Abstract
A Ni-catalyzed asymmetric reductive cross-coupling of heteroaryl iodides and α-chloronitriles has been developed. This method furnishes enantioenriched α,α-disubstituted nitriles from simple organohalide building blocks. The reaction tolerates a variety of heterocyclic coupling partners, including pyridines, pyrimidines, quinolines, thiophenes, and piperidines. The reaction proceeds under mild conditions at room temperature, and precludes the need to pre-generate organometallic nucleophiles.
Graphical abstract
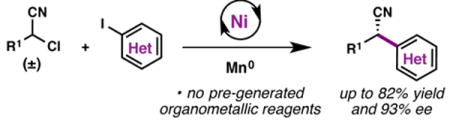
In recent years, Ni-catalyzed reductive cross-coupling reactions have experienced a surge of development.1 These transformations forge C–C bonds between two organic electrophiles, employing a stoichiometric reductant (usually Zn0 or Mn0) to turn over the Ni catalyst. An appealing aspect of this chemistry is that sec-alkyl electrophiles are competent reaction partners,2 and in some cases, these reactions can be rendered enantioselective by use of an appropriate chiral ligand.3 However, the scope of C(sp3) electrophiles used in these asymmetric transformations has so far been limited to α-substituted benzyl chlorides. Moreover, the asymmetric cross-coupling of heteroaryl electrophiles – a substrate class that would be of high value for medicinal chemists4 – has proven challenging.5 In this communication, we report the Ni-catalyzed asymmetric reductive cross-coupling between α-chloronitriles and heteroaryl iodides, a reaction that provides access to a variety of enantioenriched heterocyclic products.
In considering the development of new C(sp3) electrophiles for Ni-catalyzed reductive cross-coupling reactions, we became interested in the use of α-chloronitriles. Nitriles are valuable synthetic intermediates that serve as precursors to amines, carboxylic acids, carboxamides, aldehydes, ketones, and alcohols.6 The cyano group is also found in a number of natural products and medicinal compounds.7 However, there are few transition metal-catalyzed cross-coupling methods to directly prepare enantioenriched α,α-disubstituted nitriles. In 2010, Falck and coworkers published a Pd-catalyzed stereospecific Suzuki cross-coupling of α-cyanohydrin triflates.8 Two years later, Fu and Choi reported a highly enantioselective Ni-catalyzed Negishi coupling between racemic α-bromonitriles and arylzinc reagents.9,10 However, neither report included heteroaryl nucleophiles as part of their substrate studies.
We envisioned that a Ni-catalyzed asymmetric reductive cross-coupling between α-chloronitriles and heteroaryl halides could provide access to a complementary scope of synthetically useful products. Furthermore, α-chloronitriles have not been developed as C(sp3) electrophiles for Ni-catalyzed reductive cross-coupling reactions (even in the racemic sense).11 Thus, the successful development of this transformation would expand the scope of reductive cross-coupling reactions to new and synthetically versatile classes of electrophiles.
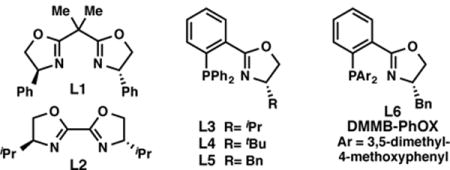
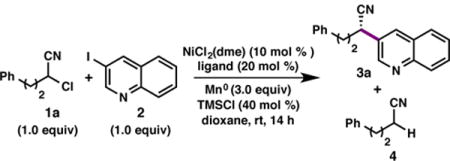
We began our investigations with the coupling between α-chloronitrile 1a and 3-iodoquinoline (2). When the reaction was conducted in DMA with chiral BOX ligand L1 and TMSCl to activate Mn0, no product was formed (Table 1, entry 2). Rather, the α-chloronitrile was rapidly consumed, generating the hydrodehalogenation product 4-phenylbutyronitrile (4). A solvent screen revealed that 3a forms in trace yield and 38% ee when dioxane is used as solvent (entry 3); chiral BiOX ligand L2 provided slightly improved yield (entry 4). We hypothesized that more electron-rich ligands might accelerate the rate of oxidative addition of 2 to a LNi(0) complex, relative to the rate of hydrodehalogenation and decomposition reactions of 1, thereby improving the yield of 3a.12 Consistent with this hypothesis, phosphino-oxazoline (PhOX) ligands were found to provide improved reactivity, with BnPhOX L5 furnishing 3a in 52% yield and 77% ee (entry 7). Further ligand optimization identified DMMB-PhOX L6 as providing the best combination of yield and selectivity (entry 1). A study of additional reaction parameters revealed that: 1) use of Zn0 instead of Mn0 provides the product in lower yield and ee (entry 12), and 2) 2-bromo-4-phenylbutanenitrile (5) suffers from facile hydrodehalogenation and elimination under the reaction conditions, providing 3a in only 9% yield and 84% ee (entry 16). Additives that have been shown to improve the yields of reductive cross-electrophile coupling were also investigated (entries 13–15).3a,5a The addition of NaBF4 provided 3 in comparable yield and improved selectivity (entry 14); however, further studies revealed that for many substrates, NaBF4 provides no added benefits. Erring toward the use of fewer reagents, NaBF4 was only added for the cross-coupling of certain more challenging substrates, as indicated in the following Tables. The exact role of NaBF4 in these transformations is unknown at this time.5a
Table 1.
Optimization of reaction conditions.a
| |||||
|---|---|---|---|---|---|
| Entrya | Ligand | Deviation from Standard Conditions | Yield 3 (%)b |
Yield 4 (%)b |
ee 3 (%)c |
| 1 | L6 | None | 78 | 20 | 84 |
| 2 | L1 | DMA instead of dioxane | 0 | 62 | – |
| 3 | L1 | – | <5 | 32 | 38 |
| 4 | L2 | – | 17 | 22 | 9 |
| 5 | L3 | – | 25 | 40 | 83 |
| 6 | L4 | – | 0 | 32 | – |
| 7 | L5 | – | 52 | 35 | 77 |
| 8 | L6 | No Ni | 0 | 0 | – |
| 9 | L6 | No Mn0 | 0 | 0 | – |
| 10 | L6 | No TMSCl | <5 | <5 | 82 |
| 11 | – | No Ligand | 4 | 23 | 0 |
| 12 | L6 | Zn0 instead of Mn0 | 25 | 32 | 10 |
| 13 | L6 | TFA (0.4 equiv) | 48 | 37 | 78 |
| 14 | L6 | NaBF4 (1.0 equiv) | 76 | 24 | 90 |
| 15 | L6 | NaI (0.25 equiv) | 71 | 29 | 84 |
| 16 | L6 | RBrCN (5) instead of 1 | 9 | 36 | 84 |
Reactions conducted under inert atmosphere on 0.1 mmol scale for 14 h.
Determined by 1H NMR versus an internal standard.
Determined by SFC using chiral stationary phase.
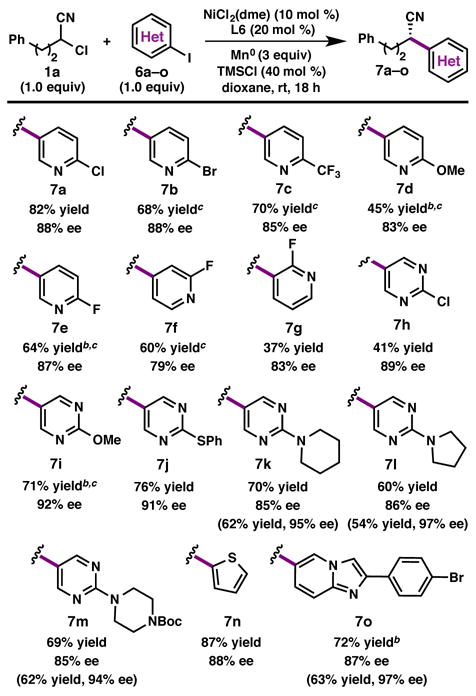
Having optimized the reaction parameters for the coupling between 1a and 2, we sought to probe the scope of the heteroaryl partner (Table 2). We were pleased to find that a variety of heteroaryl iodides undergo cross-coupling to furnish the α,α-disubstituted nitriles in good yields and with high enantioinduction. The reaction demonstrates good chemoselectivity, with no coupling observed at the 2-position of 2-bromo- or 2-chloro-5-iodopyridine (see products 7a and 7b).13 Whereas substitution meta to the iodide was tolerated (7f), a decrease in yield was observed when the substituent was ortho to the iodide (7g). Iodo-pyridines or -pyrimidines lacking substitution at C2 were poor substrates, presumably due to the increased Lewis basicity of the nitrogen. A variety of C2-substituted pyrimidines, as well as 2-iodothiophene and a 6-imidazopyridine also undergo cross-coupling, delivering products in good yield and with high enantioinduction (7h–7o). Importantly, many of the products were easily recrystallized to afford highly enantioenriched (>95% ee) material with excellent recovery. For less-reactive heteroaryl iodides, competitive hydrodehalogenation of 1a resulted in decreased yields; this could be mitigated in most cases by using two equivalents of the iodide partner.14
Table 2.
Scope of heteroaryl iodide.a
|
Reaction conducted on 0.2 mmol scale. Isolated yields are provided; ee is determined by SFC using chiral stationary phase. Values in parentheses are yield and ee following a single recrystallization of the product.
2.0 equiv heteroaryl iodide used.
1.0 equiv NaBF4 added.
We also investigated the scope of the α-chloronitrile (1). In general, less sterically-encumbered substrates provide products in good yield and modest enantioselectivity, while more bulky substrates provide the product with good enantioselectivity and slightly more modest yields. Nonetheless, the reaction exhibits notable functional group tolerance, including carbamates (3h and 3i), esters (3f) and a primary alkyl chloride (3g). Recrystallization of nitrile 3e provided crystals suitable for X-ray diffraction analysis, which allowed us to assign the stereochemistry as the (S)-configuration.15
The enantioenriched α,α-disubstituted nitriles produced in this reaction serve as versatile synthetic intermediates. For example, hydrogenation of 2-piperidyl-pyrimidine 7k under standard conditions provides phenethylamine 8 in excellent yield and with no erosion of ee (Scheme 2). The two step sequence involving cross-coupling and hydrogenation represents an straightforward approach to the synthesis of this bioactive class of molecule. The same substrate can be subjected to Pt-catalyzed hydrolysis16 to afford carboxamide 9 in high yield and with complete stereoretention. Alternatively, reduction of thiophene-containing nitrile 7n with DIBAL-H furnished the enantioenriched aldehyde 10 in excellent yield and with minor erosion of ee.
Scheme 2.
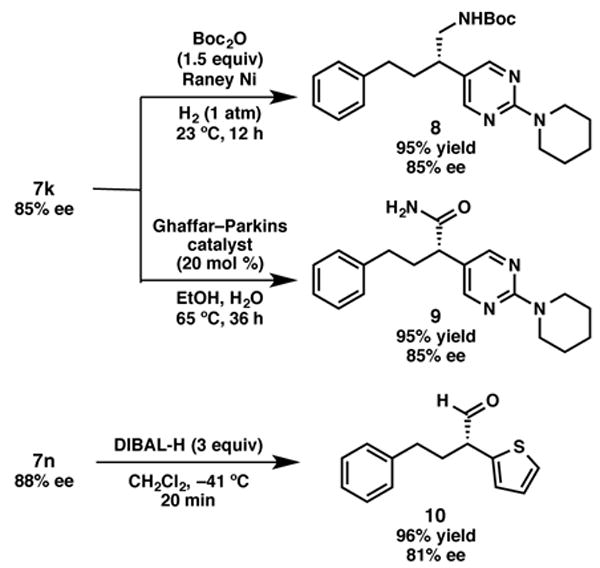
Derivatization of α,α-disubstituted nitriles.
Several experiments were conducted to interrogate the potential mechanism of this transformation. To probe whether the oxidative addition of the α-chloronitrile proceeds by a radical pathway, cyclopropyl-containing substrate 11 was prepared and subjected to the reaction conditions (Scheme 3). Ring-opened coupling product 12 was obtained in 21% yield as a 1:1 mixture of cis and trans isomers, consistent with a radical intermediate.17 None of the corresponding cyclopropane-containing product was observed. Despite this evidence for a radical intermediate, the reaction proceeds with comparable efficiency in the presence of 50 mol % of common radical inhibitors, such as 2,6-bis(1,1-dimethylethyl)-4-methylphenol (BHT) or dihydroanthracene (DHA).18 The latter finding is inconsistent with cage-escaped radicals expected in a radical chain mechanism, although more studies are needed to fully elucidate the reaction pathway.19
Scheme 3.
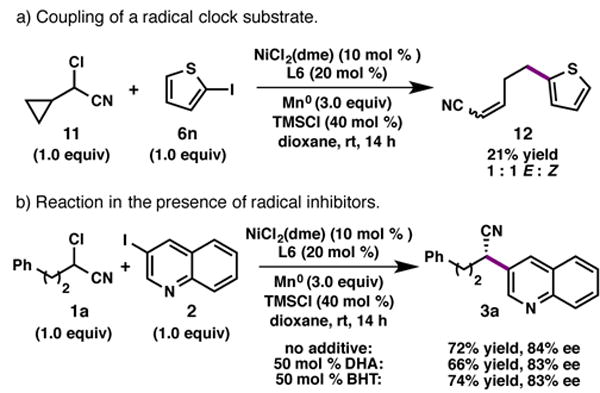
Mechanistic experiments.
In conclusion, a Ni-catalyzed asymmetric reductive cross-coupling between α-chloronitriles and heteroaryl iodides has been developed. A new chiral PHOX ligand was identified that provides α,α-disubstituted nitriles in good yields and with high enantioinduction. This is the first example of a Ni-catalyzed asymmetric reductive cross-coupling reaction that tolerates N- and S-heterocyclic coupling partners, and demonstrates the feasibility of developing related transformations of electrophiles containing Lewis basic functional groups. The development of new asymmetric reductive cross-coupling reactions as well as mechanistic investigations are the subject of ongoing research in our laboratory.
Supplementary Material
Scheme 1.
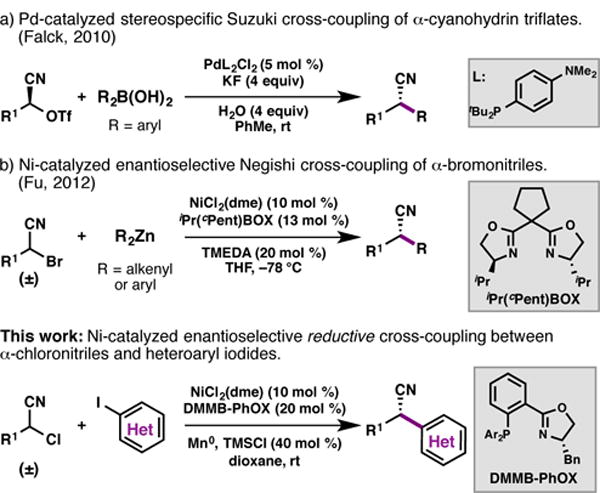
Transition metal-catalyzed cross-coupling reactions of α-cyano electrophiles.
Table 3.
Scope of α-chloronitriles.a
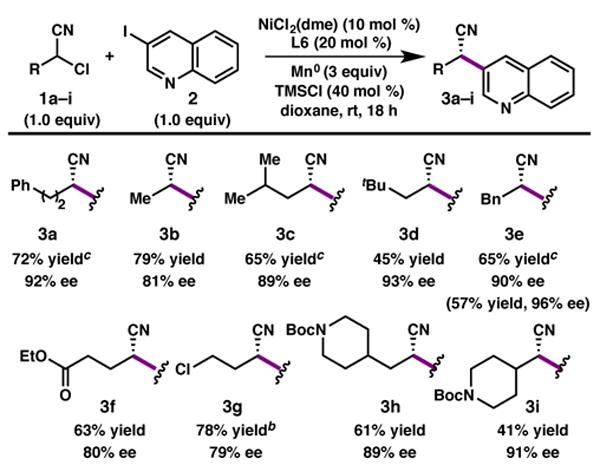
|
Reaction conducted on 0.2 mmol scale. Isolated yields are provided; ee is determined by SFC using chiral stationary phase. Values in parentheses are yield and ee following a single recrystallization of the product.
2.0 equiv heteroaryl iodide used.
1 equiv NaBF4 added.
Acknowledgments
We thank Prof. Brian Stoltz, Dr. Scott Virgil, and the Caltech Center for Catalysis and Chemical Synthesis for access to analytical equipment. We also thank Dr. Alan Cherney and Robert Scanes for helpful discussions. S.E.R. is a Camille Dreyfus Teacher-Scholar and an American Cancer Society Research Scholar. Financial support from NIH (GM111805-01), Amgen, Novartis, and Eli Lilly is gratefully acknowledged.
Footnotes
Supporting Information. Detailed experimental procedures, compound characterization data, 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Everson DA, Weix DJ. J Org Chem. 2014;79:4793. doi: 10.1021/jo500507s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Knappke CE, Grupe S, Gartner D, Corpet M, Gosmini C, Jacobi von Wangelin A. Chem Eur J. 2014;20:6828. doi: 10.1002/chem.201402302. [DOI] [PubMed] [Google Scholar]; (c) Moragas T, Correa A, Martin R. Chem Eur J. 2014;20:8242. doi: 10.1002/chem.201402509. [DOI] [PubMed] [Google Scholar]; (d) Weix DJ. Acc Chem Res. 2015 doi: 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Durandetti M, Gosmini C, Périchon J. Tetrahedron. 2007;63:1146. [Google Scholar]; (b) Everson DA, Shrestha R, Weix DJ. J Am Chem Soc. 2010;132:920. doi: 10.1021/ja9093956. [DOI] [PubMed] [Google Scholar]; (c) Yu X, Yang T, Wang S, Xu H, Gong H. Org Lett. 2011;13:2138. doi: 10.1021/ol200617f. [DOI] [PubMed] [Google Scholar]; (d) Wotal AC, Weix DJ. Org Lett. 2012;14:1476. doi: 10.1021/ol300217x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wu F, Lu W, Qian Q, Ren Q, Gong H. Org Lett. 2012;14:3044. doi: 10.1021/ol3011198. [DOI] [PubMed] [Google Scholar]
- 3.(a) Cherney AH, Kadunce NT, Reisman SE. J Am Chem Soc. 2013;135:7442. doi: 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]; (b) Cherney AH, Reisman SE. J Am Chem Soc. 2014;136:14365. doi: 10.1021/ja508067c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ackerman LK, Anka-Lufford LL, Naodovic M, Weix DJ. Chem Sci. 2015;6:1115. doi: 10.1039/c4sc03106g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For analyses of heterocycles in drug candidates, see:; (a) Roughley SD, Jordan AM. J Med Chem. 2011;42:3451. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]; (b) Vitaku E, Smith DT, Njardarson JT. J Med Chem. 2014;57:10257. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 5.The asymmetric Ni-catalyzed reductive cross-coupling of (E)-2-(2-bromovinyl)furan is the only previously reported example of a heterocyclic substrate (see ref. 3b). For examples of non-asymmetric Ni-catalyzed reductive cross-coupling of heterocyclic electrophiles, see:; (a) Molander GA, Traister KM, O’Neill BT. J Org Chem. 2014;79:5771. doi: 10.1021/jo500905m. [DOI] [PubMed] [Google Scholar]; (b) Molander GA, Traister KM, O’Neill BT. J Org Chem. 2015;80:2907. doi: 10.1021/acs.joc.5b00135. [DOI] [PubMed] [Google Scholar]; (c) Wang S, Qian Q, Gong H. Org Lett. 2012;14:3352. doi: 10.1021/ol3013342. [DOI] [PubMed] [Google Scholar]; (d) Molander GA, Wisniewski SR, Traister KM. Org Lett. 2014;16:3692. doi: 10.1021/ol501495d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murahashi S-I, editor. Science of Synthesis. Vol. 19 Georg Thieme Verlag; Stuttgart, Germany: 2004. [Google Scholar]
- 7.(a) Fleming FF. Nat Prod Rep. 1999;16:597. [Google Scholar]; (b) Fleming FF, Yao L, Ravikumar PC, Funk L, Shook BC. J Med Chem. 2010;53:7902. doi: 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He A, Falck JR. J Am Chem Soc. 2010;132:2524. doi: 10.1021/ja910582n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J, Fu GC. J Am Chem Soc. 2012;134:9102. doi: 10.1021/ja303442q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacMillan and coworkers recently reported an enantioselective cross-coupling of primary α-bromonitriles with aldehydes under photoredox organocatalysis.; Welin ER, Warkentin AA, Conrad JC, MacMillan DWC. Angew Chem Int Ed. doi: 10.1002/anie.201503789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.For a single example of a Ni-catalyzed electrochemical cross-coupling of 2-chloropropanenitrile and (E)-(2-bromovinyl)benzene, see:; Cannes C, Condon S, Durandetti M, Perichon J, Nedelec JY. J Org Chem. 2000;65:4575. doi: 10.1021/jo000182f. [DOI] [PubMed] [Google Scholar]
- 12.Hartwig JF. Organotranisition Metal Chemistry. University Science Books; Mill Valley, CA: 2010. [Google Scholar]
- 13.This chemoselectivity is notable given these prior reports of Ni-catalyzed reductive cross-couplings of 2-chloropyridines:; (a) Gosmini C, Bassene-Ernst C, Durandetti M. Tetrahedron. 2009;65:6141. [Google Scholar]; (b) Everson DA, Buonomo JA, Weix DJ. Synlett. 2014;25:233. doi: 10.1055/s-0033-1340151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Electron deficient (non-hetero) aryl iodides are also competent coupling partners, providing the products in good yields but more modest enantioselectivity. For example, 1-iodo-4-(trifluoromethyl)benzene couples to 3a in 76% yield and 70 % ee
- 15.See Supporting Information
- 16.(a) Ghaffar T, Parkins AW. Tetrahedron Lett. 1995;36:8657. [Google Scholar]; (b) Ghaffar T, Parkins AW. J Mol Catal A: Chem. 2000;160:249. [Google Scholar]
- 17.Nonhebel DC. Chem Soc Rev. 1993;5:293. [Google Scholar]
- 18.Addition of TEMPO to the reaction mixture results in poor conversion, presumably from catalyst inhibition. There is no evidence of trapping of carbon-based radicals.
- 19.For mechanistic investigations of related Ni-catalyzed reactions, see:; (a) Ren Q, Jiang F, Gong H. J Organomet Chem. 2014;770:130. [Google Scholar]; (b) Biswas S, Weix DJ. J Am Chem Soc. 2013;135:16192. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gutierrez O, Tellis JC, Primer DN, Molander GA, Kozlowski MC. J Am Chem Soc. 2015;137:4896. doi: 10.1021/ja513079r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


