial cells (MacDonald et al., 2006; Doherty et al., 2009; Logan et al., 2012) while also overexpressing caInR and then assessed clearance of severed OR85e axons. Notably, we found that inhibiting Draper expression was sufficient to block clearance of axonal debris, despite activating glial ILS (p<0.0001) (Figure 3M and 3N). Taken together, our data suggest that glial InR/Akt1 activity promotes proper phagocytic clearance of damaged axons through STAT92E-dependent transcription of the engulfment receptor Draper.
ILS is required in adult ensheathing glia and not astrocytes for proper clearance of ORN axonal debris
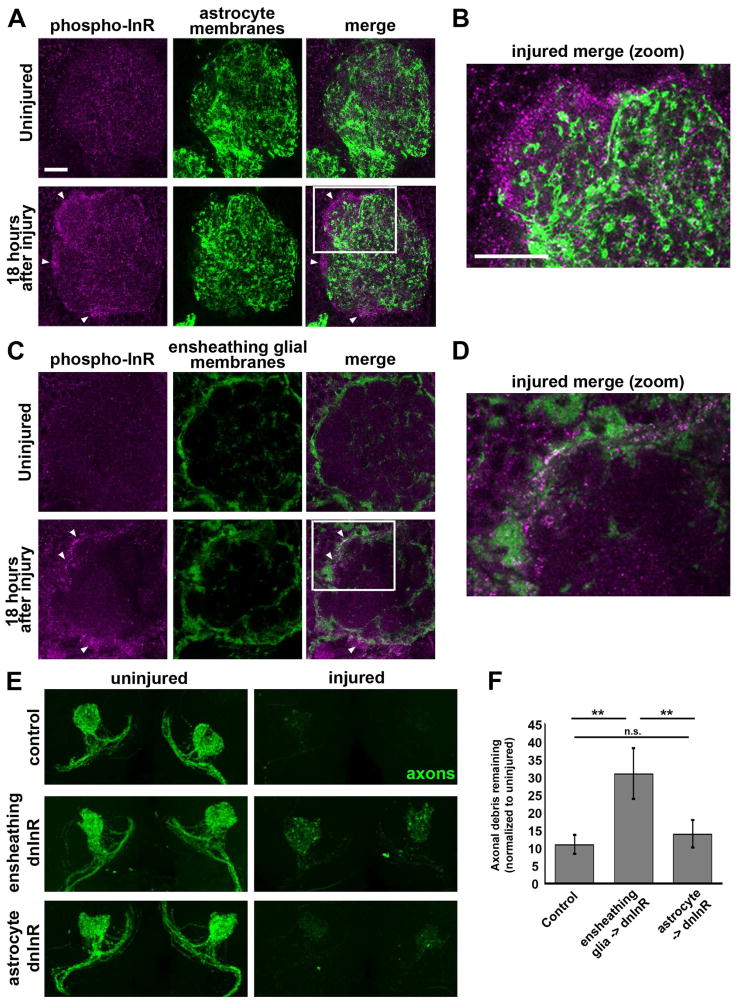
In the adult CNS, neuropil regions, including the antennal lobes, contain two major glial subtypes: ensheathing glia, which enwrap projections, and astrocytes (Awasaki et al., 2008; Doherty et al., 2009; Edwards and Meinertzhagen, 2010; Omoto et al., 2015). Draper is expressed in ensheathing glia, as well as the cell body glia that surround neuronal cell bodies in the cortex of the brain, but is not detectable in adult astrocytes (Doherty et al., 2009). To determine if ILS activity is increased in ensheathing glia and/or astrocytes following ORN axotomy, we expressed membrane-tethered GFP (UAS-mCD8::GFP) in astrocytes with alrm-Gal4 (Doherty et al., 2009) or in ensheathing glia with TIFR-Gal4 (Yao et al., 2007; Ziegenfuss et al., 2012) and immunostained for phospho-InR 18 hours after antennal nerve axotomy. We found that the increased phospho-InR signal largely overlapped with ensheathing glial membranes and not astrocytic membranes (white arrowheads, Figure 4A, B). Next, we expressed dnInR in each subtype and assessed clearance of GFP-labeled OR85 axons after maxillary nerve axotomy and, importantly, found that significantly more axonal debris persisted following ensheathing glial, but not astrocyte, expression of dnInR (Figure 4E and 4F). Together, these results indicate that InR activity is specifically required in ensheathing glia and may be selectively increased in this particular glial subtype after axon injury to drive Draper-dependent engulfment of degenerating axons in the adult brain.
Figure 4. InR is required in ensheathing glia, not astrocytes, for proper clearance of degenerating axonal debris.
(A) Representative confocal images of single antennal lobes. Brains immunostained with anti-phospho-InR (magenta) reveal little overlap between increased InR activity (white arrowheads) and astrocyte membranes labeled with mCD8::GFP (green). (B) Zoomed image of region outlined in A (white box). (C) Representative confocal images of single antennal lobes. Brains immunostained with anti-phospho-InR (magenta) reveal overlap between increased InR activity (white arrowheads) and ensheathing glial membranes labeled with mCD8::GFP (green). (D) Zoomed image of region outlined in C (white box). (E) Maximum intensity projections of OR85e axons. (F) Quantification of GFP+ OR85e axonal debris in E. **p < 0.01. N ≥ 16 for each condition. Scale bar =20 μm. Pooled data plotted as mean ± SEM. Genotypes: A, B: (UAS-mCD8::GFP/+; alrm-Gal4/+). C, D: (UAS-mCD8::GFP/+; TIFR-Gal4/+), InRRNAi. E, F: control (OR85e-mCD8::GFP, tub-Gal80ts/+; InRex15/+), ensheathing dnInR (OR85e-mCD8::GFP, tub-Gal80ts/+; TIFR-Gal4, InRex15/UAS-Dominant Negative InR), astrocyte dnInR (OR85e-mCD8::GFP, tub-Gal80ts/+; alrm-Gal4, InRex15/UAS-Dominant Negative InR).
Dense core vesicles are distributed along adult olfactory receptor neuron axons
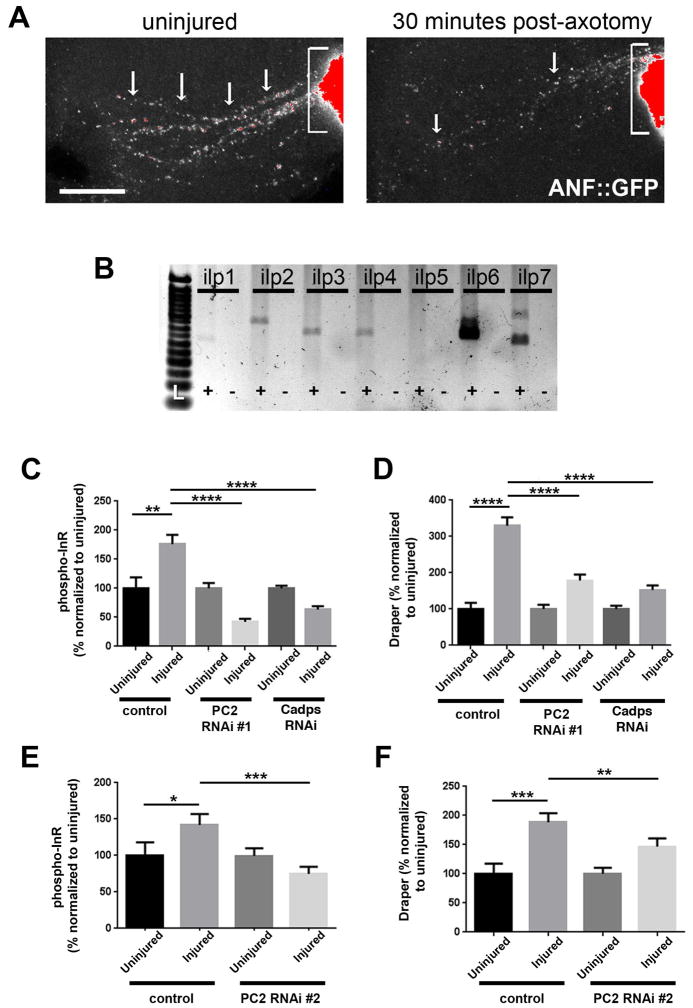
The Drosophila InR can be activated by eight unique insulin-like peptide (ilp) ligands (ilp1-ilp8) (Garelli et al., 2012; Gronke et al., 2010). Several neuropeptides are expressed in adult antennal lobes (Carlsson et al., 2010), and transcripts for ilp6 and ilp8 have been detected in adult ORNs (Menuz et al., 2014). Moreover, ilp4 is detectable by immunostaining in the adult brain, including the antennal lobes (Gronke et al., 2010). We wondered if severed ORN axons might release ilps to activate InR on ensheathing glia. First, we employed a common strategy to visualize neuropeptide distribution in ORN axons before and after axotomy. Neuropeptides, including ilps, are packaged into dense core vesicles (DCVs) for release. Atrial natriuretic factor (ANF)-tagged to GFP (ANF::GFP) is loaded into and released from DCVs along with endogenous neuropeptides and reductions in ANF::GFP-positive vesicles track with high levels of neuropeptide release (Husain and Ewer, 2004; Rao et al., 2001). We used OR22a-Gal4 to express ANF::GFP in antennal ORNs and imaged uninjured and injury brains. ANF::GFP was highly enriched at synaptic terminals in OR22a glomeruli (brackets, Figure 5A). We also observed discrete GFP+ puncta along the length of the axons (arrows). We observed a 65% reduction in ANF::GFP+ vesicles along OR22a axonal tracts 30 minutes after antennal nerve axotomy (Figure 5A), suggesting that DCVs are released from injured axons shortly after injury.
Figure 5. Knockdown of PC2 or Cadps in olfactory neurons attenuates phospho-InR activation and Draper upregulation in ensheathing glia.
(A) GFP-tagged atrial natriuretic factor (ANF::GFP) neuropeptide distribution along uninjured OR22a axons (white arrows). Grayscale converted confocal images (red represents maximum intensity) are shown. Fewer ANF::GFP+ puncta (65%) are observed 30 minutes after antennal nerve axotomy. Synaptic rich OR22a glomeruli are highlighted with brackets. Genotype: y, w, UAS-ANF::GFP/+; OR22a-Gal4/+; N>15 brains for each condition. Scale bar =20 microns. (B) PCR analysis of ilp1–7 ligands in w1118 adult third antennal segments. +/− correspond to + and − RT samples. L= molecular ladder. (C, E) Quantification of phosphor-InR in dorsal antennal lobe ensheathing glia 18 hrs after antennal nerve axotomy. N ≥ 18 for each condition. (D, F) Quantification of Draper levels in dorsal antennal lobe ensheathing glia 18 hrs after antennal nerve axotomy. N ≥ 21 for each condition. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Pooled data plotted as mean ± SEM. Genotypes in B–E: control (UAS-dicer2/+; orco-Gal4/+), PC2 RNAi #1 (UAS-dicer2/UAS-PC2RNAi VDRC 110788; orco-Gal4/+); PC2 RNAi #2 (UAS-dicer2/+; orco-Gal4/UAS-PC2RNAi Bl28583); CadpsRNAi (UAS-dicer2/UAS-CadpsRNAi VDRC 110055; orco-Gal4/+).
amontillado and Cadps are required in ORNs are for glial InR activation and Draper upregulation after injury
To identify additional ilps (insulin-like peptides) expressed in adult ORNs, we performed RT-PCR for ilps1–7 on RNA isolated from the third antennal segments of adult Drosophila, which houses all of the antennal ORN cell bodies (~1,200). We detected transcripts for all 7 ilps, with the exception of ilp5 (Figure 5B). Next, we knocked down the expression of each ilp ligand in ORNs by expressing dsRNA with the pan-ORN driver orco-Gal4 (Larsson et al., 2014). Although we observed a trend towards attenuated Draper upregulation after olfactory nerve axotomy in some genotypes, these results were not statistically significantly (not shown).
Notably, there are robust compensatory mechanisms in place to boost the activity of ILS in flies when suppressed. One notable strategy is to raise the levels of one or more ilp ligands when the expression of other ilps is genetically inhibited (Broughton et al, 2008; Sousa-Nunes et al 2011; Wigby et al, 2011). Thus, we took an alternative approach to broadly inhibit ilps/neuropeptides in ORNs. Ilps, like many neuropeptides, are initially formed as inactive precursors, which require proteolytic processing to form biologically active ligands. We tested two different RNAi lines that target unique regions of subtilisin-like proprotein convertases 2 (PC2), also known as amontillado in flies, which is involved in ilp proprotein cleavage (Rayburn et al., 2003; Rhea et al., 2010). We expressed each RNAi (Bl 28583 or VDRC 110788) under the control of orco-Gal4 and tubulin-Gal80ts to temporally restrict Gal4 activity in adult ORNs. We found that expression of PC2RNAi significantly inhibited phospho-InR increases and Draper upregulation in ensheathing glial regions after antennal nerve axotomy (Figure 5C–F). Next, we targeted calcium-activated protein for secretion (Cadps), which is essential for DCV release (Renden et al., 2001; Wong et al., 2012; Wong et al., 2015). We expressed CadpsRNAi (VDRC 110055) in all ORNs with orco-Gal4 and found that phospho-InR and Draper increases after axon injury were also attenuated (Figure 5C, D). Collectively, these results suggest that severed ORNs may be an important source of secreted neuropeptide signals that elicit InR-dependent glial responses to axon degeneration.
DISCUSSION
Here, we show that the insulin-like signaling (ILS) pathway required for two key events associated with glial responses to acute axon injury in the adult Drosophila brain: upregulation of the conserved engulfment receptor Draper and efficient phagocytic engulfment of degenerating axons. Previous studies ranging from invertebrates to humans have linked ILS and innate immunity. In Drosophila, the ILS pathway is an important homeostatic regulator of hemocyte responses to injury. Similar to our findings, repression of ILS inhibits responses to epidermal damage, including hemocyte recruitment to injury sites (Karpac et al., 2011). IGF-1 treatment of human phagocytes in vitro also boosts phagocytic activity. For example, in cultured neutrophils, IGF-1 induces upregulation of CD11b, a component of complement receptor 3, which functions as a widespread phagocytic pattern recognition receptor to facilitate recognition and engulfment of bacteria and protein aggregates (Bierknes and Aarskog, 1995; Inoue et al., 1998). Our work now provides in vivo evidence that ILS positively drives phagocytosis by regulating expression of a key pro-engulfment factor and suggests that this is an evolutionarily conserved signaling mechanism to regulate both professional and unprofessional phagocytes.
Maintenance of basal Draper levels requires phosphatidylinositol-3 kinase (PI3K) signaling (Doherty et al., 2014). Although the InR can signal through PI3K, we did not detect a significant reduction in basal Draper levels (at the transcript or protein level) following InR knockdown/inhibition in adult glia, which argues against InR as the primary upstream receptor maintaining basal Draper expression via PI3K. Instead, we find that glial InR is required for transcriptional induction of draper after axotomy and for recruitment of Draper on severed axons, revealing the InR to be an upstream regulator of Draper expression in the context of neural injury. The transcription factor STAT92E is similarly required to boost Draper levels in local ensheathing glia post-injury through a positive Draper/STAT92E autoregulatory loop (Doherty et al., 2014). Interestingly, in addition to the highly conserved canonical PI3K/Akt cascade, STAT has also been identified as a signaling effector of the Insulin Receptor and the IGF-1 receptor (Himpe and Kooijman, 2009; Taniguchi et al., 2006); coupling of canonical and non-canonical signal transduction pathways provides spatial and temporal refinement of insulin-like signaling cascades. Since we find that basal Draper levels are normal in glial InR-depleted flies, it is unlikely that the failure of glia to upregulate draper-I or activate STAT92E-dependent transcription (Figure 3) is due to deficient pre-injury Draper levels. It will be important to define the signaling cascades, direct and indirect, that connect activation of glial InR/Akt1 with acute draper upregulation, Draper recruitment to degenerating axons and timely phagocytic removal of cellular debris. Based on the strong conservation of ILS and the Draper/MEGF10/Jedi glial engulfment pathway across species, we speculate that ILS could similarly be a positive regulator of mammalian glial phagocytic activity, in part through regulated expression of MEGF10 and/or Jedi.
Our analysis of ANF::GFP, combined with our CadpsRNAi results, supports a model in which DCVs are released from severed axons. DCVs can be released extra-synaptically, including along axons following robust stimulation protocols (Bospoort et al. 2012; Matsuda et al., 2009), and CAPS1 is required for DCV fusion from extrasynaptic sites in mammalian neurons (Farina et al., 2015). Future work will provide important insight into the intrinsic axonal changes, including calcium-dependent mechanisms, that may promote DCV and neuropeptide release after injury. Our PC2 and Cadps loss of function results indicate that perturbing neuropeptide processing or DCV release attenuates InR signaling and Draper upregulation in local ensheathing glia. These phenotypes were not replicated in ilp-depleted flies, but it will important to determine if systematic disruption of a single ilp (or even several ilp ligands) in ORNs triggers compensatory responses, like those described in other areas of the CNS. For example, medial neurosecretory cells in the dorsal region of the fly brain express ilp2, ilp3 and ilp5, but knockdown of ilp2 in these cells results in increased production of ilp3 and ilp5, which functionally compensates for the loss of ilp2 (Broughton et al., 2008). Moreover, it remains to be determined if ilps are expressed uniformly throughout the antennal lobes or if different ilps are variably expressed in subsets of ORNs. We also cannot exclude that an as yet to be identified neuropeptide activates ensheathing glial InR after ORN axotomy. Finally, it will be important to also explore the possibility that glia-glia ILS communication, for example between local astrocytes and ensheathing glia, occurs after injury.
In invertebrates and mice, ILS is required in axons to execute a normal Wallerian degeneration program through the production of reactive oxygen species following neurotoxic insults (Calixto et al., 2012). It is interesting to speculate that the ILS pathway serves multiple roles at CNS trauma sites to simultaneously ensure that a) axons degenerate promptly and b) local glia respond robustly to efficiently clear degenerating material. Whether this occurs in a variety of contexts (axotomy, neurodegenerative disease, etc.) remains to be explored. Nevertheless, there is growing interest in understanding the relationship between adult ILS pathways and neurological disorders since defects in insulin pathways and metabolic diseases are associated with increased risk of neurodegenerative diseases (Bassil et al., 2014; Fernandez et al., 2012; Russo et al., 2005). Long-term changes in insulin/IGF signaling (e.g. ILS resistance) likely alter basal health and metabolic integrity of the nervous system (Bassil et al., 2014; Broughton and Partridge, 2009; Piriz et al., 2011), but our findings now provide in vivo evidence that highlights the importance of the glial ILS pathway as a critical neuroprotective signaling system in the damaged brain.
EXPERIMENTAL PROCEDURES
Fly Stocks
The following fly strains were used: repo-Gal4 (Leiserson et al., 2000). w1118; OR85e-mCD8::GFP, tubulin-Gal80ts; (Ziegenfuss et al., 2012). InRex15 (Song et al., 2003). y1 w1118; UAS-InR.K1409A (dominant negative InR, Bl 8253) (Demontis and Perrimon, 2009). w1118; UAS-mCD8::GFP, repo-gal4. y1 v1; UAS-InRRNAi (Bl 31037). w1118; UAS-LacZ::NLS (Bl 3955), y1 v1; UAS-AktRNAi (Bl 31701), 10XSTAT92E-dGFP (Back et al., 2007). y1 w1118; UAS-Akt1 (Bl 8191). y1 w1118; UAS-InR (Bloomington stock 8262). y1 w1118; UAS-InR.del (constitutively active InR, Bloomington stock 8248). w1118; TIFR-Gal4 (Yao et al., 2007). y1 w1118; DraperRNAi (MacDonald et al., 2006). UAS-ANF::GFP (Bl 7001). OR22a-Gal4 (Dobritsa et al., 2003). UAS-PC2RNAi (Bl 28583). UAS-PC2RNAi (VDRC 110788) (Dietzl et al., 2007). orco-Gal4 (Bl 23292) (Larsson et al., 2004). UAS-dicer2 (Bl 24650) (Dietzl et al., 2007). UAS-CadpsRNAi (VDRC 110055).
Olfactory Neuron Injuries, Dissection, and Analysis
Maxillary and antennal nerve transections, adult fly brain dissections, and whole brain antibody staining were performed using previously described methods (MacDonald et al., 2006). See Supplemental Experimental Procedures.
Confocal Microscopy
All immunostained brains were imaged on a Zeiss LSM 700 with a Zeiss 40X 1.4NA oil immersion plan-apochromatic lens. Brains within each experiment were mounted under a single #1.5 cover glass and imaged on the same day with the same confocal microscope settings (laser power, photomultiplier tube gain, offset, filter configuration. The Z step in all experiments was 0.76 microns (pinhole 1.5 micron optical slice). Pixel dimensions ranged from 100nm – 230nm.
Statistical Analysis
GraphPad Prism software was used to perform: Ordinary 1-way analysis of variance tests, Kruskall-Wallis 1-way analysis of variance tests, two-tailed Student’s t-tests, two-tailed Welch’s t-tests, two-tailed Mann-Whitney U-tests, Dunnett’s multiple comparisons post hoc tests and Holm-Sidak multiple comparisons post hoc tests. Assumptions of normality were tested with the D’Agostino-Pearson normality test. Where applicable, outliers were identified using the ROUT method. In some analyses, log-transformations were uniformly performed on otherwise non-Gaussian data sets to allow for the appropriate use of parametric tests. When assumptions of normality could not be met for a given data set, non-parametric tests were used. Each N =1 sample number represents pooled measurements taken from independent animals.
Supplementary Material
Highlights.
InR and Akt are activated in ensheathing glia after axotomy
InR/Akt signaling is required for upregulation draper
InR/Akt induction of draper is required for glial clearance of degenerating axons
Acknowledgments
We thank Leslie Pick, Tzumin Lee, the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947), the Bloomington Drosophila Stock Center (NIH P40OD018537) and the Vienna Drosophila Resource Center for flies. We thank members of the Logan lab for discussions, Marc Freeman and Jolanda Muenzel for manuscript comments and Erika Petitt for excellent technical assistance. This work was supported by NIH Grant R01 NS079387-01 (M.A.L), NIH Grant P30NS069346 P30 (M.A.L.), the Medical Research Foundation of Oregon (S.D.S and M.A.L), Fred W. Fields Foundation (M.A.L), Glenn/AFAR (M.D.P) and NIH Training Grant T32AG023477 (M.D.P).
Footnotes
Supplemental information includes Supplemental Experimental Procedures, four figures and one table and can be found with this article online.
AUTHOR CONTRIBUTIONS
D.T.M. conceived and performed experiments, analyzed data and wrote the manuscript. M.D.P. designed and performed experiments and analyzed data. S.D.S. designed and performed experiments, analyzed data and wrote the manuscript. J.D. performed experiments. M.A.L. conceived the project, designed and performed experiments, analyzed data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Gonzalez A-N, Hidalgo A. Nuclear Receptors and Clearance of Apoptotic Cells: Stimulating the Macrophage’s Appetite. Front Immunol. 2014;5:211. doi: 10.3389/fimmu.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–53. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz D, Leyssen M, Koch M, Yan J, Srahna M, Sheeba V, Fogle KJ, Holmes TC, Hassan BA. Axonal injury and regeneration in the adult brain of Drosophila. J Neurosci. 2008;28:6010–21. doi: 10.1523/JNEUROSCI.0101-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Landreth GE. Inflammation, apoptosis, and Alzheimer’s disease. Neuroscientist. 2002;8:276–283. doi: 10.1177/1073858402008003013. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Bonafè M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285: E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Bassil F, Fernagut PO, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118: 1–18. doi: 10.1016/j.pneurobio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Bjerknes R, Aarskog D. Priming of human polymorphonuclear neutrophilic leukocytes by insulin-like growth factor I: increased phagocytic capacity, complement receptor expression, degranulation, and oxidative burst. J Clin Endocrinol Metab. 1995;80: 1948–55. doi: 10.1210/jcem.80.6.7775645. [DOI] [PubMed] [Google Scholar]
- Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS One. 2008;11 doi: 10.1371/journal.pone.0003721. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- Calixto A, Jara JS, Court FA. Diapause formation and downregulation of insulin-like signaling via DAF-16/FOXO delays axonal degeneration and neuronal loss. PLoS Genet. 2012;8:e1003141. doi: 10.1371/journal.pgen.1003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantera R, Barrio R. Do the genes of the innate immune response contribute to neuroprotection in Drosophila? J Innate Immun. 2015;7:3–10. doi: 10.1159/000365195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson MA, Diesner M, Schachtner J, Nassal DR. Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J Comp Neurol. 2010;16: 3359–3380. doi: 10.1002/cne.22405. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Núñez A, Torres-Aleman I. Brain repair and neuroprotection by serum insulin-like growth factor I. Mol Neurobiol. 2003;2: 153–62. doi: 10.1385/MN:27:2:153. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Spuch C, Bohl D, Heard JM, Torres-Aleman I. Blockade of the insulin-like growth factor I receptor in the choroid plexus originates Alzheimer’s-like neuropathology in rodents: New cues into the human disease? Neurobiology of Aging. 2006;27:1618–1631. doi: 10.1016/j.neurobiolaging.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis F, Perrimon N. Integration of Insulin receptor/Foxo signaling and dMyc activity during muscle growth regulates body size in Drosophila. Development. 2009;136: 983–93. doi: 10.1242/dev.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Tasdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci. 2009;29: 4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Sheehan AE, Bradshaw R, Fox AN, Lu T-Y, Freeman MR. PI3K signaling and Stat92E converge to modulate glial responsiveness to axonal injury. PLoS Biol. 2014;12:e1001985. doi: 10.1371/journal.pbio.1001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol. 2010;90: 471–97. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JI, Elguero EJ, Tran JA, Sinatra V, Feany MB, McCall K. Defective Phagocytic Corpse Processing Results in Neurodegeneration and Can Be Rescued by TORC1 Activation. J Neurosci. 2016;36: 3170–83. doi: 10.1523/JNEUROSCI.1912-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Bonini NM. Axon degeneration and regeneration: insights from Drosophila models of nerve injury. Annu Rev Cell Dev Biol. 2012;28: 575–597. doi: 10.1146/annurev-cellbio-101011-155836. [DOI] [PubMed] [Google Scholar]
- Farina M, van de Bospoort R, He E, Persoon CM, van Weering JR, Broeke JH, Verhage M, Toonen RF. CAPS-1 promotes fusion competence of stationary dense-core vesicles in presynaptic terminals of mammalian neurons. Elife. 2015;4 doi: 10.7554/eLife.05438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SB, Blundon MA, Klovstad MS, Schüpbach T. Modulation of gurken translation by insulin and TOR signaling in Drosophila. J Cell Sci. 2012;125: 1407–19. doi: 10.1242/jcs.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13: 225–39. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38: 567–80. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336:579–82. doi: 10.1126/science.1216735. [DOI] [PubMed] [Google Scholar]
- Giunti D, Parodi B, Cordano C, Uccelli A, Kerlero de Rosbo N. Can we switch microglia’s phenotype to foster neuroprotection? Focus on multiple sclerosis. Immunology. 2014;141:328–339. doi: 10.1111/imm.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147: 867–83. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Grönke S, Clarke D-F, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T, Zhao T, Hewes RS. Insulin signaling regulates neurite growth during metamorphic neuronal remodeling. Biol Open. 2014;3: 81–93. doi: 10.1242/bio.20136437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Progress in Neurobiology. 2003;70:443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7:494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himpe E, Kooijman R. Insulin-like growth factor-I receptor signal transduction and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK-STAT) pathway. Biofactors. 2009;35: 76–81. doi: 10.1002/biof.20. [DOI] [PubMed] [Google Scholar]
- Hua K, Forbes ME, Lichtenwalner RJ, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I alters oligodendrocyte turnover in the corpus callosum. Glia. 2009;57: 1062–71. doi: 10.1002/glia.20829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain QM, Ewer J. Use of targetable gfp-tagged neuropeptide for visualizing neuropeptide release following execution of a behavior. J Neurobiol. 2004;59(2):181–91. doi: 10.1002/neu.10309. [DOI] [PubMed] [Google Scholar]
- Inoue T, Saito H, Matsuda T, Fukatsu K, Han I, Furukawa S, Ikeda S, Muto T. Growth hormone and insulin-like growth factor I augment bactericidal capacity of human polymorphonuclear neutrophils. Shock. 1998;10: 278–84. doi: 10.1097/00024382-199810000-00008. [DOI] [PubMed] [Google Scholar]
- Kannan K, Fridell YW. Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction. Front Physiol. 2013;16 doi: 10.3389/fphys.2013.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Forero MG, Fenton JC, Hidalgo A. The glial regenerative response to central nervous system injury is enabled by pros-notch and pros-NFκB feedback. PLoS Biol. 2011;9(8):e1001133. doi: 10.1371/journal.pbio.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Younger A, Jasper H. Dynamic coordination of innate immune signaling and insulin signaling regulates systemic responses to localized DNA damage. Dev Cell. 2011;20: 841–54. doi: 10.1016/j.devcel.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver MB, Huang Z, Hardy RW. Insect antiviral innate immunity: pathways, effectors, and connections. J Mol Biol. 2013;425: 4921–36. doi: 10.1016/j.jmb.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurant E. Keeping the CNS clear: glial phagocytic functions in Drosophila. Glia. 2011;59(9):1304–11. doi: 10.1002/glia.21098. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–14. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lee YM, Sun YH. Drosophila as a model to study the role of glia in neurodegeneration. J Neurogenet. 2015;29(2–3):69–79. doi: 10.3109/01677063.2015.1076816. [DOI] [PubMed] [Google Scholar]
- Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000;28: 793–806. doi: 10.1016/s0896-6273(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Logan MA, Freeman MR. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 2007;3: 63–74. doi: 10.1017/S1740925X07000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan MA, Hackett R, Doherty J, Sheehan A, Speese SD, Freeman MR. Negative regulation of glial engulfment activity by Draper terminates glial responses to axon injury. Nat Neurosci. 2012;15: 722–730. doi: 10.1038/nn.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, Bellen HJ. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell. 2015;160(1–2):177–90. doi: 10.1016/j.cell.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31: 647–56. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Marr MT, 2nd1, D’Alessio JA, Puig O, Tjian R. IRES-mediated functional coupling of transcription and translation amplifies insulin receptor feedback. Genes Dev. 2007;21: 175–83. doi: 10.1101/gad.1506407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29(45):14185–98. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302: 1765–8. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Menuz K, Larter NK, Park J, Carlson JR. An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. 2014;10(11):e1004810. doi: 10.1371/journal.pgen.1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltiadous P, Stamatakis A, Stylianopoulou F. Neuroprotective effects of IGF-I following kainic acid-induced hippocampal degeneration in the rat. Cell Mol Neurobiol. 2010;3: 347–60. doi: 10.1007/s10571-009-9457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B, Carson R, Hume RI, Collins CA. Sodium and potassium currents influence Wallerian degeneration of injured Drosophila axons. J Neurosci. 2013;33(48):18728–39. doi: 10.1523/JNEUROSCI.1007-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S, Ghosh-Roy S, Loison F, Li Y, Jia Y, Harris C, Williams DA, Luo HR. PTEN negatively regulates engulfment of apoptotic cells by modulating activation of Rac GTPase. J Immunol. 2011;187: 5783–94. doi: 10.4049/jimmunol.1100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Omoto JJ, Yogi P, Hartenstein V. Origin and development of neuropil glia of the Drosophila larval and adult brain: Two distinct glial populations derived from separate progenitors. Dev Biol. 2015;404(2):2–20. doi: 10.1016/j.ydbio.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46: 96–9. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Puig O, Mattila J. Understanding Forkhead box class O function: lessons from Drosophila melanogaster. Antioxid Redox Signal. 2011;14: 635–647. doi: 10.1089/ars.2010.3407. [DOI] [PubMed] [Google Scholar]
- Rayburn LY, Gooding HC, Choksi SP, Maloney D, Kidd AR, 3rd, Siekhaus DE, Bender M. amontillado, the Drosophila homolog of the prohormone processing protease PC2, is required during embryogenesis and early larval development. Genetics. 2003;2163(1):227–37. doi: 10.1093/genetics/163.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Lang C, Levitan ES, Deitcher DL. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J Neurobiol. 2001;49(3):159–72. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- Renden R, Berwin B, Davis W, Ann K, Chin CT, Kreber R, Ganetzky B, Martin TF, Broadie K. Drosophila CAPS is an essential gene that regulates dense-core vesicle release and synaptic vesicle fusion. Neuron. 2001;31(3):421–37. doi: 10.1016/s0896-6273(01)00382-8. [DOI] [PubMed] [Google Scholar]
- Rhea JM, Wegener C, Bender M. The proprotein convertase encoded by amontillado (amon) is required in Drosophila corpora cardiaca endocrine cells producing the glucose regulatory hormone AKH. PLoS Genet. 6(5):e1000967. doi: 10.1371/journal.pgen.1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz JR, Sorribes A, de Polavieja GG, Fernandez-Funez P, Ferrus A. Age-independent synaptogenesis by phosphoinositide 3 kinase. Martin-Pena A, Acebes A. J Neurosci. 2006;26(40):10199–208. doi: 10.1523/JNEUROSCI.1223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney TM, Freeman MR. Drosophila models of neuronal injury. Ilar J. 2014;54:291–295. doi: 10.1093/ilar/ilt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nässel DR, Lee C-H, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145: 133–44. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26: 916–43. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- Scheib JL, Sullivan CS, Carter BD. Jedi-1 and MEGF10 signal engulfment of apoptotic neurons through the tyrosine kinase Syk. J Neurosci. 2012;32: 13022–13031. doi: 10.1523/JNEUROSCI.6350-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi C, Stecca C, Iacomino A, Steardo L. Role of astrocytes in major neurological disorders: the evidence and implications. IUBMB Life. 2013;65:957–961. doi: 10.1002/iub.1223. [DOI] [PubMed] [Google Scholar]
- Song J, Wu L, Chen Z, Kohanski RA, Pick L. Axons guided by insulin receptor in Drosophila visual system. Science. 2003;300:502–505. doi: 10.1126/science.1081203. [DOI] [PubMed] [Google Scholar]
- Sousa-Nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 2011;471(7339):508–12. doi: 10.1038/nature09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7: 85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Torres-Aleman I. Targeting insulin-like growth factor-1 to treat Alzheimer’s disease. Expert Opin Ther Targets. 2007;11:1535–1542. doi: 10.1517/14728222.11.12.1535. [DOI] [PubMed] [Google Scholar]
- Ugur B, Chen K, Bellen HJ. Drosophila tools and assays for the study of human diseases. Dis Model Mech. 2016;9(3):235–44. doi: 10.1242/dmm.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Bospoort R, Farina M, Schmitz SK, de Jong A, de Wit H, Verhage M, Toonen RF. Munc13 controls the location and efficiency of dense-core vesicle release in neurons. J Cell Biol. 2012;199(6):883–91. doi: 10.1083/jcb.201208024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, Shaw RJ, Yates JR, Fischer WH, Thomas JB, Montminy M. A Hormone-Dependent Module Regulating Energy Balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz C, Köhler K, Hafen E, Stocker H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genet. 2009;5:e1000596. doi: 10.1371/journal.pgen.1000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S, Slack C, Grönke S, Martinez P, Calboli FC, Chapman T, Partridge L. Insulin signalling regulates remating in female Drosophila. Proc Biol Sci. 2011;278:424–31. doi: 10.1098/rspb.2010.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Zhou C, Shakiryanova D, Lloyd TE, Deitcher DL, Levitan ES. Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell. 2012;148(5):1029–38. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Cavolo SL, Levitan ES. Synaptic neuropeptide release by dynamin-dependent partial release from circulating vesicles. Mol Biol Cell. 2015;26(13):2466–2474. doi: 10.1091/mbc.E15-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-H, Bellmunt E, Scheib JL, Venegas V, Burkert C, Reichardt LF, Zhou Z, Fariñas I, Carter BD. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat Neurosci. 2009;12: 1534–1541. doi: 10.1038/nn.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51: 1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Yao Y, Wu Y, Yin C, Ozawa R, Aigaki T, Wouda RR, Noordermeer JR, Fradkin LG, Hing H. Antagonistic roles of Wnt5 and the Drl receptor in patterning the Drosophila antennal lobe. Nature Neuroscience. 2007;10:1423–1432. doi: 10.1038/nn1993. [DOI] [PubMed] [Google Scholar]
- Ziegenfuss JS, Doherty J, Freeman MR. Distinct molecular pathways mediate glial activation and engulfment of axonal debris after axotomy. Nat Neurosci. 2012;15(7):979–87. doi: 10.1038/nn.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.