Abstract
Background
Lymphatic malformations (LMs) are congenital malformations of the lymphatic system that commonly affect the head and neck region and cause marked cosmetic and functional complications. In this pilot study, we present eight children with LMs treated using an herbal medicine for this indication.
Methods
Between January 2009 and May 2014, eight children (four boys, four girls) with LMs were treated using oral administration of an herbal medicine, Eppikajyutsuto (TJ‐28; Tsumura, Tokyo, Japan), as monotherapy.
Results
Four of the cases were macrocystic and four were mixed micro‐ and macrocystic. The mean treatment duration was 7.2 ± 2.9 months (range 5–12 mos). The mean LM volume shrinkage on magnetic resonance imaging was 54.5 ± 38.3% (macrocystic 73.6 ± 27.0%; mixed micro‐ and macrocystic 35.4 ± 41.5%). One of four macrocystic lesions had a marked reduction, two had a moderate reduction, and one had no response. A marked reduction was observed in three of the four mixed micro‐ and macrocystic cases; the other mixed cystic case had no response. The treatment was well tolerated, without severe adverse events.
Conclusions
This preliminary study demonstrates the beneficial effects of TJ‐28. Further evaluations of this therapeutic modality are warranted.
Lymphatic malformations (LMs) are congenital malformations of the lymphatic system that commonly affect the head and neck regions 1, 2 and cause marked cosmetic and functional complications. Treatment for LMs includes observation, sclerotherapy, and surgical excision 1, 2, 3, 4, 5. More recently, sirolimus 6 and sildenafil 7 have been proposed as potential medications for LMs, but they have the potential for adverse events and are not always effective.
The regulatory effects of the herbal medicine Eppika‐jyutsuto (TJ‐28; Tsumura Co., Tokyo, Japan) have been previously reported in Japan 8, 9. TJ‐28 has been shown to be effective in reducing and eliminating excessive fluids in patients with inflammatory joint disorders and edema with nephritis and nephrotic syndrome. Ogawa‐Ochiai et al 9 reported the first case of a child with LM treated with TJ‐28 and Ogikenchuto (TJ‐98; Tsumura Co.). TJ‐28 was used because LMs can be considered to be a mass of accumulated water.
We present the findings of our pilot study in children with LMs treated with TJ‐28.
Patients and Methods
Between January 2009 and May 2014, eight children (four boys, four girls) with LMs were treated with herbal medicines at the Departments of Pediatric Surgery and Innovative Kampo Medicine, Kurume University School of Medicine. The age of the patients ranged from 3 months to 4 years (mean 24.6 ± 17.6 mos, median 24.5 mos). Four patients were younger than 2 years old. All parents of the patients provided written informed consent. Four LMs cases were macrocystic and four were mixed micro‐ and macrocystic, including one with combined capillary–lymphatic malformation. All of the patients presented with a chief complaint of swelling LMs. No patients experienced complications, such as airway obstruction, swallowing dysfunction, speech disturbance, or compression of other organs.
None of the patients received any other treatments before being treated with TJ‐28. The herbal medicine was prescribed as monotherapy at a dose of 0.3 g/kg/day for patients weighing less than 25 kg and 7.5 g/day twice a day for those weighing 25 kg or more. The safety of TJ‐28 in children has not been fully established because of sparse usage in children, but guidelines allow for its use in infants and children under the careful observation of a medical doctor. Therefore a medical doctor typically administers TJ‐28 to patients ranging in age from infant to adult in Japan. Because the optimal dose of TJ‐28 has not been defined for children, it is often administered at 0.1 to 0.3 g/kg/day in Japan. All patients were admitted to the hospital every 8 weeks for evaluation of medication compliance; response to treatment, including lesional size; and side effects such as fever, pain, heart disease, and epigastric distress. Routine laboratory monitoring was not performed unless there were signs or symptoms indicating a reason to do so. All patients underwent magnetic resonance imaging (MRI) before treatment and at least 3 months after therapy to assess treatment response. The LM volume index (LVI) was defined as length (mm) times width (mm) times depth (mm). The degree of reduction was defined as postadministration LVI divided by preadministration LVI.
The outcome of this study was the rate of LM reduction as observed using MRI. The degree of reduction was defined as complete (total disappearance), marked (<50% of initial volume), moderate (50–89% of initial volume), or no response (≥90% of initial volume). Data are given as means ± SD. A paired t‐test was used when appropriate, and p < 0.05 was considered to be statistically significant. All of the statistical analyses were performed using the JMP software package (SAS Institute, Cary, NC).
Results
The patient profiles are shown in Table 1. The mean duration of herbal treatment administration was 7.2 ± 2.9 months (median 10.2 mos, range 5–12 mos). The mean rate of LM reduction was 54.5 ± 38.3% (median 53.1%, p = 0.01). There were significant differences in the degree of LM reduction. For instance, the mean rate of LM reduction for the macrocystic lesions was 73.6 ± 27.0% (median 77.1%, p = 0.14) (Fig. 1) and that for the mixed micro‐ and macrocystic lesions was 35.4 ± 41.5% (median 15.8%, p = 0.05) (Fig. 2). Comparing the response rates between the lesion types, one of four macrocystic lesions exhibited a marked reduction, two a moderate reduction, and one no response. In contrast, a marked reduction was seen in three of four mixed micro‐ and macrocystic lesions, with only one mixed micro‐ and macrocystic lesion showing no response. Patients 5, 6, and 7 discontinued TJ‐28 after follow‐up MRI. The mean duration of follow‐up after herbal treatment discontinuation was 7.3 ± 1.2 months (range 6–8 mos) after the study period. No enlargement of the LMs was noted after discontinuation. Other cases continued TJ‐28 administration after the study period.
Table 1.
Profiles of the Patients with Lymphatic Malformations
| Patient no./sex | Age of start treatment | Localization | Symptoms | Duration of treatment (mos) | Tumor size (mm) | Reduction ratio (%) | |
|---|---|---|---|---|---|---|---|
| Before administration | After administration | ||||||
| Macrocystic | |||||||
| 1/M | 4 mos | Head | Swelling | 11 | 46 × 20 × 40 | 28 × 15 × 38 | 38.7 |
| 2/M | 2 yrs, 5 mos | Neck | Swelling | 8 | 18 × 38 × 41 | 13 × 34 × 55 | 86.7 |
| 3/F | 2 yrs, 10 mos | Neck | Swelling | 7 | 20 × 17 × 15 | 19 × 16 × 17 | 101.3 |
| 4/M | 1 yr, 8 mos | Chest | Swelling | 5 | 51 × 11 × 36 | 44 × 10 × 31 | 67.5 |
| Mixed micro‐ and macrocystic | |||||||
| 5/F | 1 yr, 1 mos | Shoulder | Swelling | 12 | 25 × 20 × 26 | 11 × 8 × 11 | 12.4 |
| 6/M | 3 yrs, 5 mos | Chest | Swelling | 5 | 60 × 24 × 62 | 16 × 16 × 56 | 16.1 |
| 7/F | 4 yrs, 4 mos | Cheek | Swelling | 5 | 98 × 35 × 26 | 46 × 30 × 10 | 15.5 |
| 8a/F | 3 mos | Chest | Swelling | 5 | 38 × 24 × 37 | 38 × 28 × 31 | 97.7 |
Combined with capillary‐lymphatic malformation.
Figure 1.
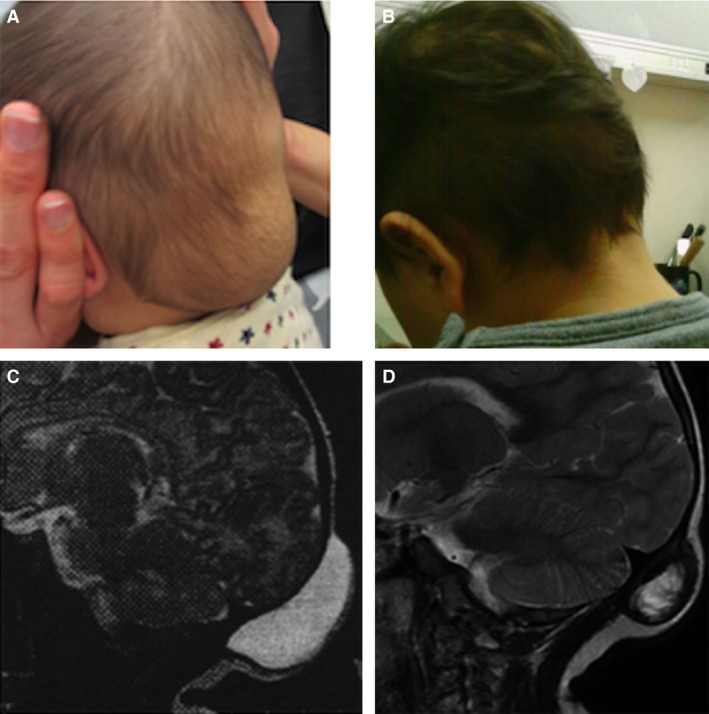
Clinical photographs and magnetic resonance imaging (MRI) (T2) obtained before and after 11 months of therapy in patient 1: (A) before treatment, (B) after treatment, (C) MRI before treatment, (D) MRI after treatment.
Figure 2.
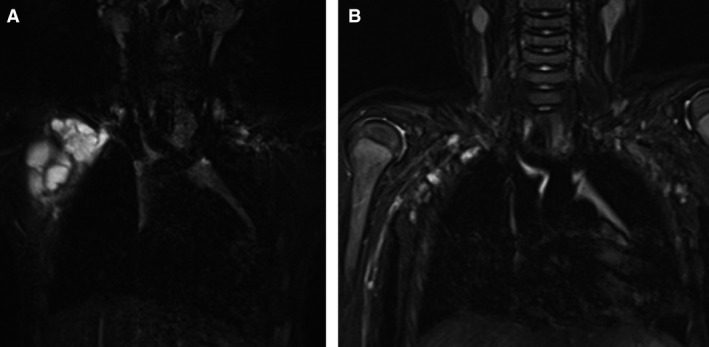
Magnetic resonance imaging (MRI) (T2) obtained before and after 5 months of treatment in patient 6: (A) before treatment, (B) after treatment.
Although the LM in patient 1 became transiently enlarged because of an upper respiratory viral infection, the volume of the LM was significantly less after the infection than before. The LMs in patients 3 and 8 showed no apparent response in size, but they had a protruding appearance before treatment that became less noticeable, more superficial, and flatter as the patients grew in height and gained weight during this study.
Discussion
There are several treatment options for LMs, including surgery and sclerotherapy 1, 2, 3, 4, 5, laser therapy 10, and pharmacotherapy such as sirolimus 6, sildenafil 7, and interferon‐alpha treatment 11. None of these treatments is uniformly effective, and all have potential side effects. Certain herbal medicines have been used as alternative therapy for LMs. Ogawa‐Ochiai et al 9 reported the first case of a patient with an LM who was effectively treated with herbal medicines, specifically TJ‐28 and TJ‐98 combined therapy, after OK‐432 sclerotherapy.
Typically TJ‐28 is administered at 7.5 g/day divided into three doses for adults; 7.5 g contains 3.25 g of dried extracts obtained from mixed raw herbs in the following amounts: 8.0 g of Gypsum fibrosum, “Sekko;” 6.0 g of Ephedra herba, “Mao;” 4.0 g of Atractylodis lanceae rhizome, “Sojutsu;” 3.0 g of Zizyphi fructus,“Taiso;” 2.0 g of Glycyrrhizae radix, “Kanzo;” and 1.0 g of Zingiberis rhizome,“Shokyo.” All herbal extracts originate from China. TJ‐28 has been shown to be effective at reducing or eliminating excessive fluid in patients with inflammatory joint disorders and edema with nephritis and nephrotic syndrome. Based on these observations, we used TJ‐28 for LMs.
The ephedra herb is the main ingredient in TJ‐28 and is known to induce pharmacologic effects beyond its sympathomimetic activities, such as antiinflammatory 12, antianaphylactic 13, antimicrobial, and antihistamine effects 14. Pseudoephedrine, a component of the ephedra herb, has inhibitory effects on acute inflammation. Furthermore, ephedra alkaloids, such as D‐pseudoephedrine and L‐norephedrine, exhibit inhibitory effects on the fibroblast proliferation induced by TJ‐28 13. These findings, especially the inhibition of prostaglandin E2 (PGE2) biosynthesis, indicate the possible antiinflammatory effects of the ephedra herb. In one study, children with LMs were found to have higher plasma vascular endothelial growth factor (VEGF) levels than controls 15. In addition, the ephedra herb suppresses the lipopolysaccharide‐induced expression of cyclooxygenase 2 (COX‐2) proteins and activation of the inhibitor of κ B/nuclear factor‐κ B–dependent signaling pathway in C6 cells in vivo 16, 17. VEGF and COX‐2 play a role in the development and growth of the vascular endothelial system. It has recently been reported that patients with LMs have somatic activating mutations in phosphatidylinositol‐4,5‐bisphospate 3‐kinase, catalytic subunit alpha, which encodes the catalytic subunit of the phosphatidylinositol 3‐kinase 18. It is possible that the inhibition and suppression of PGE2 and COX‐2 by the ephedra herb might also inhibit the mechanistic target of rapamycin pathway.
Ephedra herbal treatments should not be used in elderly patients or individuals with ischemic heart disease or low appetite, because of the risk of tachycardia and hypertension 19. Because most children often do not have risk factors such as ischemic heart disease or epigastric dysfunction, this medication is likely to be safe in children, but more evidence is needed to support this as a safe treatment in children.
Because TJ‐28 prevents the accumulation of lymphatic fluid in LMs, TJ‐28 treatment may reduce the volume of these lesions. Because less lymphatic fluid is present in mixed micro‐ and macrocystic LMs than in macrocystic LMs, mixed micro‐ and macrocystic LMs were much more responsive to herbal treatment than macrocystic LMs.
At our institution, herbal treatment was tried as a first‐line treatment. Based on our findings, further studies are warranted.
The copyright line for this article was changed on 26 August 2016 after original online publication.
References
- 1. Okazaki T, Iwatani S, Yanai T et al. Treatment of lymphangioma in children: our experience of 128 cases. J Pediatr Surg 2007;42:386–389. [DOI] [PubMed] [Google Scholar]
- 2. Baskota DK, Singh BB, Sinha BK. OK‐432: an effective sclerosing agent for the treatment of lymphangiomas of head and neck. Kathmandu Univ Med J 2007;5:312–317. [PubMed] [Google Scholar]
- 3. Ogita S, Tsuto T, Tokiwa K et al. Intracystic injection of OK‐432: a new sclerosing therapy for cystic hygroma in children. Br J Surg 1987;74:690–691. [DOI] [PubMed] [Google Scholar]
- 4. Rebuffini E, Zuccarino L, Grecchi E et al. Picibanil (OK‐432) in the treatment of head and neck lymphangiomas in children. Dent Res J (Isfahan) 2012;9:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rozman Z, Thambidorai RR, Zaleha AM et al. Lymphangioma: is intralesional bleomycin sclerotherapy effective? Biomed Imaging Interv J 2011;7:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lackner H, Karastaneva A, Schwinger W et al. Sirolimus for the treatment of children with various complicated vascular anomalies. Eur J Pediatr 2015;174:1579–1584. [DOI] [PubMed] [Google Scholar]
- 7. Danial C, Tichy AL, Tariq U et al. An open‐label study to evaluate sildenafil for the treatment of lymphatic malformations. J Am Acad Dermatol 2014;70:1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohnishi N, Nagasawa K, Yokoyama T. The verification of regulatory effects of Kampo formulations on body fluid using model mice [in Japanese]. J Med Pharm Soc WAKAN‐YAKU 2000;17:131–136. [Google Scholar]
- 9. Ogawa‐Ochiai K, Sekiya N, Kasahara Y et al. A case of mediastinal lymphangioma successfully treated with Kampo medicine. J Altern Complement Med 2011;17:563–565. [DOI] [PubMed] [Google Scholar]
- 10. Lai CH, Hanson SG, Mallory SB. Lymphangioma circumscriptum treated with pulsed dye laser. Pediatr Dermatol 2001;18:509–510. [DOI] [PubMed] [Google Scholar]
- 11. Souza RJ, Tone LG. Treatment of lymphangioma with alpha‐2a‐interferon. J Pediatr (Rio J) 2001;77:139–142. [DOI] [PubMed] [Google Scholar]
- 12. Kasahara Y, Hikino H, Tsurufuji S et al. Antiinflammatory actions of ephedrines in acute inflammations. Planta Med 1985;51:325–331. [PubMed] [Google Scholar]
- 13. Konno C, Mizuno T, Hikino H. Isolation and hypoglycemic activity of ephedrans A, B, C, D and E, glycans of ephedra distachya herbs. Planta Med 1985;2:162–163. [DOI] [PubMed] [Google Scholar]
- 14. Shiroishi H, Terasawa K, Fuse S et al. Inhibitor effects on fibroblast proliferation of Eppi‐ka‐jyutu‐to, ephedra herba and constitution of ephedra [in Japanese]. J Med Pharm Soc WAKAN‐YAKU 1991;8:153–161. [Google Scholar]
- 15. Sidle DM, Maddalozzo J, Meier JD et al. Altered pigment epithelium‐derived factor and vascular endothelial growth factor levels in lymphangioma pathogenesis and clinical recurrence. Arch Otolaryngol Head Neck Surg 2005;131:990–995. [DOI] [PubMed] [Google Scholar]
- 16. Yeom MJ, Lee HC, Kim GH et al. Anti‐arthritic effects of Ephedra sinica STAPF herb‐acupuncture: inhibition of lipopolysaccharide‐induced inflammation and adjuvant‐induced polyarthritis. J Pharmacol Sci 2006;100:41–50. [DOI] [PubMed] [Google Scholar]
- 17. Aoki K, Yamakuni T, Yoshida M et al. Ephedorae herba decreases lipopolysaccharide‐induced cyclooxgenase‐2 protein expression and NF‐kappaB‐dependent transcription in C6 rat glioma cells. J Pharmacol Sci 2005;98:327–330. [DOI] [PubMed] [Google Scholar]
- 18. Luks VL, Kamitaki N, Vivero MP et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somaticmutations in PIK3CA. J Pediatr 2015;166:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med 2000;343:1833–1838. [DOI] [PubMed] [Google Scholar]


