Abstract
Objectives:
The authors present the case of a 3-year-old girl with a history of complicated surgery for removing a third branchial cleft fistula.
Methods:
An endoscopic approach using N-butyl-2-acrylate and metacrilosisolfolane glue (GLUBRAN 2) to seal the fistula was performed.
Results:
The clinical and radiological 6-year follow-up confirmed the absence of the fistulous orifice and the persistence of scar due to previous open-neck surgical procedures.
Conclusion:
endoscopic Glubran 2 sealing has been an effective treatment procedure for branchial fistula.
Keywords: Branchial abnormalities, pyriform sinus fistula, endoscopic cauterization, recurrent fistula, third branchial anomalies
Introduction
The management of third and fourth branchial anomalies is controversial; various treatment techniques have been reported in the literature.1–13 The authors present the case of a 3-year-old girl with a third branchial cleft fistula successfully treated by endoscopic Glubran 2 sealing.
Case report
A 3-year-old girl with a long history of neck mass with fistulous orifice and an erythematous halo located along the anterior border of the left sternocleidomastoid muscle (Figure 1) was referred to the Maxillo-Facial Department of Sapienza University.
Figure 1.
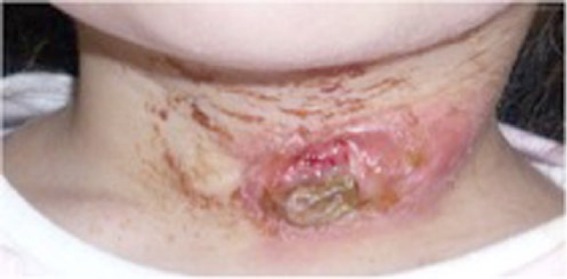
A 3-year-old patient. Fistulous orifice and erythematous halo located in the anterior border of the left sternocleidomastoid muscle.
Two previous open-neck surgical procedures had been performed to remove a third branchial cleft fistula 1 year and 6 months before coming to us because she had complained of continuous discharge of fluids after swallowing liquids. Magnetic resonance imaging (MRI) revealed a neck mass of 3.5 cm in diameter located in the left neck. For this reason, the patient underwent surgery to remove the fistulized lesion by open-neck surgical procedure.
After 10 months, a fourth operation was required for recurrence of symptoms. After 8 months, an MRI revealed an expansive lesion of 2.5 cm (Figure 2) located medially to the left sternocleidomastoid muscle, with offshoots to the left antero-lateral wall of the hypopharynx.
Figure 2.
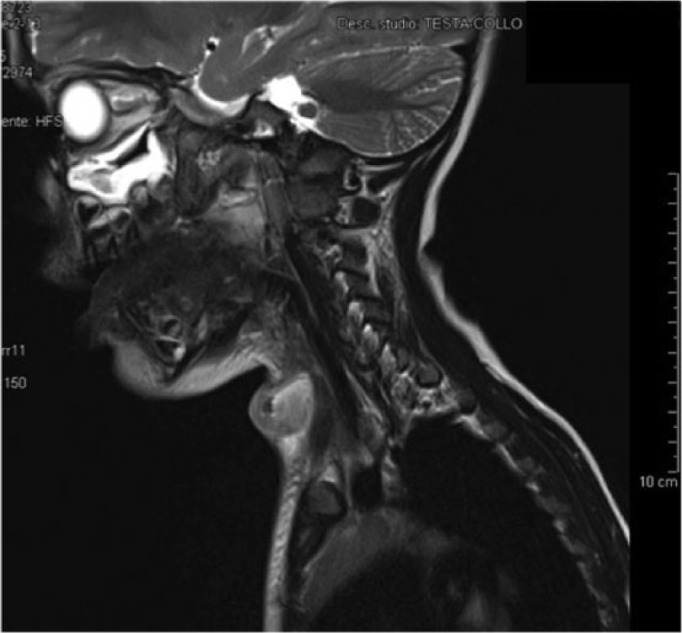
MRI reveals the presence of pyriform sinus fistula and the extension of abscess toward pyriform fossa.
Further clinical controls revealed the reappearance of the fistulous orifice and leakage of liquid food material and saliva during swallowing as confirmed by barium swallow test (Figure 3). Following failure of the repeated open-neck surgical procedures, an endoscopic approach was planned.
Figure 3.
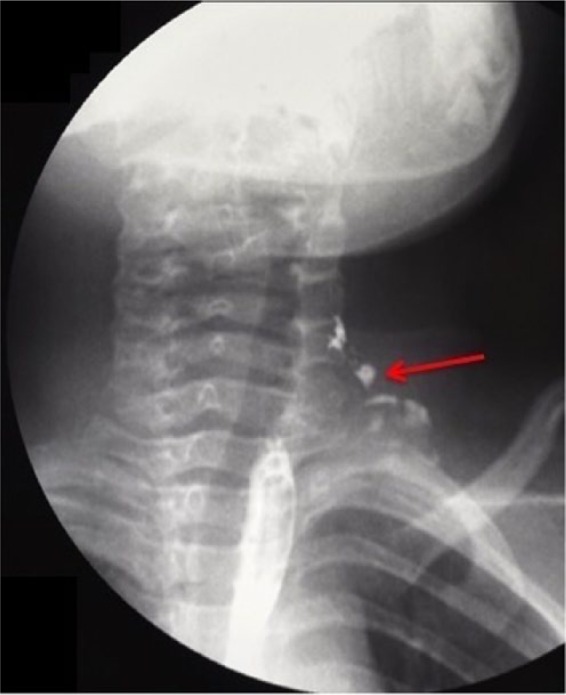
Pyriform sinus fistula demonstrated by contrast swallow study.
After endoscopical identification of the internal orifice (Figure 4(a)), the fistula was catheterized (Figure 4(b)) and injected with 1 mL of Glubran 2 (Gem s.r.l. Laboratories, Viareggio, Italy), a N-butyl-2-acrylate and metacrilosisolfolane glue. At discharge, fistulous drainage had ceased and radiologic examination showed the absence of leakage (Figure 5). The clinical and radiological 6-year follow-up confirmed the absence of the fistulous orifice and the persistence of scar due to previous open-neck surgical procedures (Figure 6).
Figure 4.
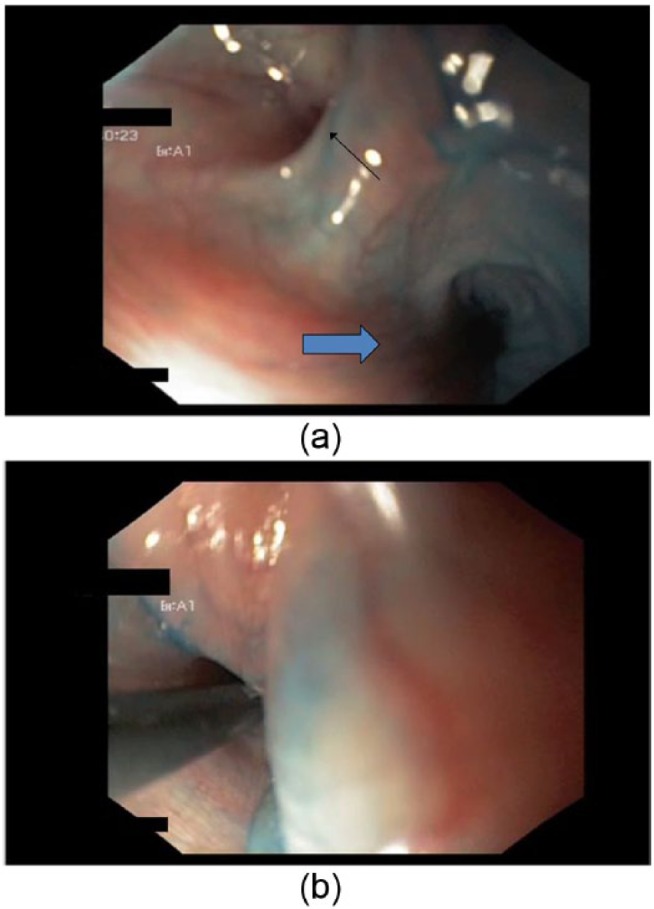
(a) Endoscopical identification of the internal orifice of the fistula (little arrow) and the entrance of the esophagus (blue arrow) (b) Insertion of the probe into the fistula.
Figure 5.
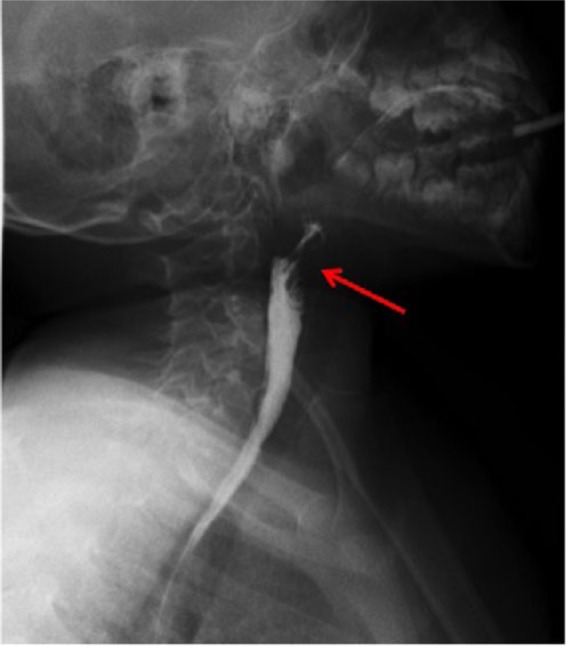
Barium swallow test after endoscopic chemo-cauterization demonstrates the absence of leakage.
Figure 6.
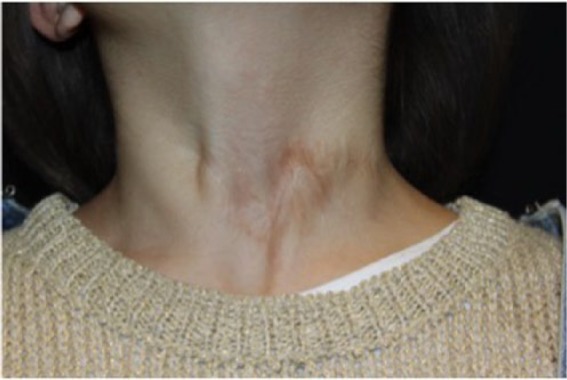
Disappearance of fistulous orifice and detail of cutaneous scar after 6 years of follow-up.
Discussion
The branchial apparatus undergoes development and differentiation between the third and seventh weeks of gestation. The third and fourth branchial anomalies represent only 2%–8% and 1%–4% of all branchial anomalies, respectively.3 Third branchial arch gives rise to the stylopharyngeal muscle, internal carotid artery, ninth cranial nerve, greater horn and body of the hyoid bone. The third branchial pouch gives rise to the inferior parathyroid gland and the thymus. The third branchial fistulas are rare and generally originate from the upper lateral wall pyriform sinus, piercing the thyrohyoid membrane, pass behind the internal carotid artery, loop around hypoglossal nerve, go deep to the glossopharyngeal nerve and over superior laryngeal nerve, descend posterior to internal and common carotid artery, pierce the platysma and emerge on the skin near the mid-to-lower third of anterior border of the sternocleidomastoid muscle.
A sinus or cyst might be found at any point along this course. Similar to third branchial abnormalities, fourth branchial fistulas arise on the skin near the junction of lower and middle thirds of the anterior border of sternocleidomastoid muscle.
Due to the similarity of their clinical presentation, some authors have even suggested grouping them as the same entity. The distinction between third and fourth branchial fistulas can only be established during dissection. The difference lies in the relationship to the superior and recurrent laryngeal nerves: fistulas of the third one pass superficial to both the superior and recurrent, while those of the fourth pass deep below the superior but superficial to the recurrent. Moreover, third branchial arch fistulas generally originate from the upper lateral wall of the pyriform sinus as opposed to fourth branchial arch anomalies which originate from the apex (caudal end) of the pyriform sinus.
Based on surgical findings, the patient in our case was diagnosed with a third branchial arch fistula.
Clinical presentation includes recurrent cervical inflammatory swelling, neck abscess, needing repeated incision and drainage and acute suppurative thyroiditis. The neonatal form manifests as a very large cervical mass that compresses the surrounding structures. There is often a bubble of air visible within the mass on imaging. The mass may cause respiratory distress with stridor and feeding difficulties.4
The suspicion of branchial cleft sinus or fistula can usually be made clinically. Various investigations are helpful in improving the diagnostic yield, including ultrasonography, computed tomography (CT), CT-fistulography and MRI.1–5 A fistulogram is frequently performed to assess the extent of the fistulous tract by injecting a water-soluble iodinated contrast medium and is considered the gold standard for diagnosing branchial abnormalities.
Because of their rare occurrence, consistent recommendations on management are lacking. Although the literature3–13 shows that complete removal of the fistula is the most commonly used treatment, surgery must be performed carefully because of the anatomical proximity to the superior and recurrent laryngeal nerves, esophagus, trachea and, in case of fourth branchial fistula, aorta. Furthermore, previous infections and scar tissue make dissections difficult. Re-treatment of recurrent branchial cleft deformities is a challenge owing to the presence of a large amount of scarring and inflammatory tissues around the recurrent lesion and to the difficulty in distinguishing normal tissue and avoiding damage to important structures.
A number of treatment techniques have been reported in the literature, from surgery to endoscopy as an adjuvant or exclusive method. The combination of the two techniques proved helpful in decreasing the recurrence rate.3–13
Endoscopy alone represents an alternative treatment method. Through endoscopic approach, cauterization of the fistulous tract opening in the pyriform sinus can be performed, avoiding risks of an open-neck surgical procedure.
Several methods for cauterization have been described, including electro-cauterization, low-power diode or CO2 laser, chemo-cauterization, or application of fibrin glue or silver nitrate, nevertheless there is no consensus as to which method is superior.10,11 Nicoucar et al. comparing open surgery and endoscopic approach showed that the failure rate after cauterization is comparable to traditional open-neck surgery (18% vs 15%). They suggested that complications after surgery occurred more often in children aged 8 years or younger, and complete excision of the entire fistula tract during a quiescent period remains the treatment of choice for the management of third branchial anomalies in children aged 8 years and older, recommending endoscopic cauterization in children 8 years or younger.12
Derks et al.13 observed that the success rate of cauterization as a definite treatment seems to be comparable to the success rate of open surgery with the advantage that this procedure is minimally invasive, has a lower complication rate and could potentially be performed simultaneously with incision and drainage of the abscess.
Glubran 2 is a synthetic surgical glue, with hemostatic, adhesive, sealer and bacteriostatic properties; it has been applied for treating spinal tumors, extra-cerebral tumors, cerebral and spinal arteriovenous malformations and dural fistulae.14,15
Up to now, Glubran 2 has never been used for the treatment of congenital branchial abnormalities, but this case illustrates that Glubran injection may be an easy and safe technique in small children to successfully close recalcitrant branchial fistula. In our experience, endoscopic cauterization with Glubran 2 may represent a valid method as first approach to branchial fistulas and particularly useful in recurrence.
Conclusion
In our experience, endoscopic Glubran 2 sealing has been an effective treatment procedure for branchial fistula. Further studies are expected in order to add evidence to more standardized protocols for managing patients with branchial anomalies especially in young patients and also to treat fistula recurrence.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
Patient consent: Patient’s legally authorized representative agreed to report this case and to publish clinical pictures.
References
- 1. Schroeder JW, Jr, Mohyuddin N, Maddalozzo J. Branchial anomalies in the pediatric population. Otolaryngol Head Neck Surg 2007; 137: 289–295. [DOI] [PubMed] [Google Scholar]
- 2. Kajosaari L, Mäkitie A, Salminen P, et al. Second branchial cleft fistulae: patient characteristics and surgical outcome. Int J Pediatr Otorhinolaryngol 2014; 78(9): 1503–1507. [DOI] [PubMed] [Google Scholar]
- 3. Goff CJ, Allred C, Glade RS. Current management of congenital branchial cleft cysts, sinuses, and fistulae. Curr Opin Otolaryngol Head Neck Surg 2012; 20(6): 533–539. [DOI] [PubMed] [Google Scholar]
- 4. Wasson J, Blaney S, Simo R. A third branchial pouch cyst presenting as stridor in a child. Ann R Coll Surg Engl 2007; 89(1): W12–W14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mou JW, Chan KW, Wong YS, et al. Recurrent deep neck abscess and pyriform sinus tract: a 15-year review on the diagnosis and management. J Pediatr Surg 2014; 49(8): 1264–1267. [DOI] [PubMed] [Google Scholar]
- 6. Lee ST, Krishnan MM. Branchial fistula: a review. Singapore Med J 1991; 32: 50–52. [PubMed] [Google Scholar]
- 7. Blackwell KE, Calcaterra TC. Functional neck dissection for treatment of recurrent branchial remnants. Arch Otolaryngo Head Neck Surg 1994; 120: 417–421. [DOI] [PubMed] [Google Scholar]
- 8. Cai Q, Pan Y, Xu Y, et al. Resection of recurrent branchial cleft deformity using selective neck dissection technique. Int J Pediatr Otorhinolaryngol 2014; 78(7): 1071–1073. [DOI] [PubMed] [Google Scholar]
- 9. Xiao X, Zheng S, Zheng J, et al. Endoscopic-assisted surgery for pyriform sinus fistula in children: experience of 165 cases from a single institution. J Pediatr Surg 2014; 49(4): 618–621. [DOI] [PubMed] [Google Scholar]
- 10. Proske JM, Vons C. Transgastric laparoscopic approach for resection of hemorrhagic Dieulafoy’s vascular malformation. Surg Endosc 2004; 18(3): 554–556. [DOI] [PubMed] [Google Scholar]
- 11. Stenquist M, Juhlin C, Aström G, et al. Fourth branchial pouch sinus with recurrent deep cervical abscesses successfully treated with trichloroacetic acid cauterization. Acta Otolaryngol 2003; 123(7): 879–882. [DOI] [PubMed] [Google Scholar]
- 12. Nicoucar K, Giger R, Jaecklin T, et al. Management of congenital third branchial arch anomalies: a systematic review. Otolaryngol Head Neck Surg 2010; 142: 21–28.3. [DOI] [PubMed] [Google Scholar]
- 13. Derks LS, Veenstra HJ, Oomen KP, et al. Surgery versus endoscopic cauterization in patients with third or fourth branchial pouch sinuses: a systematic review. Laryngoscope 2016; 126(1): 212–217. [DOI] [PubMed] [Google Scholar]
- 14. Raffi L, Simonetti L, Cenni P, et al. Use of Glubran 2 acrylic glue in interventional neuroradiology. Neuroradiology 2007; 49(10): 829–836. [DOI] [PubMed] [Google Scholar]
- 15. Desal HA, Toulgoat F, Raoul S, et al. Brain arteriovenous malformations technical note of endovascular treatment with Glubran. Interv Neuroradiol 2005; 11(Suppl. 1): 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]


