Abstract
Economic evaluation using dynamic transmission models is important for capturing the indirect effects of infectious disease interventions. We examine the use of these methods in low‐ and middle‐income countries, where infectious diseases constitute a major burden. This review is comprised of two parts: (1) a summary of dynamic transmission economic evaluations across all disease areas published between 2011 and mid‐2014 and (2) an in‐depth review of mosquito‐borne disease studies focusing on health economic methods and reporting. Studies were identified through a systematic search of the MEDLINE database and supplemented by reference list screening. Fifty‐seven studies were eligible for inclusion in the all‐disease review. The most common subject disease was HIV/AIDS, followed by malaria. A diverse range of modelling methods, outcome metrics and sensitivity analyses were used, indicating little standardisation. Seventeen studies were included in the mosquito‐borne disease review. With notable exceptions, most studies did not employ economic evaluation methods beyond calculating a cost‐effectiveness ratio or net benefit. Many did not adhere to health care economic evaluations reporting guidelines, particularly with respect to full model reporting and uncertainty analysis. We present a summary of the state‐of‐the‐art and offer recommendations for improved implementation and reporting of health economic methods in this crossover discipline. © 2016 The Authors. Health Economics published by John Wiley & Sons Ltd.
Keywords: economic evaluation, dynamic transmission modelling, low income, infectious disease
1. Introduction
In recent years development health assistance has grown to exceed US$ 30 billion annually, of which 35% is directed towards three infectious diseases: HIV/AIDS, malaria and tuberculosis (Dieleman et al., 2014). Nevertheless, for these and other infectious diseases, financing is insufficient for all potential interventions. Good decision‐making on how best to spend available resources can make a substantial difference to population health. Healthcare economic evaluation can identify where to direct resources so that health gains are maximised by appraising the health return on investment for technologies and services (Briggs et al., 2006; Drummond et al., 2005). However, economic evaluation of infectious diseases can be complex because of the additional indirect effects of infectious disease interventions. That is, a treated or prevented case is a direct outcome in itself but may also reduce disease transmission including mediation by host immunity and drug resistance. Commonly used tools of health economic evaluation modellers such as decision trees or ‘Markov’ models seldom capture these transmission effects. Transmission effects are the focus of the usually separate field of infectious disease mathematical modelling, which aims to simulate disease transmission in a human population based on human behaviour, biology and epidemiology. By incorporating information on intervention costs and cost of illness, transmission models can be used for economic evaluation, thus capturing both the direct and indirect effects of an infectious disease intervention in the evaluation. This is particularly important for the evaluation of interventions and policies that seek to reduce the transmission of disease, such as mass vaccination, in contrast to interventions that principally seek to improve direct health outcomes without necessarily impacting disease transmission, such as case management of severe illness. While these joint models can be complex and computationally intensive, they are becoming more widely used as the computing capacity readily available to researchers continues to rise.
In 2011, Jit and Brisson published an introduction to methods for modelling infectious diseases for decision analysis (a broader discipline that encompasses economic evaluation) (Jit and Brisson, 2011), and the following year a working group report by Pitman et al., offered some ‘best practices’ in dynamic transmission modelling for pharmacoeconomics (Pitman et al., 2012). In this review, we use the term dynamic transmission economic evaluation (DT‐EE) and define as a modelling analysis where i) the force of infection is dependent on the model state in a previous time step and ii) that makes a comparison of the costs and effects of one or more interventions or events. DT‐EE methodology has thus far been developed primarily by researchers in high income country (HIC) settings. However infectious diseases are overwhelmingly a problem of low‐ and middle‐income countries (LMICs), where evidence‐based decision‐making stands to make a far greater impact on health. In particular, mosquito‐borne diseases such as malaria and dengue are a major burden in LMICs yet almost absent from HICs. In recent years, resources available for the control and elimination of mosquito‐borne diseases, particularly malaria, have increased considerably. Political commitment to the elimination of malaria is strong, but the practical approach to achieve this remains unclear.
To the knowledge of the authors, there are no previous literature reviews on the state‐of‐the‐art of DT‐EE, whether in HICs or LMICs. This review aims to examine the literature base of such studies in LMICs and examines the scope, methods and reporting of such studies from a health economist's perspective.
2. Methods
The review is comprised of two parts: first, a broad summary of DT‐EE studies in all infectious disease areas published in the peer‐reviewed literature and second, a more in‐depth review of DT‐EE studies of mosquito‐borne disease interventions. The two‐part review provides both an understanding of the application of these methods across varied disease‐specific research ‘silos’ as well as a more detailed review of mosquito‐borne disease studies.
Studies are included in the general review if (1) the paper compares costs and effects of an infectious disease intervention; (2) the model includes a dynamic force of infection (i.e. incidence, a rate at which the susceptible population acquire the infection) that depends on the prevalence of infection at a previous time point; (3) the paper was published between 1 January 2011 and 31 May 2014; and (4) the study focuses partially or entirely on a population in a LMIC according to World Bank definitions (World Bank, 2015). The time period for inclusion in the all‐disease review is restricted to recent years because of the time required to screen articles against the inclusion criteria and to focus on the contemporary state‐of‐the‐art DT‐EE. Studies are included in the review of mosquito‐borne diseases if they meet previous criteria 1–3 and evaluate one or more interventions against a mosquito‐borne disease. In this part of the review the publication year restriction is relaxed to include studies published at any time.
2.1. Search strategy
The literature search is outlined in Figure 1. The MEDLINE database was searched for and was applied to the MEDLINE database. There are four main components to this initial search:
Cost OR economic* (wildcard is used to include word extensions), AND;
Infectious OR communicable, AND;
Dynamic OR transmission, AND;
Date: January 2011–May 2014
Figure 1.
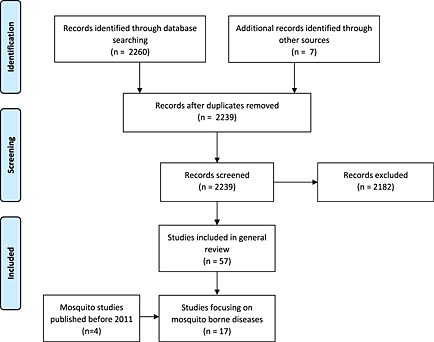
Database search and screening of identified records
The terms ‘cost’ and ‘economic*’ were restricted to title or abstract, while other terms were applied to any field. This search strategy aims to be as sensitive as possible while returning a feasible number of abstracts to be screened. Secondly, the initial search results were supplemented by a series of additional disease‐specific searches:
(Transmission OR dynamic) AND ((compartmental model OR stochastic*) OR individual‐based model) AND
Cost [title/abstract] AND effect*
Date: January 2011–May 2014
Specific disease, for example, HIV OR AIDS OR HIV/AIDS
Disease‐specific search terms included HIV/AIDS, malaria, tuberculosis, influenza, schistosomiasis, polio, respiratory syncytial virus, hepatitis, human papillomavirus, measles, rabies, cholera, pneumococcal disease, meningococcal disease, dengue, rabies, yellow fever and ebola.
Further studies were identified by screening the reference lists of eligible studies or in other relevant reviews (Gomez et al., 2013; Pérez Velasco et al., 2012; Reiner et al., 2013). Because of the nature of the inclusion criteria, abstracts were often insufficient to judge eligibility and full‐text screening was frequently necessary to determine eligibility for inclusion. A cross check of the search was performed on a comprehensive database of health economic evaluations, produced for another paper in this supplement (Pitt et al., 2016). No additional articles meeting the inclusion criteria were identified.
The review of mosquito‐borne disease DT‐EEs includes all those in the all‐disease review as well as additional studies published prior to 2011. Additional studies were identified through disease‐specific searches outlined in Figure 1 and screening of reference lists of identified studies and a recent review of mosquito‐borne disease transmission models (Reiner et al., 2013).
2.2. Data extraction
Both the general and mosquito‐borne disease reviews include data extraction on six fields: disease, intervention, model, outcome measure, sensitivity analysis and journal (Table 1). Some studies included more than one disease, outcome measure or sensitivity analysis therefore totals may exceed 100%. Data extraction for both the general and mosquito‐borne disease reviews was undertaken independently by two reviewers. Differing results were resolved by discussion until consensus was reached.
Table 1.
Data extracted for all‐disease review
| Field | Definition |
|---|---|
| 1. Disease | The infectious disease(s) subject to analysis |
| 2. Intervention | The health care technology or programme subject to analysis |
| 3. Model | The type of model used. Options include the following: |
| ‐ Deterministic compartmental | |
| ‐ Stochastic compartmental | |
| ‐ Individual‐based model | |
| ‐ Multi‐model (two or more of the previously mentioned) | |
| 4. Outcome measure | The metric used to quantify human health. Options include the following: |
| ‐ Disability‐adjusted life years (DALYs) | |
| ‐ Quality‐adjusted life years (QALYs) | |
| ‐ Life years (LYs) | |
| ‐ Infections averted | |
| ‐ Number of deaths or mortality rate | |
| ‐ Net health or monetary benefit (NHB or NMB) | |
| ‐ Fixed endpoint (e.g. elimination) | |
| 5. Sensitivity or uncertainty analysis | The approach taken to quantify potential variation in model results. Options include the following: |
| ‐ Univariate deterministic | |
| ‐ Multivariate or scenario | |
| ‐ Probabilistic | |
| ‐ Structural | |
| 6. Journal | The name of the publication featuring the study |
Reporting standards have been developed within the field of health care economic evaluation so that readers might appraise the methodological integrity of a study. The most recent and widely accepted are the Consolidated Healthcare Economic Evaluation Reporting Standards (CHEERS) guidelines (Husereau et al., 2013). In the mosquito‐borne disease review, all studies, including supplementary materials, were reviewed against the CHEERS guidelines. Data extraction focused on reporting of the basic economic evaluation framing indicators such as perspective, cost details, model description and sensitivity or uncertainty analyses (points 6, 8, 9, 13b, 15, 16, 17 and 20b). In addition, the use of advanced techniques was recorded including probabilistic decision analysis (such as cost‐effectiveness acceptability curves), value of information analysis, resource allocation modelling or programme budgeting and marginal analysis and spatial analysis.
The synthesis of the review examines whether relevant health economic methods are commonly employed and well reported and makes recommendations for future studies.
3. Results
3.1. Search results
The database searches identified 2260 published studies for screening (Figure 1). A total of 57 DT‐EEs were identified for the all‐disease review (Agusto and Adekunle, 2014; Alistar et al., 2014a, 2014b; Babigumira et al., 2011; Bärnighausen et al., 2012; Bishai et al., 2011; Briët et al., 2013; Briet and Chitnis, 2013; Briët and Penny, 2013; Carrasco et al., 2011; Ciaranello et al., 2011; Cremin et al., 2013; Crowell et al., 2013; Durham et al., 2013; Dye, 2013; Eaton et al., 2014; Enns et al., 2011; Fitzpatrick et al., 2014; Freiesleben de Blasio et al., 2014; Giglio et al., 2012; Gomez et al., 2012; Granich et al., 2012; Hontelez et al., 2011, 2013; Hutton and Brandeau, 2013; Kato et al., 2013; Kawai et al., 2012; Keebler et al., 2014; Levin et al., 2011; Li et al., 2012; Long and Stavert, 2013; Luz et al., 2011; Maire et al., 2011; Mbah et al., 2013a, 2013b; Mbonigaba, 2013; Menzies et al., 2012; Nichols et al., 2013; Okell et al., 2014; Okosun et al., 2011, 2013; Okosun and Makinde, 2012; Palombi et al., 2012; Prinja et al., 2011; Ross et al., 2011; Sardar et al., 2013; Sartori et al., 2012; Scott Braithwaite et al., 2014; Stuckey et al., 2014; Terris‐Prestholt et al., 2014; Vanni et al., 2012; Verguet et al., 2013; von Wyl et al., 2012; Wagner and Blower, 2012; Walensky et al., 2012, 2013; Winetsky et al., 2012).
From this set, 13 studies of mosquito‐borne diseases, plus a further 4 studies of mosquito‐borne diseases published prior to 2011, were included in a more detailed review (Briët et al., 2013; Briet and Chitnis, 2013; Briët and Penny, 2013; Crowell et al., 2013; Durham et al., 2013; Tediosi et al., 2009; Laxminarayan, 2004; Laxminarayan et al., 2006; Luz et al., 2011; Maire et al., 2011; Okell et al., 2014; Okosun et al., 2011, 2013; Okosun and Makinde, 2012; Ross et al., 2011; Stuckey et al., 2014; Tediosi et al., 2006).
3.2. Summary of recent dynamic transmission economic evaluations in LMICs
By far the most common disease studied in DT‐EEs was HIV/AIDS (n = 30, 53%), followed by malaria (n = 11, 19%). A range of interventions were studied including vaccination (n = 14, 25%) and pharmaceutical therapy as either treatment (n = 7, 12%), prophylaxis (n = 5, 9%) or mass administration (including mass screening and treatment) (n = 4, 8%). Eighteen studies used a model to consider multiple interventions simultaneously (32%). As with economic evaluation in other disease areas, pharmaceuticals and other health technologies are better represented than non‐technological interventions (Drake et al., 2012). This review finds only one study that focuses on a non‐technological intervention (Enns et al., 2011).
The majority of studies (n = 33, 58%) used a deterministic compartmental model, while 18 studies (32%) used an individual‐based model. Three studies (5%) used a stochastic implementation of a compartmental model, and a further three studies (5%) deployed multiple model structures. A variety of outcome metrics were reported, the most common of which were infections averted (n = 40, 69%) and DALYs (n = 22, 38%). The majority of studies (n = 41, 72%) reported two or more outcome measures. Elimination was used as an outcome metric by two studies (4%). While not all studies performed explicit sensitivity analysis, all went some way towards exploring variation in results. The majority of studies conducted univariate sensitivity analysis (n = 32, 78%), multivariate or scenario analysis (n = 27, 66%) and/or probabilistic analysis (n = 19, 46%). Probabilistic analysis was in some cases incorporated into the transmission model from the outset rather than conducted as a post hoc sensitivity or uncertainty analysis. Results for the all‐disease review are summarised in Table 2. Studies were published in a wide range of journals. The most common journal was PLoS One (n = 8, 14%). PLoS Medicine, Vaccine, AIDS and Malaria Journal had all published four studies each (7%).
Table 2.
Summary of dynamic transmission economic evaluations in low‐ and middle‐income contexts (all diseases, 2011 to May 2014)
| Field | Frequency | % |
|---|---|---|
| Disease | ||
| Cholera | 1 | 2% |
| Dengue | 2 | 4% |
| HIV | 26 | 46% |
| Human papilloma virus | 2 | 4% |
| Malaria | 11 | 19% |
| Measles | 3 | 5% |
| Pandemic influenza | 1 | 2% |
| Rabies | 1 | 2% |
| Seasonal influenza | 1 | 2% |
| Tuberculosis | 2 | 4% |
| Hepatitis A | 1 | 2% |
| Hepatitis B | 1 | 2% |
| Herpes simplex virus | 1 | 2% |
| HIV and tuberculosis | 2 | 4% |
| HIV and schistosomiasis | 2 | 4% |
| 57 | 100% | |
| Intervention type | ||
| Contact reduction | 1 | 2% |
| Diagnostic | 3 | 5% |
| Mass treatment | 2 | 4% |
| Mass screening and treatment | 2 | 4% |
| Multiple | 18 | 32% |
| Prophylaxis | 5 | 9% |
| Screening | 1 | 2% |
| Treatment | 7 | 12% |
| Vaccine | 14 | 25% |
| Vector control | 4 | 7% |
| 57 | 100% | |
| Primary outcome | ||
| Disability‐adjusted life year | 22 | 38% |
| Elimination | 2 | 3% |
| Infections averted | 40 | 69% |
| Life year | 12 | 21% |
| Mortality | 16 | 28% |
| Net health benefit | 4 | 7% |
| Net monetary benefit | 4 | 7% |
| Quality‐adjusted life year | 14 | 24% |
| 114 | 197% | |
| Model type | ||
| Deterministic compartmental | 33 | 58% |
| Stochastic compartmental | 3 | 5% |
| Individual | 18 | 32% |
| Multi‐model | 3 | 5% |
| 57 | 100% | |
| Sensitivity analysis | ||
| Univariate | 32 | 78% |
| Multivariate or scenario | 27 | 66% |
| Probabilistic | 19 | 46% |
| Structural | 5 | 12% |
| 83 | 202% |
3.3. Review of mosquito‐borne disease studies
Of the 17 studies of mosquito‐borne diseases, 15 consider malaria interventions and two dengue interventions (Table 3). A range of interventions were evaluated including vaccination (n = 4, 24%), treatment (n = 3, 18%), vector control (n = 4, 24%), mass screening (n = 1, 6%) and intermittent preventive treatment (n = 1, 6%). Some studies evaluated multiple integrated approaches (n = 4, 24%). The evaluation perspective and time horizon were generally identifiable but often not explicitly stated. The same number of studies purported to take a societal perspective (n = 7, 41%) as a provider perspective (n = 7, 41%), but it was not always clear that all relevant societal costs, such as patient financial costs and the opportunity costs of patient and caregivers' time were included in studies reporting a societal perspective. Time horizons varied considerably, and most studies did not include time horizon in the sensitivity analysis. The most common outcome metrics reported were DALYs (n = 9) and infections averted (n = 9). No studies explicitly cited elimination as an outcome metric; four studies (24%) use the relatively uncommon net health benefit metric. Clear and detailed costing information is only reported in full in a minority of studies. Cost information is in some cases fragmented in different sections of the paper or supporting documents and is usually not tabulated with the other model parameters.
Table 3.
Review of dynamic transmission economic evaluations in low‐ and middle‐income countries (mosquito‐borne diseases, any publication year)
| First author | Year | Country or region | CHEERS review | ||||
|---|---|---|---|---|---|---|---|
| Perspective (6.) | Intervention (7.) | Time horizon (8.) | Health outcome measure (10.) | Describes costs including sources and approximations using opportunity cost (13b.) | |||
| Okell | 2014 | Africa | Provider | Treatment | 5 years | Infections averted | Clear description of costs and sources |
| Stuckey | 2014 | Kenya | Societal | Multiple | 5 years | DALY and infections averted and mortality | Clear description of costs and sources |
| Briët (a) | 2013 | Not specified | Provider (‘health system’) | Vector control | Lifetime of the intervention | DALY and NHB | Refers to previous paper |
| Briët (b) | 2013 | Not specified | Provider (health system) | Vector control | Lifetime of the intervention | DALY and infections averted and NHB | Some information but not comprehensive |
| Briët (c) | 2013 | Not specified | Provider (health system) | Vector control | 60 years | DALY and NHB | Some information but not comprehensive |
| Crowell | 2013 | Sub‐Saharan Africa | Provider | MSAT | 1 year | Infections averted | Clear description of costs and sources |
| Okosun (a) | 2013 | Not specified | Not found | Multiple | 140 days | Infections averted | Little information |
| Durhama | 2013 | Brazil | Societal (but only cost of vaccine and cost of illness) | Vaccine | 73 years | DALY and NHB | Some information. Cites a study on the cost of dengue treatment in the Americas, little information on processing or generalisability |
| Okosun (b) | 2012 | Not specified | Not found | Multiple | 1 year | Infections averted | Little information |
| Maire | 2011 | Sub‐Saharan Africa | Societal | Vaccine | 10 years | DALY | Clear description of costs and sources |
| Ross | 2011 | Sub‐Saharan Africa | Provider (implied) | Intermittent presumptive treatment | 10 years | DALY | Some information. Describes intervention costs but does not report case management unit costs in the methods |
| Okosun (c) | 2011 | Not specified | Not found | Multiple | 100 days | Infections averted | Little information |
| Luza | 2011 | Brazil | Societal | Vector control | 5 years | DALY | Clear description of costs and sources (in web appendix) |
| Tediosi (a) | 2009 | Tanzania | Societal | Vaccine | 10 years | DALY and infections averted | Clear description of costs and sources, although referencing is relied on |
| Tediosi (b) | 2006 | Tanzania | Societal | Vaccine | 20 years | DALY and life years and infections averted and mortality | Clear description of costs and sources, although referencing is relied on (reference paper is part of the same journal supplement) |
| Laxminarayan (a) | 2006 | Sub‐Saharan Africa | Societal (implied) | Treatment | 10 years | Mortality | Clear description of costs and sources, although cost parameters are not tabulated |
| Laxminarayan (b) | 2004 | Sub‐Saharan Africa | Provider (implied) | Treatment | 5, 10 and 20 years | Infections treated | Clear description of costs and sources, although cost parameters are not tabulated |
| Describe and justify model (15.) | Parameter uncertainty for all parameters and structural uncertainty (20b.) | Advanced Analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| First author | Model type | Pre‐published model (Y/N) | Sensitivity analysis methods used | Includes cost parameters? | Probabilistic decision analysis (Y/N) | Value of information analysis (Y/N) | Resource allocation or programme budgeting (Y/N) | Spatial modelling (Y/N) |
| Okell | Individual | Y | Univariate and probabilistic | N | N | N | N | Y |
| Stuckey | Individual | Y | Univariate and probabilistic and structural | Only univariate | N | N | N | N |
| Briët (a) | Individual | Y | Probabilistic | N | N | N | N | N |
| Briët (b) | Individual | Y | Probabilistic | N | N | N | N | N |
| Briët (c) | Individual | Y | Scenario and probabilistic and structural | N | N | N | N | N |
| Crowell | Individual | Y | Multivariate | N | N | N | N | N |
| Okosun (a) | Deterministic | N | Scenario | N | N | N | N | N |
| Durhama | Deterministic | N | Scenario and probabilistic | Y | Y | N | N | N |
| Okosun (b) | Deterministic | N | Scenario | N | N | N | N | N |
| Maire | Individual | Y | Probabilistic | Y | Y | Y | N | N |
| Ross | Individual | Y | Multivariate | Y | N | N | N | N |
| Okosun (c) | Deterministic | N | Scenario | N | N | N | N | N |
| Luza | Deterministic | Y | Probabilistic | Y | Y | N | N | N |
| Tediosi (a) | Individual | Y | Multivariate | Mentioned but not quantified in main paper | N | N | N | N |
| Tediosi (b) | Individual | Y | Scenario | Y | N | N | N | N |
| Laxminarayan (a) | Deterministic | Y | Scenario | Y | N | N | N | N |
| Laxminarayan (b) | Deterministic | N | Univariate | Y | N | N | N | N |
Dengue studies, all others are on malaria.
Half the malaria studies (n = 9, 53%) used versions of the same ‘open malaria’ individual‐based model. One other malaria study (6%) used a different individual‐based model, while the remaining five malaria studies and two dengue studies (12%) used deterministic compartmental models. The majority of studies (n = 11, 65%) referred to previously published work for a full description of the transmission model. In some cases, but not all, a brief description of the model was provided. Conversely, the three studies (18%) by Okosun and colleagues focused primarily on reporting the description and behaviour of the model, such as the identification of equilibria, model boundaries and optimal control points (Okosun et al., 2011, 2013; Okosun and Makinde, 2012). In these studies, less attention was paid to the description of and justification of economic or operational factors. A majority of studies conducted a multivariate or scenario sensitivity analysis (n = 10, 59%), and just under half conducted probabilistic analysis (n = 8, 47%). A common theme in the quantification of parameter sensitivity or uncertainty is that studies frequently focus principally on epidemiological parameters. In the majority of studies (n = 10, 59%), cost parameters were not included in the sensitivity or uncertainty analysis or were treated separately. The most common funder was the Bill and Melinda Gates Foundation (n = 7, 41%). One study (6%) was funded by a commercial bed net manufacturer.
The majority of studies (n = 14, 82%) did not use advanced health economic methods. The study by Maire et al. is a notable exception as it used a complex dynamic transmission model to undertake probabilistic decision analysis and also performed a value of information analysis (Maire et al., 2011). The two dengue studies also employed probabilistic decision analysis and presented results in terms of a cost‐effectiveness acceptability curve (Durham et al., 2013; Luz et al., 2011). Okell et al. undertook spatially explicit analysis, applying their model to country‐specific data in sub‐Saharan Africa (Okell et al., 2014). No studies included resource allocation or programme budgeting in their analysis.
4. Discussion
This review outlines the literature base for dynamic transmission economic evaluation (DT‐EE) in LMICs and appraises reporting practices and health economic methods for a smaller number of studies addressing mosquito‐borne disease.
The first section of the review outlines the scope of the literature across all disease areas. The majority of studies consider either HIV or malaria. Both diseases are the focus of major global efforts to reduce disease transmission and both are relatively well financed. The potential impact of efficient resource allocation is therefore greater and research funds are more readily available. There were notably fewer studies on other major infectious disease burdens in LMICs, including tuberculosis, pneumonia and diarrhoeal disease. This may be to be because of challenges in modelling disease transmission, particularly for pneumonia and diarrhoeal disease where multiple aetiologies exist. Tuberculosis, like HIV and malaria, has a dedicated modelling consortium to support a range of modelling studies including DT‐EEs (Dowdy et al., 2014).
The role of DT‐EE is particularly important in the evaluation of vaccinations and in the comparison of multiple interventions. For single intervention evaluations, vaccination was the most common intervention. This is not surprising given the level of investment in the development and implementation of vaccination programmes and that key benefits of vaccination programmes include herd immunity ; a reduction in disease transmission that is best captured using dynamic transmission modelling. The largest share of studies evaluated multiple interventions and intervention combinations rather than single interventions. Transmission models are well suited to incorporating diverse modes of action for various interventions simultaneously. Alternative health economic models such as decision trees are usually developed for a specific intervention and although they can be used for comparing multiple interventions, modifying them to include additional interventions and interactions between interventions can be difficult and cumbersome.
The second stage of the review appraises health economic methods and reporting for set of mosquito‐borne disease studies using existing guidelines for reporting economic evaluations (Husereau et al., 2013), explores the handling of parameter uncertainty as well as the use of advanced economic methods and makes recommendations for future DT‐EEs.
Comprehensive reporting is key to good quality economic evaluation. In some cases, very few of the CHEERS checklist points were reported well. While this is understandable in that these studies often originate in the discipline of mathematical modelling, which does not share these reporting norms, transmission modelling studies that make comparisons of cost and health impact are de facto economic evaluations. This review finds three areas where reporting can be improved: evaluation perspective, costing and model description.
The perspective of the study should be clearly stated and applied equally to costs and effects. Costing methods and data sources should be clearly described including the approach to discounting, currency exchanges and a justification of what resources were costed. Several studies reference previously published work to describe the transmission model. Full reporting of all model and analytical information in the main body of a paper may not be feasible if the journal does not support technical appendices. However, without clear communication of methods to the reader, complex models must be taken on trust; where possible model description should be reported in full. Overall, adherence to the best practices for undertaking and reporting economic evaluation would improve quality and communication in DT‐EEs. Publication of these studies is across a wide range of journals with diverse specialisations. If the rate of publication continues to rise, a dedicated journal may help to improve methodology and reporting practices.
Analysis and communication of uncertainty is particularly important in LMIC DT‐EEs. A potential risk in layering complex analyses on complex models is that detail and complexity can create a perception of validity. Moreover, in LMICs, data collection systems face considerable challenges and the resulting data sets may be far from robust. Thorough sensitivity or uncertainty analysis is therefore essential. While all studies in this review conducted sensitivity analysis to some degree, in many cases the approach was partial or treated economic and epidemiological parameters separately. This may lead to misinterpretation if confidence or credible intervals are reported for a cost‐effectiveness ratio, but only epidemiological uncertainty is included. Pitman et al. point out that a comprehensive probabilistic analysis may also be problematic if parameter values cannot be assumed to be independent, that is, if there are unidentified joint parameters (Pitman et al., 2012). However if researchers can address this, then probabilistic analysis is likely to be the best approach to quantifying parameter uncertainty. Indeed, parameter fitting processes more common in transmission modelling methods offer some options for fitting correlated parameters (Li et al., 2010). Otherwise, a univariate analysis that includes both economic and epidemiological parameters can highlight key determinants of model uncertainty, allowing direct comparison of uncertainty or sensitivity for all model parameters. A comprehensive appraisal of parameter uncertainty can be supplemented but not replaced, by further scenario or multivariate sensitivity analysis. This can, for example, elucidate operational decisions such as seasonal timing of vaccination or required vaccine uptake at different assumptions of efficacy.
Box 1: Recommendations for dynamic transmission economic evaluations:
All epidemiological and economic parameters are fully described and tabulated.
All studies report a basic description of the model structure and key assumptions. For journals that support web appendices, the transmission model should be described in full including model equations, software platform and all analytical processes involved in parameterisation.
Economic parameters are included along with epidemiological and other parameters in sensitivity and uncertainty analyses.
Where appropriate, studies go beyond simple cost‐effectiveness ratio or net benefit calculation and employ advanced economic evaluation methods.
If publications in this area continue to rise, a dedicated journal could improve methodology and reporting standards.
Most studies did not employ advanced health economic methods and went no further with economic analysis than calculating disaggregated costs and effects, a cost‐effectiveness ratio or net benefit. Those that did (Luz et al., 2011; Maire et al., 2011) illustrate some of the advantages of probabilistic decision analysis, including cost‐effectiveness acceptability curves (CEACs), and value of information analysis. For example, the inclusion of CEACs goes some way towards dealing with the uncertainty related to the fact that cost‐effectiveness thresholds for LMICs are usually not well defined. Studies that rely on a single threshold value undermine efforts made to produce more precise estimates of costs and effects through dynamic transmission modelling, by introducing considerable unquantified uncertainty in the cost‐effectiveness threshold chosen (Drake, 2014; Lubell, 2014; Marseille et al., 2015; Revill et al., 2014). The presentation of CEACs helps to illustrate the impact of cost‐effectiveness threshold choice.
Value of information analysis can indicate the potential value of further research into a decision problem. The key piece of information provided by value of information analyses is typically an estimate of the monetary value of completely reducing uncertainty in the decision problem, known as the expected value of perfect information (EVPI). If the cost of further research to reduce this uncertainty is greater than this value, then the research is not warranted, and conversely, if the value of information is much greater than the cost of research, then further research could be of benefit, depending on the extent to which the uncertainty could be resolved (Briggs et al., 2006). Maire et al. estimate the EVPI, of a pre‐erythrocytic malaria vaccine to be $ 1.9 billion, suggesting that further research to better inform the decisions could be worth investment. This type of analysis can be extended to estimate the expected value of perfect parameter information, the value of reducing uncertainty in a specific parameter, providing more detailed information on the value of uncertainty relating to specific parameter, for example, the potential value of reduced uncertainty in vaccine effectiveness. In general, the application of advanced economic evaluation methods in more DT‐EEs could yield useful results.
The two studies by Laxminaryan et al. are worth noting in that they tackle the critical question of antimicrobial resistance (Laxminarayan, 2004; Laxminarayan et al., 2006). Dynamic transmission models are an important tool in modelling the spread of antimicrobial resistance, and the incorporation of costs into these models has the potential to quantify the economic impact in addition to the health impact. This is a critical component in the evaluation of diagnostics and other interventions that can mitigate the emergence and spread of resistance. The capacity to incorporate the impact of interventions on the dynamics of drug resistance is a further example of the flexibility of dynamic transmission modelling.
This review has several limitations. The set of studies identified was reviewed with the aim of describing the scope, methods and reporting practices of DT‐EEs from the perspective of a health economist. An evaluation of transmission modelling methods was beyond the scope of this review. Correctly identifying DT‐EEs is a challenge as there is no specific or succinct label for this type of study. Studies can originate from mathematical modelling or economic evaluation disciplines, and each has its own standards and reporting norms. Studies that are a combination of both transmission modelling and economic evaluation methods often do not signpost this clearly in the abstract, and it was frequently necessary to refer to the full text. Even then, eligibility for inclusion in the review was not always clear as economic evaluations that do not dynamically model disease transmission also use terms such as ‘dynamic’ and ‘transmission’ (not incorrectly) to describe aspects of their models. The full all‐disease review is therefore limited in its time period because of the challenges of the search and screening processes. Despite the challenges in the search, this study identifies two literature sets representing a powerful yet relatively uncommon combination of methods. To the authors' knowledge, this the first review of this literature base.
DT‐EE is an emerging field at the intersection of two disciplines and is particularly relevant to LMICs, where infectious diseases constitute an enormous burden on human health. This review outlines the current landscape in this field and identifies priority areas to improve the implementation of methodology and reporting.
Conflict of Interest
The authors declare that they are aware of no potential conflicts.
Original Publication
This review is original work and has not been submitted for publication elsewhere.
Ethics Statement
This is a desk‐based literature review using only publicly available sources. No specific ethical review was obtained for this study.
Acknowledgements
The authors are grateful to Catherine Pitt, Anna Vassall and Matthew Quaife for insightful comments on an earlier draft of the manuscript.
TLD, LJW and YL are supported by research grants from Three Millennium Development Goal Fund (3MDG) and the Bill and Melinda Gates Foundation (BMGF). Mahidol‐Oxford Tropical Medicine Research Unit is supported by the Wellcome Trust.
Drake, T. L. , Devine, A. , Yeung, S. , Day, N. P. J. , White, L. J. , and Lubell, Y. (2016) Dynamic Transmission Economic Evaluation of Infectious Disease Interventions in Low‐ and Middle‐Income Countries: A Systematic Literature Review. Health Econ., 25: 124–139. doi: 10.1002/hec.3303.
The copyright line for this article was changed on 21 January 2016 after original online publication.
Footnotes
In health economics, a Markov model typically uses a pre‐defined force of infection. Dynamic transmission models may also hold Markov properties but are not usually referred to as Markov models.
Population health impact arising from mass vaccination is greater than the sum of individual vaccine protection. Unvaccinated individuals also benefit from immunity of the ‘herd’.
References
- Agusto FB, Adekunle AI. 2014. Optimal control of a two‐strain tuberculosis‐HIV/AIDS co‐infection model. Biosystems 119: 20–44. DOI:10.1016/j.biosystems.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Alistar S, Grant P, Bendavid E. 2014a. Comparative effectiveness and cost‐effectiveness of antiretroviral therapy and pre‐exposure prophylaxis for HIV prevention in South Africa. BMC Medicine 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alistar SS, Owens DK, Brandeau ML. 2014b. Effectiveness and cost effectiveness of oral pre‐exposure prophylaxis in a portfolio of prevention programs for injection drug users in mixed HIV epidemics. PLoS ONE 9: e86584. DOI:10.1371/journal.pone.0086584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babigumira JB, Levin A, Burgess C, Garrison LP, Bauch CT, Braka F, Mbabazi WB, Nabyonga JO, Simons E, Dabbagh A. 2011. Assessing the cost‐effectiveness of measles elimination in uganda: local impact of a global eradication program. Journal of Infectious Diseases 204: S116–S123. DOI:10.1093/infdis/jir132. [DOI] [PubMed] [Google Scholar]
- Bärnighausen T, Bloom DE, Humair S. 2012. Economics of antiretroviral treatment vs. circumcision for HIV prevention. Proceedings of the National Academy of Sciences of the United States of America 109: 21271–21276. DOI:10.1073/pnas.1209017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishai D, Johns B, Nair D, Nabyonga‐Orem J, Fiona‐Makmot B, Simons E, Dabbagh A. 2011. The cost‐effectiveness of supplementary immunization activities for measles: a stochastic model for Uganda. Journal of Infectious Diseases 204: S107–S115. DOI:10.1093/infdis/jir131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briet OJ, Chitnis N. 2013. Effects of changing mosquito host searching behaviour on the cost effectiveness of a mass distribution of long‐lasting, insecticidal nets: a modelling study. Malaria Journal 12: 215 DOI:10.1186/1475-2875-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briët OJ, Penny MA. 2013. Repeated mass distributions and continuous distribution of long‐lasting insecticidal nets: modelling sustainability of health benefits from mosquito nets, depending on case management. Malaria Journal 12: 401 DOI:10.1186/1475-2875-12-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briët OJ, Penny MA, Hardy D, Awolola TS, Bortel WV, Corbel V, Dabiré RK, Etang J, Koudou BG, Tungu PK, Chitnis N. 2013. Effects of pyrethroid resistance on the cost effectiveness of a mass distribution of long‐lasting insecticidal nets: a modelling study. Malaria Journal 12: 77 DOI:10.1186/1475-2875-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs A, Sculpher M, Claxton K. 2006. Decision Modelling for Health Economic Evaluation(1st edn). Oxford University Press: UK. [Google Scholar]
- Carrasco LR, Lee VJ, Chen MI, Matchar DB, Thompson JP, Cook AR. 2011. Strategies for antiviral stockpiling for future influenza pandemics: a global epidemic‐economic perspective. Journal of the Royal Society Interface 8: 1307–1313. DOI:10.1098/rsif.2010.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaranello AL, Lockman S, Freedberg KA, Hughes M, Chu J, Currier J, Wood R, Holmes CB, Pillay S, Conradie F, McIntyre J, Losina E, Walensky RP. 2011. First‐line antiretroviral therapy after single‐dose nevirapine exposure in South Africa: a cost‐effectiveness analysis of the OCTANE trial. AIDS (London, England) 25: 479–492. DOI:10.1097/QAD.0b013e3283428cbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremin I, Alsallaq R, Dybul M, Piot P, Garnett G, Hallett TB. 2013. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS (London, England) 27: 447–458. DOI:10.1097/QAD.0b013e32835ca2dd. [DOI] [PubMed] [Google Scholar]
- Crowell V, Briët O, Hardy D, Chitnis N, Maire N, Pasquale A, Smith T. 2013. Modelling the cost‐effectiveness of mass screening and treatment for reducing Plasmodium falciparum malaria burden. Malaria Journal 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman JL, Graves CM, Templin T, Johnson E, Baral R, Leach‐Kemon K, Haakenstad AM, Murray CJL. 2014. Global health development assistance remained steady in 2013 but did not align with recipients’ disease burden. Health Affairs (Millwood) 33: 878–886. DOI:10.1377/hlthaff.2013.1432. [DOI] [PubMed] [Google Scholar]
- Dowdy DW, Houben R, Cohen T, Pai M, Cobelens F, Vassall A, Menzies NA, Gomez GB, Langley I, Squire SB, White R, for the TB MAC meeting participants . 2014. Impact and cost‐effectiveness of current and future tuberculosis diagnostics: the contribution of modelling. The International Journal of Tuberculosis and Lung Disease 18: 1012–1018. DOI:10.5588/ijtld.13.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. 2014. Priority setting in global health: towards a minimum DALY value. Health Economics 23: 248–252. DOI:10.1002/hec.2925. [DOI] [PubMed] [Google Scholar]
- Drake TL, Chalabi Z, Coker R. 2012. Cost‐effectiveness analysis of pandemic influenza preparedness: what's missing? Bulletin of the World Health Organization 90: 940–941. DOI:10.2471/BLT.12.109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MF, Sculpher MJ, Torrance GW. 2005. Methods for the Economic Evaluation of Health Care Programs. Oxford University Press: UK. [Google Scholar]
- Durham D, Mbah M, Medlock J, Luz P, Meyers L, Paltiel A, Galvani A. 2013. Dengue dynamics and vaccine cost‐effectiveness in Brazil. Vaccine 31: 3957–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C. 2013. Making wider use of the world's most widely used vaccine: Bacille Calmette–Guérin revaccination reconsidered. Journal of the Royal Society Interface 10: 20130365: . DOI:10.1098/rsif.2013.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, Cori A, Hontelez JAC, Humair S, Kerr CC, Klein DJ, Mishra S, Mitchell KM, Nichols BE, Vickerman P, Bakker R, Bärnighausen T, Bershteyn A, Bloom DE, Boily M‐C, Chang ST, Cohen T, Dodd PJ, Fraser C, Gopalappa C, Lundgren J, Martin NK, Mikkelsen E, Mountain E, Pham QD, Pickles M, Phillips A, Platt L, Pretorius C, Prudden HJ, Salomon JA, van de Vijver DAMC, de Vlas SJ, Wagner BG, White RG, Wilson DP, Zhang L, Blandford J, Meyer‐Rath G, Remme M, Revill P, Sangrujee N, Terris‐Prestholt F, Doherty M, Shaffer N, Easterbrook PJ, Hirnschall G, Hallett TB. 2014. Health benefits, costs, and cost‐effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Global Health 2: e23–e34. DOI:10.1016/S2214-109X(13)70172-4. [DOI] [PubMed] [Google Scholar]
- Enns EA, Brandeau ML, Igeme TK, Bendavid E. 2011. Assessing effectiveness and cost‐effectiveness of concurrency reduction for HIV prevention. International Journal of STD and AIDS 22: 558–567. DOI:10.1258/ijsa.2011.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick MC, Hampson K, Cleaveland S, Mzimbiri I, Lankester F, Lembo T, Meyers LA, Paltiel AD, Galvani AP. 2014. Cost‐effectiveness of canine vaccination to prevent human rabies in rural Tanzania. Annals of Internal Medicine 160: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiesleben de Blasio B, Flem E, Latipov R, Kuatbaeva A, Kristiansen IS. 2014. Dynamic modeling of cost‐effectiveness of rotavirus vaccination, Kazakhstan. Emerging Infectious Diseases 20: 29–37. DOI:10.3201/eid2001.130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio N, Gentile A, Lees L, Micone P, Armoni J, Reygrobellet C, Crepey P. 2012. Public health and economic benefits of new pediatric influenza vaccination programs in Argentina. Human Vaccines and Immunotherapeutics 8: 312–322. DOI:10.4161/hv.18569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez GB, Borquez A, Caceres CF, Segura ER, Grant RM, Garnett GP, Hallett TB. 2012. The potential impact of pre‐exposure prophylaxis for HIV prevention among men who have sex with men and transwomen in Lima, Peru: a mathematical modelling study. PLoS Medicine 9: e1001323. DOI:10.1371/journal.pmed.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. 2013. The cost and impact of scaling up pre‐exposure prophylaxis for hiv prevention: a systematic review of cost‐effectiveness modelling studies. PLoS Medicine 10: e1001401. DOI:10.1371/journal.pmed.1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granich R, Kahn JG, Bennett R, Holmes CB, Garg N, Serenata C, Sabin ML, Makhlouf‐Obermeyer C, De Filippo Mack C, Williams P, Jones L, Smyth C, Kutch KA, Ying‐Ru L, Vitoria M, Souteyrand Y, Crowley S, Korenromp EL, Williams BG. 2012. Expanding ART for treatment and prevention of HIV in South Africa: estimated cost and cost‐effectiveness 2011–2050. PLoS ONE 7: e30216. DOI:10.1371/journal.pone.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hontelez JAC, Nagelkerke N, Bärnighausen T, Bakker R, Tanser F, Newell M‐L, Lurie MN, Baltussen R, de Vlas SJ. 2011. The potential impact of RV144‐like vaccines in rural South Africa: a study using the STDSIM microsimulation model. Vaccine 29: 6100–6106. DOI:10.1016/j.vaccine.2011.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hontelez JAC, Lurie MN, Barnighausen T, Bakker R, Baltussen R, Tanser F, Hallett TB, Newell M‐L, de Vlas SJ. 2013. Elimination of HIV in South Africa through expanded access to antiretroviral therapy: a model comparison study. PLoS Medicine 10 DOI:10.1371/journal.pmed.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. 2013. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMC Medicine 11: 80 DOI:10.1186/1741-7015-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton DW, Brandeau ML. 2013. Too much of a good thing? When to stop catch‐up vaccination. Medical Decision Making : An International journal of the Society for Medical Decision Making 33: 920–936. DOI:10.1177/0272989X13493142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jit DM, Brisson M. 2011. Modelling the epidemiology of infectious diseases for decision analysis. PharmacoEconomics 29: 371–386. DOI:10.2165/11539960-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Granich R, Bui DD, Tran HV, Nadol P, Jacka D, Sabin K, Suthar AB, Mesquita F, Lo YR, Williams B. 2013. The potential impact of expanding antiretroviral therapy and combination prevention in Vietnam: towards elimination of HIV transmission. Journal of Acquired Immune Deficiency Syndromes 63: e142–e149. DOI:10.1097/QAI.0b013e31829b535b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, de Araujo GTB, Fonseca M, Pillsbury M, Singhal PK. 2012. Estimated health and economic impact of quadrivalent HPV (types 6/11/16/18) vaccination in Brazil using a transmission dynamic model. BMC Infectious Diseases 12: 250 DOI:10.1186/1471-2334-12-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebler D, Revill P, Braithwaite S, Phillips A, Blaser N, Borquez A, Cambiano V, Ciaranello A, Estill J, Gray R, Hill A, Keiser O, Kessler J, Menzies NA, Nucifora KA, Vizcaya LS, Walker S, Welte A, Easterbrook P, Doherty M, Hirnschall G, Hallett TB. 2014. Cost‐effectiveness of different strategies to monitor adults on antiretroviral treatment: a combined analysis of three mathematical models. Lancet Global Health 2: e35–e43. DOI:10.1016/S2214-109X(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R. 2004. Act Now or Later? Economics of Malaria Resistance. American Journal of Tropical Medicine and Hygiene 71: 187–195. [PubMed] [Google Scholar]
- Laxminarayan R, Over M, Smith DL. 2006. Will a global subsidy of new antimalarials delay the emergence of resistance and save lives? Health Affairs (Millwood) 25: 325–336. DOI:10.1377/hlthaff.25.2.325. [DOI] [PubMed] [Google Scholar]
- Levin A, Burgess C, Garrison LP, Bauch C, Babigumira J, Simons E, Dabbagh A. 2011. Global eradication of measles: an epidemiologic and economic evaluation. Journal of Infectious Diseases 204: S98–S106. DOI:10.1093/infdis/jir096. [DOI] [PubMed] [Google Scholar]
- Li G, Rabitz H, Yelvington PE, Oluwole OO, Bacon F, Kolb CE, Schoendorf J. 2010. Global sensitivity analysis for systems with independent and/or correlated inputs. Journal of Physical Chemistry A 114: 6022–6032. DOI:10.1021/jp9096919. [DOI] [PubMed] [Google Scholar]
- Li J, Gilmour S, Zhang H, Koyanagi A, Shibuya K. 2012. The epidemiological impact and cost‐effectiveness of HIV testing, antiretroviral treatment and harm reduction programs. AIDS (London, England) 26: 2069–2078. DOI:10.1097/QAD.0b013e3283574e54. [DOI] [PubMed] [Google Scholar]
- Long EF, Stavert RR. 2013. Portfolios of biomedical HIV interventions in South Africa: a cost‐effectiveness analysis. Journal of General Internal Medicine 28: 1294–1301. DOI:10.1007/s11606-013-2417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubell Y. 2014. Investment in malaria elimination: a leap of faith in need of direction. Lancet Global Health 2: e63–e64. DOI:10.1016/S2214-109X(14)70005-1. [DOI] [PubMed] [Google Scholar]
- Luz PM, Vanni T, Medlock J, Paltiel AD, Galvani AP. 2011. Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet 377: 1673–1680. DOI:10.1016/S0140-6736(11)60246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire N, Shillcutt S, Walker D, Tediosi F, Smith T. 2011. Cost‐effectiveness of the introduction of a pre‐erythrocytic malaria vaccine into the expanded program on immunization in sub‐Saharan Africa: analysis of uncertainties using a stochastic individual‐based simulation model of Plasmodium falciparum malaria. Value in Health : The Journal of the International Society for Pharmacoeconomics and Outcomes Research 14: 1028–1038. [DOI] [PubMed] [Google Scholar]
- Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. 2015. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bulletin of the World Health Organization 93: 118–124. DOI:10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbah M, Kjetland E, Atkins K, Poolman E, Orenstein E, Meyers L, Townsend J, Galvani A. 2013a. Cost‐effectiveness of a community‐based intervention for reducing the transmission of Schistosoma haematobium and HIV in Africa. Proceedings of the National Academy of Sciences of the United States of America 110: 7952–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbah M, Poolman E, Atkins K, Orenstein E, Meyers L, Townsend J, Galvani A. 2013b. Potential cost‐effectiveness of schistosomiasis treatment for reducing HIV transmission in Africa—the case of Zimbabwean women. PLoS Neglected Tropical Diseases 7: e2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonigaba J. 2013. Modeling the Cost‐effectiveness of HIV/AIDS Interventions in Different Socio‐economic Contexts in South Africa, (2007–2020). Mediterranean Journal of Social Sciences 4: 587. [Google Scholar]
- Menzies NA, Cohen T, Lin H‐H, Murray M, Salomon JA. 2012. Population health impact and cost‐effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS Medicine 9: e1001347. DOI:10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BE, Boucher CAB, van Dijk JH, Thuma PE, Nouwen JL, Baltussen R, van de Wijgert J, Sloot PMA, van de Vijver DAMC. 2013. Cost‐effectiveness of pre‐exposure prophylaxis (PrEP) in preventing HIV‐1 infections in rural zambia: a modeling study. PLoS ONE 8: e59549. DOI:10.1371/journal.pone.0059549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okell LC, Cairns M, Griffin JT, Ferguson NM, Tarning J, Jagoe G, Hugo P, Baker M, D'Alessandro U, Bousema T, Ubben D, Ghani AC. 2014. Contrasting benefits of different artemisinin combination therapies as first‐line malaria treatments using model‐based cost‐effectiveness analysis. Nature Communications 5: 5606 DOI:10.1038/ncomms6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okosun KO, Makinde OD. 2012. On a drug‐resistant malaria model with susceptible individuals without access to basic amenities. Journal of Biological Physics 38: 507–530. DOI:10.1007/s10867-012-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okosun KO, Ouifki R, Marcus N. 2011. Optimal control analysis of a malaria disease transmission model that includes treatment and vaccination with waning immunity. Biosystems 106: 136–145. DOI:10.1016/j.biosystems.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Okosun KO, Rachid O, Marcus N. 2013. Optimal control strategies and cost‐effectiveness analysis of a malaria model. Biosystems 111: 83–101. DOI:10.1016/j.biosystems.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Palombi L, Bernava GM, Nucita A, Giglio P, Liotta G, Nielsen‐Saines K, Orlando S, Mancinelli S, Buonomo E, Scarcella P, Altan AMD, Guidotti G, Ceffa S, Haswell J, Zimba I, Magid NA, Marazzi MC. 2012. Predicting trends in HIV‐1 sexual transmission in sub‐Saharan Africa through the Drug Resource Enhancement Against AIDS and Malnutrition model: antiretrovirals for 5 reduction of population infectivity, incidence and prevalence at the district level. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America 55: 268–275. DOI:10.1093/cid/cis380. [DOI] [PubMed] [Google Scholar]
- Pérez Velasco R, Praditsitthikorn N, Wichmann K, Mohara A, Kotirum S, Tantivess S, Vallenas C, Harmanci H, Teerawattananon Y. 2012. Systematic review of economic evaluations of preparedness strategies and interventions against influenza pandemics. PLoS ONE 7: e30333. DOI:10.1371/journal.pone.0030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, Brisson M. 2012. Dynamic transmission modeling a report of the ISPOR‐SMDM Modeling Good Research Practices Task Force Working Group–5. Medical Decision Making 32: 712–721. DOI:10.1177/0272989X12454578. [DOI] [PubMed] [Google Scholar]
- Pitt C, Goodman C, Hanson K. 2016. Economic evaluation in global perspective: A bibliometric analysis of the recent literature. Health Economics 25: S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinja S, Bahuguna P, Rudra S, Gupta I, Kaur M, Mehendale SM, Chatterjee S, Panda S, Kumar R. 2011. Cost effectiveness of targeted HIV prevention interventions for female sex workers in India. Sexually Transmitted Infections 87: 354–361. DOI:10.1136/sti.2010.047829. [DOI] [PubMed] [Google Scholar]
- Reiner R, Perkins T, Barker C, Niu T, Chaves LF, Ellis A, George D, Menach A, Pulliam J, Bisanzio D, Buckee C, Chiyaka C, Cummings D, Garcia A, Gatton M, Gething P, Hartley D, Johnston G, Klein E, Michael E, Lindsay S, Lloyd A, Pigott D, Reisen W, Ruktanonchai N, Singh B, Tatem A, Kitron U, Hay S, Scott T, Smith D. 2013. A systematic review of mathematical models of mosquito‐borne pathogen transmission: 1970–2010. Journal of the Royal Society Interface 10: 20120921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revill P, Walker S, Claxton K, Sculpher M. 2014. Using cost‐effectiveness thresholds to determine value for money in low‐and middle‐income country healthcare systems: are current international norms fit for purpose? Research Paper No. CHE 98, University of York.
- Ross A, Maire N, Sicuri E, Smith T, Conteh L. 2011. Determinants of the cost‐effectiveness of intermittent preventive treatment for malaria in infants and children. PLoS ONE 6: e18391. DOI:10.1371/journal.pone.0018391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar T, Mukhopadhyay S, Bhowmick AR, Chattopadhyay J. 2013. An optimal cost effectiveness study on Zimbabwe cholera seasonal data from 2008–2011. PLoS ONE 8 DOI:10.1371/journal.pone.0081231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori AM, de Soárez PC, Novaes HM, Amaku M, de Azevedo RS, Moreira RC, Pereira LM, Ximenes RA, Martelli CM. 2012. Cost‐effectiveness analysis of universal childhood hepatitis A vaccination in Brazil: regional analyses according to the endemic context. Vaccine 30: 7489–7497. DOI:10.1016/j.vaccine.2012.10.056. [DOI] [PubMed] [Google Scholar]
- Scott Braithwaite R, Nucifora KA, Toohey C, Kessler J, Uhler LM, Mentor SM, Keebler D, Hallett T. 2014. How do different eligibility guidelines for antiretroviral therapy affect the cost‐effectiveness of routine viral load testing in sub‐Saharan Africa? AIDS (London, England) 28(Suppl 1): S73–S83. DOI:10.1097/QAD.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey EM, Stevenson J, Galactionova K, Baidjoe AY, Bousema T, Odongo W, Kariuki S, Drakeley C, Smith TA, Cox J, Chitnis N. 2014. Modeling the cost effectiveness of malaria control interventions in the highlands of Western Kenya. PLoS ONE 9: e107700. DOI:10.1371/journal.pone.0107700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tediosi F, Hutton G, Maire N, Smith T, Ross A, Tanner M. 2006. Predicting the cost‐effectiveness of introducing a pre‐erythrocytic malaria vaccine into the expanded program on immunization in Tanzania. American Journal of Tropical Medicine and Hygiene 75: 131–143. [DOI] [PubMed] [Google Scholar]
- Tediosi F, Maire N, Penny M, Studer A, Smith TA. 2009. Simulation of the cost‐effectiveness of malaria vaccines. Malaria Journal 8: 127 DOI:10.1186/1475-2875-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris‐Prestholt F, Foss AM, Cox AP, Heise L, Meyer‐Rath G, Delany‐Moretlwe S, Mertenskoetter T, Rees H, Vickerman P, Watts CH. 2014. Cost‐effectiveness of tenofovir gel in urban South Africa: model projections of HIV impact and threshold product prices. BMC Infectious Diseases 14: 14 DOI:10.1186/1471-2334-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni T, Mendes Luz P, Foss A, Mesa‐Frias M, Legood R. 2012. Economic modelling assessment of the HPV quadrivalent vaccine in Brazil: a dynamic individual‐based approach. Vaccine 30: 4866–4871. DOI:10.1016/j.vaccine.2012.04.087. [DOI] [PubMed] [Google Scholar]
- Verguet S, Stalcup M, Walsh JA. 2013. Where to deploy pre‐exposure prophylaxis (PrEP) in sub‐Saharan Africa? Sexually Transmitted Infections 89: 628–634. DOI:10.1136/sextrans-2012-050891. [DOI] [PubMed] [Google Scholar]
- Von Wyl V, Cambiano V, Jordan MR, Bertagnolio S, Miners A, Pillay D, Lundgren J, Phillips AN. 2012. Cost‐effectiveness of tenofovir instead of zidovudine for use in first‐line antiretroviral therapy in settings without virological monitoring. PLoS ONE 7: . DOI:10.1371/journal.pone.0042834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BG, Blower S. 2012. Universal access to HIV treatment versus universal “test and treat”: transmission, drug resistance & treatment costs. PLoS ONE 7: e41212. DOI:10.1371/journal.pone.0041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky RP, Park J‐E, Wood R, Freedberg KA, Scott CA, Bekker L‐G, Losina E, Mayer KH, Seage GR, Paltiel AD. 2012. The cost‐effectiveness of pre‐exposure prophylaxis for HIV infection in South African women. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 54: 1504–1513. DOI:10.1093/cid/cis225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, Nakamura YM, Godbole SV, Panchia R, Sanne I, Weinstein MC, Losina E, Mayer KH, Chen YQ, Wang L, McCauley M, Gamble T, Seage GR, Cohen MS, Freedberg KA. 2013. Cost‐Effectiveness of HIV treatment as prevention in serodiscordant couples. New England Journal of Medicine 369: 1715–1725. DOI:10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winetsky DE, Negoescu DM, DeMarchis EH, Almukhamedova O, Dooronbekova A, Pulatov D, Vezhnina N, Owens DK, Goldhaber‐Fiebert JD. 2012. Screening and rapid molecular diagnosis of tuberculosis in prisons in Russia and Eastern Europe: a cost‐effectiveness analysis. PLoS Medicine 9: e1001348. DOI:10.1371/journal.pmed.1001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank . Country Classifications. (Available at: http://data.worldbank.org/news/new‐country‐classifications accessed 1.26.15).


