Abstract
Purpose
Long‐acting bronchodilators, i.e. beta‐2‐agonists (LABA) and tiotropium are commonly used in COPD treatment. Choice of a specific agent is based on effectiveness and safety. Evidence yields controversial results with respect to mortality. The present study compared one‐year mortality associated to treatment with tiotropium versus LABA.
Methods
A population‐based cohort study using data from Italian health information systems was performed. Patients aged 45+ years, discharged with COPD diagnosis in 2006–2009 were identified. Through record linkage with drug claims, patients who received a first prescription of LABA or tiotropium within 6 months after discharge were enrolled. The main analysis was restricted to naïve users (no prior use of either LABA or tiotropium). We used ‘intention to treat’ (ITT) and ‘as treated’ (AT) approaches. We followed patients for a maximum of 12 months. Hazard ratios (HRs) were calculated by Cox regression including quintiles of propensity score. In sensitivity analysis patients receiving tiotropium + LABA combination were included in the tiotropium group.
Results
Among the 33 891 enrolees, 28% were exposed to Tio, 56% to LABA, 16% to both. Overall mean age was 74 years and the mortality rate was 122/1000 person‐years (py) at the ITT analysis and 108/1000 py at the AT analysis. The adjusted HR for tiotropium only compared with LABA only was 1.06 (95%CI: 0.94–1.20) at the ITT analysis and 1.00 (95%CI: 0.93–1.08) at the AT analysis. Results were robust in sensitivity analysis.
Conclusions
In this real‐world study use of tiotropium was not associated with an increased risk of one‐year mortality compared with LABA. © 2016 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons, Ltd.
Keywords: COPD, mortality, drug treatment, cohort, pharmacoepidemiology
Introduction
Chronic obstructive pulmonary disease (COPD) is a degenerating condition characterized by chronic respiratory obstruction and a leading cause of morbidity and mortality.1 For its treatment, clinical practice guidelines around the world advocate the use of bronchodilator drugs, which have been shown to be effective in reducing excarbations and mortality. 2, 3, 4, 5, 6 Long‐acting inhaled drugs are the mainstay of therapy, with long‐acting beta2‐agonsits (LABA), namely salmeterol, formoterol and, more recently, indacaterol, and long‐acting anticolinergics, with the active agent tiotropium bromide.
Clinical guidelines do not give clear indications on which of the available long‐acting agents should be preferred. Whereas effectiveness is recognized to be comparable between the different agents, 6 concerns regarding comparative safety remain,7, 8, 9, 10, 11 and clarification to this regard might make the difference when physicians have to make their choice.
Actually, anticholinergics act as muscarinic acetylcholine receptor antagonists and are applied as inhalants. Still a proportion is systematically absorbed and can account for anticholinergic side effects on tissues such as the myocardium.11 Several large randomized controlled trials show that inhaled anticholinergic agents ipratropium and tiotropium increase the risk of serious cardiovascular events, including cardiovascular mortality.12, 13
Evidence on safety aspects are available from clinical studies, yielding conflicting results.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 One of the main criticisms towards trials is their limited generalizability,9 and evidence from observational studies is highly warranted to complete the picture. In the case of tiotropium safety, the observational studies available report results for both, an increased risk of mortality and no mortality increase.25, 26, 27, 28, 29 Differences may partly be explained through methodological choices and limitations, such as lack of information on COPD diagnosis and severity, restriction to population subgroups such as older patients or short follow‐up. Thus, the available evidence does not allow a conclusive safety evaluation, and more data from sound observational studies may help to better understand tiotropium safety, especially in non‐selected patients with COPD treated in routine care, as highlighted recently in an expert opinion.11
The present study takes advantage of the dataset available through a large Italian multicentre study, including more than 60 000 patients with COPD (‘Long‐term outcomes and adverse events of therapy with inhaled corticosteroids (ICS), LABA and tiotropium in hospitalized patients with chronic obstructive pulmonary disease (COPD)—a cohort study based on health information systems in three Italian regions.’—OUTPUL).30 Beyond studying the efficacy of long‐acting respiratory treatment in this population, the setting is particularly suitable to address safety questions, for which large numbers of treated patients are needed.
The present study aimed to measure mortality occurring in a one‐year follow‐up in a cohort of adult patients discharged with a diagnosis of COPD and newly treated with tiotropium compared with patients newly treated with LABA. The hypothesis to be tested was whether treatment with tiotropium increases all‐cause mortality, compared with treatment with LABA.
Methods
Data source
We conducted a population‐based cohort study from three Italian regions (Lombardia, Emilia Romagna, Lazio) with about 19 million inhabitants, using hospital discharge, pharmacy and mortality data. The regional Hospital Information Systems (HIS) routinely collect data from all regional hospitals and include patient demographic data, admission referral source, discharge status, up to six discharge diagnoses (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM)), up to six hospital procedures (ICD‐9‐CM) and regional code of the facility. The regional drug‐dispensing registries comprise individual records for each medical prescription dispensed in public and private pharmacies, and also for drugs dispensed by the hospital's pharmacy at discharge. The registries are limited to drugs which are reimbursed by the healthcare system referring to outpatients. Drugs are identified by the national drug register code. Individual patient data and date of drug‐dispensing are reported for every prescription. The Regional Registries of Causes of Death list the causes of death coded according to the ICD‐9, for all deaths of residents of the region. Record linkage procedure allowed all the information to be integrated with the patient's socio‐demographic characteristics, any record of hospitalization and date of death. All data sources were linked with anonymous keys.
Population
From HIS, all hospital discharges for acute exacerbation of COPD, which occurred between 1 January 2006 and 31 December 2009 in patients aged 45 or over and resident in the study areas, were enrolled (moderate‐to‐severe cases of COPD). Inclusion criteria were main diagnosis of COPD or, alternatively, main diagnosis of COPD‐related causes along with a secondary diagnoses of COPD. Discharge from the first hospitalization, which met selection criteria during the enrolment period, was considered as the enrolment admission The diagnoses derived from HIS had been validated previously.31 Exclusion criteria were as follows: not being alive at discharge, secondary diagnosis of major trauma or major surgeries, cancer diagnoses in the two years preceding the enrolment admission, hospitalization for longer than 30 days (95th percentile), more than 50% of follow‐up spent in in‐patient regimen, or not being registered in the regional health care system during the study period. The same patient could contribute to more than one observations, if he/she fulfilled the inclusion criteria more than once during the study period. Specifically, once a patient's observation finished (e.g. because of discontinuation), he/she could be enrolled again after a 6‐month wash out period, contributing with his/her patient time to any exposure category (the same as before or the alternative treatment). Patients who did not claim any of the study drugs within 6 months after discharge from the enrolment hospitalization were excluded in order to exclude very particular cases, e.g. patients not spending time in outpatient settings after the acute event (e.g. might be in private rehabilitation facilities for which there is no information available in the health information systems). We favoured a more homogeneous study population, also considering that the proportion of these patients is limited, as reported by a study specifically investigating the use patterns of long‐acting bronchodilators in routine COPD care in the same study population, which reports that only 6.4% of all patients started treatment late.30 Details on ICD‐9‐CM codes for inclusion and exclusion criteria are described in the Appendix.
Exposure
Drugs under study were long‐acting beta‐agonists (LABA ATC codes: R03AC12, R03AC13, R03AC18 R03AK06 and R03AK07) and tiotropium (ATC: R03BB04). These categories were considered regardless of use of other respiratory drugs, including ICS. Exposure to one of the two categories LABA only versus tiotropium only were defined in terms of defined daily doses (DDD). The number of DDD available was translated into the number of days in which the patient was treated, counting one DDD per day and distributing all available DDD to the days of follow‐up and allowing also for the use of accumulated DDD over time. DDD of different drugs belonging to the same ATC group were cumulated. In order to identify combined drug treatments occurring through distinct claims, a ‘claim episode’ was defined, starting from the date of the first drug in study and allowing for four days to complete the dispense of all drugs to be taken contemporaneously. Follow‐up started at the end the claim episode, which was defined as the index‐date. Details on claim episode are reported in Figure 1. Periods of hospitalization during follow up were subtracted from exposure calculations, as information on therapies prescribed to inpatients were not available. Only new users of the drug were included, considering a six‐month wash‐out period before the index date during which the patient did not use any of the study drugs. Patients prescribed with both, tiotropium and LABA within the same claim episode were excluded from the main analysis and considered in sensitivity analysis.
Figure 1.
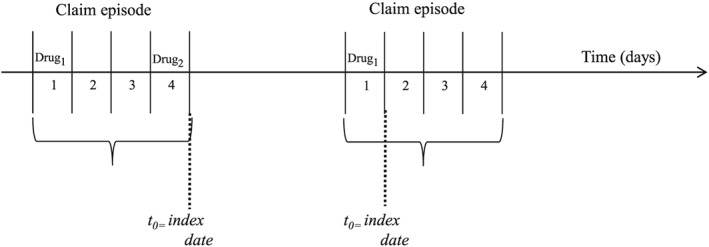
Examples of claim episode
Potential confounders
Demographic characteristics and coexisting conditions were determined from HIS, drug consumption from PHARM. The following comorbidities were retrieved from HIS, referring to the 24 months before the index date: asthma, other chronic respiratory diseases, pulmonary infections, acute pulmonary symptoms, diabetes, hypertension, ischemic heart disease, heart failure/pulmonary heart disease, other chronic heart diseases, arrhythmia, cerebrovascular diseases, peripheral vascular diseases, obesity—dyslipidemia, liver disease, chronic digestive disease (excluding liver), chronic renal diseases, neurological and muscle disease, anemia and coagulation disorders, thyroid disease, depression, psychiatric disease, peptic ulcer/esophageal reflux, rheumathologic/diffuse disease of connective tissue and HIV aids/disorders involving immune mechanism. Respiratory failure, COPD (any position), invasive respiratory procedures, staying in intensive care unit during a COPD hospitalization and use of liquid oxygen in the 12 months preceding the index prescription were considered. For details on ICD‐9‐CM codes refer to the Appendix. Drug treatment (derived from PHARM) in the 6 months prior the index date was considered for respiratory drugs other than those considered for exposure, and the overall number of all other drugs served as an indicator for polytherapy (Appendix).
Study design
Both, Intention‐to‐treat (ITT) and As‐Treated (AT) based on censoring at switching approaches were applied. In both approaches, we excluded patients not alive on the 15th day after the index date, in order to exclude extremely frail patients, allow for the drug to have an effect, and avoid to consider events associated with in‐hospital treatment for which we do not have information. In ITT, patients were classified into two groups according to their exposure on the index date. Patients were followed until death or alternatively for a maximum of 12 months. In AT we classified exposure at the index date and followed patients from index date until occurrence of death, censoring at switching or discontinuity or end of the 12‐month follow‐up, whatever happened first. Regarding discontinuation, at the end of a period of exposure (i.e. when all available DDDs were expired), a renewal grace‐time of 60 days was defined, during which the patient could claim the drug without being censored for being discontinuous. In case of switching to an alternative drug treatment, a switching‐grace time of 7 days was applied, considering a lagged effect of the drug for 1 week after its last intake (Figure 2).
Figure 2.
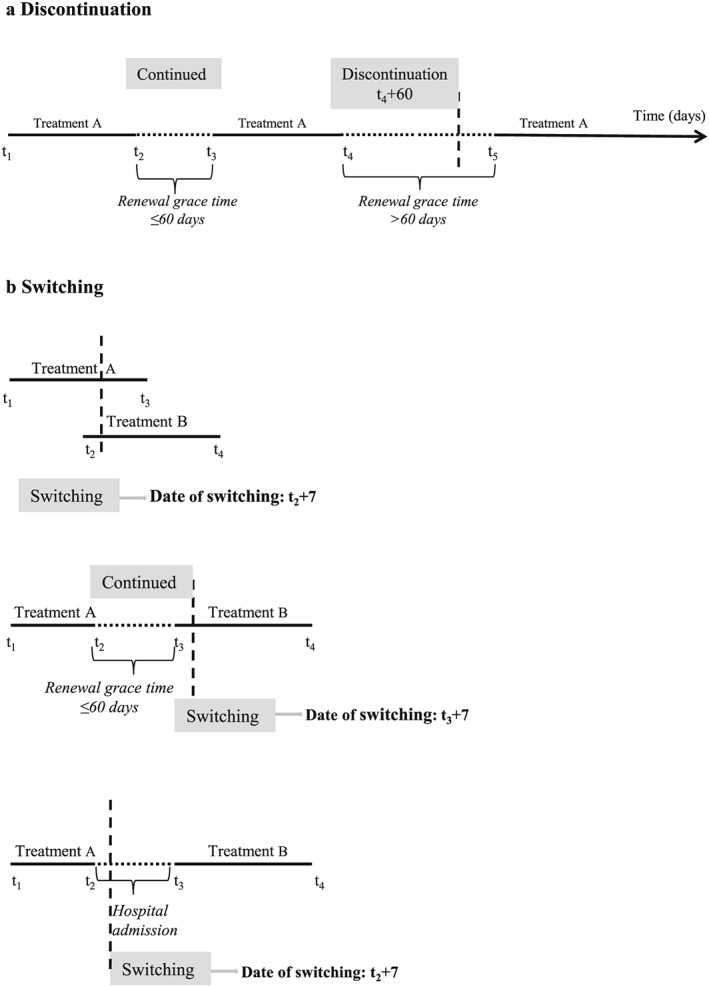
As‐treated approach: examples for censoring because of discontinuation and switching. (a) Discontinuation. (b) Switching
Statistical analysis
The mortality rate was calculated as the number of deaths, divided by the person‐time at risk. To test the association between use of tiotropium and mortality, multivariate analysis was performed using Cox proportional hazard model, producing hazard ratios (HRs) of overall mortality and 95% confidence intervals (95%CI). Adjusted estimates were obtained controlling for quintiles as a categorical variable in the propensity scores, considering also trimming. We calculated propensity scores to balance groups on baseline characteristics in order to reduce confounding by indication. The likelihood of using tiotropium was estimated, using the following characteristics (selected by a stepwise procedure): gender, age, region, drug consumption (ATC groups three digits) respiratory drugs (including ICS) and comorbidities. Patients were stratified into quintiles according to their predicted propensity score, and the predicted quintiles of the propensity score were used as a covariate together with the treatment variable to be fitted in the Cox proportional model. This method assumes that treated and control patients with the same quintile of the propensity score have the same distribution of measured baseline characteristics.32 All variables which were not balanced through the quintiles of the propensity score, were included in the model as individual potential confounders. The model also accounted for auto‐correlation of episodes referring to the same individual. Two sensitivity analyses were performed. First, patients using ICS during follow‐up were excluded. In the second analyses, we considered also patients prescribed with tiotropium and LABA within a single claim episode and considered them together with the tiotropium group. (LABA + tiotropium or tiotropium versus LABA)
Results
We selected 68 795 patients with COPD discharge in the study period. We identified 55766 treatment episodes, 33 891 of which were classified as new user episodes, generated by 24 542 patients (Figure 3). Nineteen thousand seventy‐one episodes (56.3%) were classified as LABA users, 9348 (27.6%) as tiotropium and remaining 5472 (16.1%) as LABA/tiotropium users. The mean age of the population was 74.2 (SD = 9.9) years. Table 1 describes the demographic and clinical characteristics of patients according to their exposure classification. The demographic characteristics of the study population, stratified by treatment regimen, are shown in Table 1. Most patients were males (55.1%) and residents of the Lombardia Region (43.9%).
Figure 3.
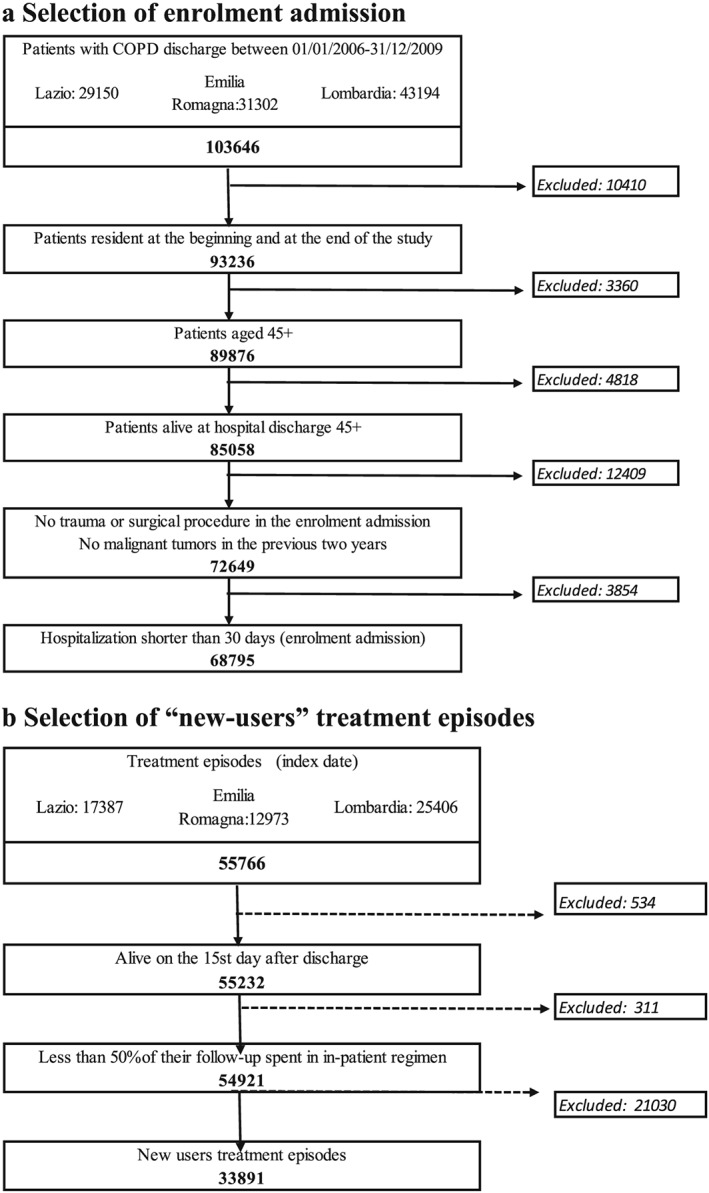
(a) Selection of enrolment admission. (b) Selection of ‘new‐users’ treatment episodes
Table 1.
Characteristics of patients with COPD according to treatment group (%)
| LABA | Tiotropium | LABA + Tiotropium | Total | |
|---|---|---|---|---|
| n = 19071 | n = 9348 | n = 5472 | n = 33891 | |
| % | % | % | % | |
| Gender | ||||
| Male | 52.4 | 56.9 | 61.2 | 55.1 |
| Female | 47.6 | 43.1 | 38.8 | 44.9 |
| Age | ||||
| 45–54 | 4.6 | 2.7 | 4.5 | 4.1 |
| 55–64 | 12.9 | 11.1 | 15.2 | 12.8 |
| 65–74 | 28.7 | 29.4 | 32.7 | 29.5 |
| 75–84 | 39.8 | 42.8 | 37.1 | 40.2 |
| 85+ | 14.0 | 14.0 | 10.5 | 13.5 |
| Residence | ||||
| Lazio | 32.9 | 32.8 | 35.1 | 33.2 |
| Emilia Romagna | 26.4 | 18.5 | 17.7 | 22.8 |
| Lombardia | 40.7 | 48.7 | 47.1 | 43.9 |
| Proxy of COPD severity (previous year)a | ||||
| Previous COPD hospitalization | 9.1 | 11.0 | 14.7 | 10.5 |
| Diagnosis of respiratory failure | 27.1 | 33.4 | 44.6 | 31.7 |
| Invasive respiratory procedures | 1.9 | 2.4 | 3.0 | 2.2 |
| Staying in intensive care unit during a COPD | 4.8 | 6.8 | 7.5 | 5.8 |
| Liquid oxygen | 10.5 | 9.0 | 44.2 | 15.5 |
| Moderate exacerbation (drug use, previous 6 months) | 15.2 | 14.4 | 15.9 | 15.1 |
| Concomitant respiratory diseases (previous year)a | ||||
| Asthma | 1.8 | 1.3 | 1.7 | 1.7 |
| Chronic respiratory disease other than COPD | 3.0 | 4.1 | 3.9 | 3.5 |
| Pulmonary infections | 9.5 | 12.9 | 14.0 | 11.2 |
| Acute pulmonary symptoms | 2.6 | 3.4 | 3.8 | 3.0 |
| Apnoea | 1.5 | 2.5 | 3.0 | 2.0 |
| History of comorbidities (previous 2 years) | ||||
| Diabetes | 12.5 | 15.5 | 17.2 | 14.1 |
| Hypertension | 28.1 | 33.1 | 34.9 | 30.6 |
| Ischemic heart disease | 12.6 | 18.7 | 14.8 | 14.6 |
| Heart failure/pulmonary heart disease | 13.4 | 19.6 | 19.5 | 16.1 |
| Other chronic heart diseases | 7.5 | 11.3 | 9.4 | 8.8 |
| Arrythmia | 11.0 | 17.7 | 13.9 | 13.3 |
| Cerebrovascular diseases | 7.8 | 8.7 | 8.2 | 8.1 |
| Peripheral vascular diseases | 3.9 | 5.4 | 5.3 | 4.5 |
| Obesity–dyslipidemia | 7.3 | 9.1 | 10.8 | 8.3 |
| Liver disease | 9.0 | 8.7 | 8.6 | 8.8 |
| Chronic digestive disease (excluding liver) | 1.2 | 1.2 | 1.4 | 1.2 |
| Chronic renal diseases | 6.1 | 9.1 | 7.8 | 7.2 |
| Neurological and muscle disease | 2.0 | 2.2 | 2.3 | 2.1 |
| Anaemia and coagulation disorders | 3.5 | 4.4 | 4.0 | 3.9 |
| Thyroid disease | 3.8 | 4.9 | 4.9 | 4.3 |
| Depression | 2.3 | 2.8 | 2.5 | 2.5 |
| Psychiatric disease | 2.7 | 2.9 | 2.5 | 2.7 |
| Peptic ulcer/oesophageal reflux | 1.1 | 1.0 | 1.3 | 1.1 |
| Rheumatologic/diffuse disease of connective tissue | 0.8 | 0.9 | 0.5 | 0.8 |
| HIV/AIDS/disorders involving immune mechanism | 0.2 | 0.1 | 0.1 | 0.2 |
| Prescriptions of drugs | ||||
| Short‐acting beta2 agonist, inhalants | 20.4 | 18.5 | 22.9 | 20.3 |
| Short‐acting beta‐2 agonists combined with glucocoticoids, inhalants or with short‐acting anticholinergics | 4.5 | 4.8 | 5.2 | 4.7 |
| Glucocorticoids, inhalants | 29.5 | 31.8 | 32.3 | 30.6 |
| Antiallergic agents, excluding corticosteroids | 14.7 | 16.5 | 18.7 | 15.8 |
| Short‐acting beta‐2 agonists for systemic use | 0.4 | 0.4 | 0.4 | 0.4 |
| Xanthines | 0.2 | 0.2 | 0.3 | 0.2 |
| Leukotriene receptor antagonists | 15.9 | 14.5 | 15.8 | 15.5 |
| Other systemic drugs for obstructive airway diseases | 2.4 | 1.7 | 1.7 | 2.1 |
| Glucocorticoids for systemic use | 25.7 | 24.5 | 25.8 | 25.4 |
| Antibacterials for systemic use | 56.4 | 56.1 | 55.3 | 56.2 |
Excluding index admission.
As compared with the patients treated with LABA, univariate analyses showed that tiotropium users were older, with a higher proportion of all variables defined as proxy of COPD severity, except for liquid oxygen, and with a higher prevalence of concomitant respiratory diseases in the previous year. Tiotropium users were more likely to have comorbidities than LABA users, in particular heart failure, other chronic heart diseases and cerebrovascular diseases. Prescription of drugs was similar between groups, except for Short‐acting beta2 agonist, inhalants, inhaled Glucocorticoids and antiallergic agents. Table 2 shows characteristics of patients with COPD according to treatment group and the expected probability of being treated with each therapy, adjusted for all characteristics measured at baseline and used in the propensity score. There were large differences in the exposure categories between treatment groups, well balanced adjusting for quintiles of the propensity score, except for beta blocking agents, ischemic heart disease, heart failure/pulmonary heart disease, other chronic heart diseases, arrhythmia, chronic renal diseases; all these latter were then included in the multivariate models as single potential confounders. Trimming at both sides of the distribution did not alter the results, and the 35 observations which would have been excluded were maintained in the analysis. In Table 3, HRs for patients treated with tioptropium versus those treated with LABA are reported. The overall rates of 1‐year all cause mortality were 13.4 per 100 person‐years (py) in the ITT approach and 12.6 per 100 py in the AT approach. Adjusted HRs were similar in both approaches and did not show any significant differences between treatments (ITT: 1.00 (95%CI:0.93–1.08), AT: 1.06 (95%CI: 0.94–1.20)). In the first sensitivity analysis, we excluded all cases that had an ICS prescription during follow‐up. This analysis produced results conflicting with primary analyses although estimates were not statistically significant. In the second analysis, comparing patients with tiotropium or tiotropium + LABA verssus LABA only, no significant differences were found (Figure 4).
Table 2.
Characteristics of patients with COPD according to treatment group, adjusted for propensity score quintiles
| LABA | Tiotropium | Adjustment for propensity score quintile | ||||
|---|---|---|---|---|---|---|
| n = 19071 | n = 9348 | p‐value | p‐value | |||
| n | % | n | % | |||
| Gender | ||||||
| Male | 9996 | 52.4 | 5323 | 56.9 | 0.000 | 0.553 |
| Female | 9075 | 47.6 | 4025 | 43.1 | ||
| Age (years) | ||||||
| 45–54 | 878 | 4.6 | 252 | 2.7 | 0.000 | 0.462 |
| 55–64 | 2458 | 12.9 | 1038 | 11.1 | ||
| 65–74 | 5467 | 28.7 | 2745 | 29.4 | ||
| 75–84 | 7592 | 39.8 | 4000 | 42.8 | ||
| 85+ | 2676 | 14.0 | 1313 | 14.0 | ||
| Residence | ||||||
| Lazio | 6280 | 32.9 | 3066 | 32.8 | 0.000 | 0.312 |
| Emilia Romagna | 5030 | 26.4 | 1730 | 18.5 | ||
| Lombardia | 7761 | 40.7 | 4552 | 48.7 | ||
| Drug (ATC group) | ||||||
| Drugs for functional gastrointestinal disorders | 368 | 1.9 | 144 | 1.5 | 0.021 | 0.771 |
| Antithrombotic agents | 9687 | 50.8 | 5436 | 58.2 | 0.000 | 0.294 |
| Cardiac therapy | 6029 | 31.6 | 3746 | 40.1 | 0.000 | 0.046 |
| Diuretics | 8606 | 45.1 | 4902 | 52.4 | 0.000 | 0.057 |
| Beta blocking agents | 2790 | 14.6 | 1971 | 21.1 | 0.000 | 0.025 |
| Lipid modifying agents | 3676 | 19.3 | 2147 | 23.0 | 0.000 | 0.224 |
| Urologicals | 2091 | 11.0 | 1056 | 11.3 | 0.402 | 0.869 |
| Corticosteroids for systemic use | 4900 | 25.7 | 2291 | 24.5 | 0.031 | 0.847 |
| Antiinflammatory and antirheumatic products | 5978 | 31.3 | 2663 | 28.5 | 0.000 | 0.656 |
| Drugs for obstructive airway diseases | 9005 | 47.2 | 4349 | 46.5 | 0.270 | 0.649 |
| Cough and cold preparations | 1276 | 6.7 | 535 | 5.7 | 0.002 | 0.905 |
| Ophthalmologicals | 1200 | 6.3 | 516 | 5.5 | 0.010 | 0.780 |
| ICS | 5622 | 29.5 | 2970 | 31.8 | 0.000 | 0.486 |
| Ipratropium bromide, oxitropium bromide | 2795 | 14.7 | 1543 | 16.5 | 0.000 | 0.404 |
| Short‐acting beta‐2 agonist, inhalants | 3892 | 20.4 | 1725 | 18.5 | 0.000 | 0.842 |
| Leukotriene receptor antagonists | 466 | 2.4 | 156 | 1.7 | 0.000 | 0.920 |
| Xanthines | 3023 | 15.9 | 1352 | 14.5 | 0.002 | 0.885 |
| Liquid oxygen | 2574 | 13.5 | 1591 | 17.0 | 0.000 | 0.111 |
| Proxy of COPD severity (previous year)a | ||||||
| Diagnosis of respiratory failure | 5164 | 27.1 | 3123 | 33.4 | 0.000 | 0.146 |
| Moderate exacerbation (drug use, previous 6 months) | 2898 | 15.2 | 1345 | 14.4 | 0.073 | 0.758 |
| Concomitant respiratory diseases (previous year)a | ||||||
| Asthma | 345 | 1.8 | 126 | 1.3 | 0.004 | 0.985 |
| Chronic respiratory disease other than COPD | 576 | 3.0 | 381 | 4.1 | 0.000 | 0.325 |
| Pulmonary infections | 1818 | 9.5 | 1204 | 12.9 | 0.000 | 0.081 |
| Sleep apnoea | 287 | 1.5 | 230 | 2.5 | 0.000 | 0.184 |
| History of comorbidities (previous 2 years) | ||||||
| Ischemic heart disease | 2407 | 12.6 | 1747 | 18.7 | 0.000 | 0.001 |
| Heart failure/pulmonary heart disease | 2554 | 13.4 | 1828 | 19.6 | 0.000 | 0.002 |
| Other chronic heart diseases | 1427 | 7.5 | 1056 | 11.3 | 0.000 | 0.004 |
| Arrythmia | 2100 | 11.0 | 261 | 2.8 | 0.000 | 0.000 |
| Cerebrovascular diseases | 1489 | 7.8 | 815 | 8.7 | 0.008 | 0.765 |
| Obesity–dyslipidemia | 1386 | 7.3 | 850 | 9.1 | 0.000 | 0.253 |
| Chronic renal diseases | 1170 | 6.1 | 855 | 9.1 | 0.000 | 0.008 |
| Thyroid disease | 731 | 3.8 | 457 | 4.9 | 0.000 | 0.233 |
| Depression | 433 | 2.3 | 261 | 2.8 | 0.008 | 0.518 |
| Peptic ulcer/oesophageal reflux | 214 | 1.1 | 93 | 1.0 | 0.330 | 0.730 |
Excluding index admission.
Table 3.
Rates and HR in patients with tiotropium versus those with LABA, using two different study designs, adjusting for quintile propensity score
| Deaths | person‐years | Ratea 100 py | HR | 95%CI | HRa | 95%CIa | |||
|---|---|---|---|---|---|---|---|---|---|
| Intention to treat | 3267 | 26784 | 12.2 | ||||||
| LABA | 2094 | 18030 | 11.6 | 1.00 | — | — | 1.00 | — | — |
| Tiotropium | 1173 | 8754 | 13.4 | 1.15 | 1.07 | 1.24 | 1.00 | 0.93 | 1.08 |
| As‐treated | 1081 | 9983 | 10.8 | ||||||
| LABA | 672 | 6727 | 9.9 | 1.00 | — | — | 1.00 | — | — |
| Tiotropium | 409 | 3256 | 12.6 | 1.26 | 1.12 | 1.43 | 1.06 | 0.94 | 1.20 |
Adjusted for propensity score quintiles plus variable non balanced (betablockers, ischemic heart disease, heart failure/pulmonary heart disease, other chronic heart diseases, arrythmia and chronic renal diseases).
Figure 4.
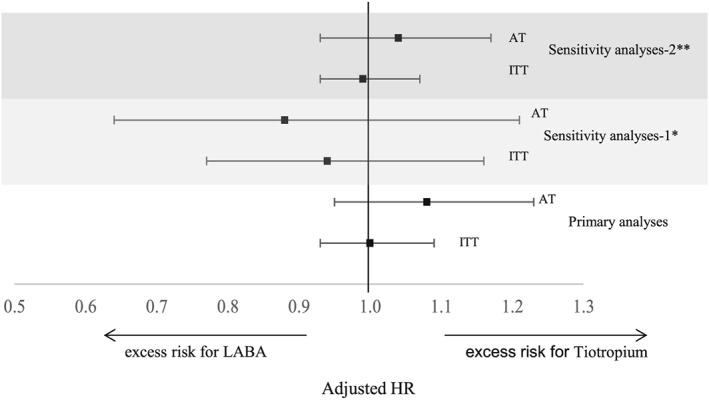
Rates and HR in sensitivity analyses, using two different study designs, adjusting for propensity score quintiles. *Sensitivity analyses compared patients with tiotropium versus LABA excluding those with prescription of ICS during follow‐up. HR adjusted for propensity score quintiles. **Sensitivity analyses compared patients with tiotropium or tiotropium + LABA versus LABA only. HR adjusted for propensity score quintiles plus variable non balanced (cardiac therapy, diuretics, betablockers, chronic respiratory disease other than COPD, ischemic heart disease, other chronic heart diseases and arrhythmia)
Discussion
The present study does not show any differences in 1‐year mortality in a cohort of adult patients discharged with a diagnosis of COPD and newly treated with tiotropium compared with patients newly treated with LABA, independently of combination with ICS. These findings were confirmed in sensitivity analyses, considering users of the combination tiotropium + LABA as exposed to tiotropium and excluding patients using ICS during follow‐up. The observed but not statistically significant increase in risk for LABA with respect to LABA + ICS was previously reported for the same study population in a paper which is in press.33
Our results are in line with a cohort study using the UK THIN primary care database in which users of tiotropium and single‐ingredient LABA had similar risk of total mortality and cardiovascular endpoints.27 Findings form other observational research reporting a reduction in mortality associated to tiotropium use, were not confirmed by the present results.25, 26, 28 A limitation of the Danish study was the lack of information on COPD severity of the included patients, which might have led to confounding by indication. Furthermore, patients were not censored at switching, but the same patient could contribute with person time to different treatments, which makes it difficult to disentangle the association between exposure and outcome.25 In the Canadian study, follow‐up was limited to 6 months and mortality reductions were shown only for combination treatment with ICS, while no differences were found between tiotropium alone and LABA.26 The UK study was prone to limitations regarding the inclusion of asthma patinets.28 A recent study performed in Canada found a lower mortality risk among patients initially prescribed with LABA than among those prescribed with tiotropium, but this study was limited to patients aged 66 and older. 29
The major strength of the present study lies in the fact that it is a large observational study, including patients in a real‐world setting, comprising adult patients, who were typically excluded from the major clinical trials on tiotropium (UPLIFT, TORCH, POET and TIOSPIR), namely those affected by acute cardiovascular diseases and renal failure. The study population was composed of medium‐severe patients with COPD, identified through hospital discharge diagnoses, which has been validated in a previous Italian study.31 Disease severity was accounted for, using information on COPD‐related conditions during previous hospitalizations, as well as use of oxygene. We also investigated the role of moderate exacerbations, defined as use of antibiotics combined with oral corticosteroids in the 6 months prior to the index date, but this variable was not associated with the exposure and consequently, was not included as a confounder. Exposure to the study drugs was evaluated in two different approaches, namely ITT and AT, and both produced very similar results. Both approaches were designed trying to counterbalance methodological pitfalls which are often criticised in pharmaco‐epidemiological cohort studies. For example, the lack of information on drug intake for patients being admitted to hospital during follow‐up was specifically addressed, subtracting inpatient periods from exposure calculations. Moreover, potential changes of drug therapy during hospitalizations occurring during follow‐up were accounted for, considering the first claim after discharge and, in case of switching with respect to the pre‐hospitalization, this was applied to the first day of hospitalisation (Figure 2b).
Accounting for the fact, that combined drug therapy may be prescribed through different prescriptions in Italy, rather than defining exposure on one single day, we considered claim episodes, which allowed for a four‐day interval to complete the combination of different drugs (e.g. tiotropium plus ICS). This time frame was defined on the basis of preliminary analysis on dates of claims of COPD drugs.
In the AT approach, one important methodological challenge is dealing with time‐related bias, which may be introduced by limitations in measuring exposure as well as by a drug's pharmacodynamic and pharmacokinetic characteristics. We applied a series of different measures to cope with that. First, we introduced a 15‐day buffer period, i.e. a minimum observation time after the start of exposure, thus allowing the drug to have an effect on a potential outcome. Second, regarding the difficulties in defining adherence, we introduced different grace‐times: the renewal grace time which accounts for the fact that on one hand, patients may not claim the drug refill immediately after they have finished their last supply, and on the other hand, stocking of a drug in a patient's house may allow for intake even in absence of a new drug claim. In case of switching, the patient was censored, but a 7‐day grace‐time was allowed for observation of a lagged effect of the drug to be associated with an outcome.
Still there are some limitations. It was not possible to investigate the association between exposure and cause specific mortality, e.g. cardiovascular causes, because cause of death is not available in Lombardia, which accounts for almost half of the study population. Other weaknesses are inherent to the data sources: healthcare databases do not provide information on individual patient dosage, and consequently exposure assessment was based on DDD, an assumption, which may not reflect individual daily drug intake for all patients. Furthermore, residual confounding cannot be ruled out completely, because information on potential confounders, such as information relating to primary care or clinical data comprising spirometry or smoking habits, were not available. However, adjusting for quintiles of the propensity score based on a variety of patient characteristics should have reasonably limited the potential impact of unadjusted confounding.
Using data referring to years prior to 2011, it was not possible to consider the soft mist inhaler device, which was marketed in Italy after the study end. Therefore, results are limited to the traditional HandiHaler® and cannot be extended to tiotropium administered through Respimat®.
Conclusion
The present study does not give evidence for an increased mortality risk of tiotropium compared with LABA. These results refer to a real‐world setting in which patients treated with tiotropium were older and sicker that patients using LABA.
Conflict of Interest
None.
Key Points.
Clinical guidelines on COPD care recommend treatment with long‐acting inhaled bronchodilators, regardless of choosing long‐acting beta‐2‐agonists or tiotropium
Evidence regarding tiotropium safety is conflicting
Mortality between tiotropium and long‐acting beta‐2‐agonists was compared in a large real‐world observational study, using intention‐to‐treat and as‐treated approaches
Our results do not show differences in 1‐year mortality comparing tiotropium to long‐acting beta‐2‐agonists.
Ethics Statement
Ethical Approval was achieved from the regional authorities.
Acknowledgements
The present research was partly funded by the Italian Medicines Agency, grant number FARM87BT93. Part of the results were submitted as an abstract to the 2014 congress of the European Respiratory Society.
Appendix Appendix.
ICD‐9‐CM codes for patient identification and selection
| ICD‐9‐CM codes | Disease |
| 490 | Bronchitis, not specified as acute or chronic |
| 491 | Chronic bronchitis |
| 492 | Emphysema |
| 494 | Bronchiectasis |
| 496 | Chronic airway obstruction, not elsewhere classified |
| 518.81 | Acute respiratory failure |
| 518.82 | Other pulmonary insufficiency, not elsewhere classified |
| 786.0 | Dyspnoea and respiratory abnormalities |
| 786.2 | Cough |
| 786.4 | Abnormal sputum |
ICD‐9‐CM codes for proxies of COPD severity, registered in the 12 months preceding the index prescription
| ICD‐9‐CM codes or ATC codes | Proxy of COPD severity |
| Refer to the preceding texts | Previous COPD hospitalization |
| 518.81–518.84 | Diagnosis of respiratory failure |
| 311, 312,967, V44.0 | Invasive respiratory procedures |
| Staying in intensive care unit during a COPD hospitalization | |
| Liquid oxygen |
ICD‐9‐CM codes for concomitant respiratory diseases, registered in the 24 months preceding the index prescription
| ICD‐9‐CM codes | Concomitant respiratory diseases |
| 493 | Asthma |
| 135, 495, 500–505, 515–517, 519, 508.1, 518.1–518.3 | Chronic respiratory disease other than COPD |
| 011, 480–487.0, 510, 511, 513 | Pulmonary infections |
| 512, 415, 786.0, 518.0 | Acute pulmonary symptoms |
| 780.51, 780.53, 780.57 | Apnoea |
ICD‐9‐CM codes for concomitant respiratory diseases, registered in the 24 months preceding the index prescription
| ICD‐9‐CM codes | Comorbidity |
| 250.0–250.9 | Diabetes |
| 401–405 | Hypertension |
| 410–414, 429.7 | Ischemic heart disease |
| 428, 416.9 | Heart failure/pulmonary heart disease |
|
429 (EXCLUDING 429.7) 093.2, 394–397.1, 424, 746.3–746.6, V42.2, V43.3, 393, 397.9, 398, 423, 391, 420, 421, 422, 425, 745, V15.1, V42.2, V43.2, V45.0, V45.81, V45.82 PROCEDURES: 36.1, 36.0,35, 37.0, 37.1, 37.3, 37.4, 37.5, 37.6, 37.9 |
Other chronic heart diseases |
| 426.0, 426.10, 426.12, 426.13, 426.7, 426.9, 427, 785.0, 996.01, 996.04, V45.0, V53.3 | Arrhythmia |
| 430–438 | Cerebrovascular diseases |
| 440–448, 557, 093.0 | Peripheral vascular diseases |
| 278.0, 272 | Obesity–dyslipidemia |
| 456.0–456.2 570–573 V42.7 | Liver disease |
| 0.70, 577.0–577.9, 555, 556 | Chronic digestive disease (excluding liver) |
|
582‐588, V42.0, V45.1, V56 PROCEDURES: 38.95, 39.95, 54.98, 55.6 |
Chronic renal diseases |
| 334.1, 342, 343, 344, 331, 332, 333.4, 333.5, 334‐335, 336.2, 340, 341, 345, 348.1, 348.3, 784.3, 356‐359 | Neurological and muscle disease |
| 280, 281, 285.9, 286, 287.1, 287.3–287.5 | Anaemia and coagulation disorders |
| 240–246 | Thyroid disease |
| 300.4, 301.12, 309.0, 309.1, 311 | Depression |
| 293.8, 295–298, 299.1, 290.0–290.4, 294.1, 331.0 | Psychiatric disease |
| 531–534, 530.81 | Peptic ulcer/oesophageal reflux |
| 710, 714 | Rheumatologic/diffuse disease of connective tissue |
| 042, 279 | HIV aids/disorders involving immune mechanism |
ATC codes for drugs prescription, registered in the 6 months preceding the index prescription
| Drug | ATC code |
| R03AC02, R03AC03, R03AC04, R03AC16, R03AC14 | Short‐acting beta‐2 agonists, inhalants |
| R03AK03 AND R03AK04 | Short‐acting beta‐2 agonists combined with glucocoticoids, inhalants or with short‐acting anticholinergics |
| R03BA | Glucocorticoids, inhalants |
| R03BB01, R03BB02 | Anticholinergics, inhalants, except tiotropium |
| R03BC | Antiallergic agents, excluding corticosteroids |
| R03CC02, R03CC04, R03CC08, R03CC13 | Short‐acting beta‐2 agonists for systemic use |
| R03DA | Xanthines |
| R03DC | Leukotriene receptor antagonists |
| H02AB | Glucocorticoids for systemic use |
| J01 | Antibacterials for systemic use |
| ATC fifth digit codes | All other drugs |
Kirchmayer, U. , Cascini, S. , Agabiti, N. , Di Martino, M. , Bauleo, L. , Formoso, G. , Voci, C. , Pistelli, R. , Patorno, E. , Davoli, M. , and , (2016) One‐year mortality associated with COPD treatment: a comparison of tiotropium and long‐acting beta2‐agonists in three Italian regions: results from the OUTPUL study. Pharmacoepidemiol Drug Saf, 25: 578–589. doi: 10.1002/pds.3961.
References
- 1. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589): 765–773 Review. [DOI] [PubMed] [Google Scholar]
- 2. Qaseem A, Wilt TJ, Weinberger SE, et al American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155(3): 179–191. [DOI] [PubMed] [Google Scholar]
- 3. O'Donnell DE, Aaron S, Bourbeau J, Hernandez P, Marciniuk D, Balter M, Ford G, Gervais A, Goldstein R, Hodder R, Maltais F, Road J, Canadian Thoracic Society. Canadian Respiratory Society Recommendations for Management of Chronic Obstructive Pulmonary Disease. Can Resp J 2003; 10(Suppl A: 11A–65A). [DOI] [PubMed] [Google Scholar]
- 4. GOLD ‐ global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (GOLD) – updated 2013. [DOI] [PubMed]
- 5. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care [Internet]. National Clinical Guideline Centre (UK). London: Royal College of Physicians (UK); 2010. Jun. [PubMed]
- 6. Kew KM, Dias S, Cates CJ. Long‐acting inhaled therapy (beta‐agonists, anticholinergics and steroids) for COPD: a network meta‐analysis. Cochrane Database Syst Rev 2014; 3 CD010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rabe KF. Anticholinergic drugs for the treatment of COPD are safe… are they? Chest 2010; 137(1): 1–3. [DOI] [PubMed] [Google Scholar]
- 8. Michele TM, Pinheiro S, Iyasu S. The safety of tiotropium‐‐the FDA's conclusions. N Engl J Med 2010; 363(12): 1097–1099. [DOI] [PubMed] [Google Scholar]
- 9. Walker S, Fingleton J, Weatherall M, et al. Limited generalisability of UPLIFT findings to clinical practice. Thorax 2013; 68(11): 1066–1067. [DOI] [PubMed] [Google Scholar]
- 10. Yohannes AM, Connolly MJ, Hanania NA. Ten years of tiotropium: clinical impact and patient perspectives. Int J Chron Obstruct Pulmon Dis 2013; 8: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tashkin DP. The safety of anticholinergic bronchodilators for the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Saf. 2015; 14(11): 1759–1772. [DOI] [PubMed] [Google Scholar]
- 12. Hilleman DE, Malesker MA, Morrow LE, Schuller D. A systematic review of the cardiovascular risk of inhaled anticholinergics in patients with COPD. Int J Chron Obstruct Pulmon Dis 2009; 4: 253–263 Epub 2009 Jul 20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh S1, Loke YK, Enright P, Furberg CD. Pro‐arrhythmic and pro‐ischaemic effects of inhaled anticholinergic medications. Thorax 2013; 68(1): 114–116. doi:10.1136/thoraxjnl-2011-201275. Epub 2012 Jul 4. [DOI] [PubMed] [Google Scholar]
- 14. Celli B, Decramer M, Leimer I, et al. Cardiovascular safety of tiotropium in patients with COPD. Chest 2010; 137(1): 20–30. [DOI] [PubMed] [Google Scholar]
- 15. Chong J, Karner C, Poole P. Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012; 9 CD009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathioudakis AG, Amanetopoulou SG, Gialmanidis IP, et al. Impact of long‐term treatment with low‐dose inhaled corticosteroids on the bone mineral density of chronic obstructive pulmonary disease patients: aggravating or beneficial? Respirology 2013; 18(1): 147–153. [DOI] [PubMed] [Google Scholar]
- 17. Rodrigo GJ, Plaza V, Castro‐Rodríguez JA. Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther 2012; 25(1): 40–47 Review. [DOI] [PubMed] [Google Scholar]
- 18. Salpeter SR, Buckley NS, Salpeter EE. Meta‐analysis: anticholinergics, but not beta‐agonists, reduce severe exacerbations and respiratory mortality in COPD. J Gen Intern Med 2006; 21(10): 1011–1019 Erratum in: J Gen Intern Med 2006;21(10):1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta‐agonists in patients with asthma and COPD: a meta‐analysis. Chest 2004; 125(6): 2309–2321. [DOI] [PubMed] [Google Scholar]
- 20. Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease. A systematic review and meta‐analysis. JAMA 2008; 300(12): 1439–1450. [DOI] [PubMed] [Google Scholar]
- 21. Tashkin DP. Long‐acting anticholinergic use in chronic obstructive pulmonary disease: efficacy and safety. Curr Opin Pulm Med 2010; 16(2): 97–105. [DOI] [PubMed] [Google Scholar]
- 22. Vogelmeier C, Hederer B, Glaab T, et al POET‐COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011; 364(12): 1093–1103. [DOI] [PubMed] [Google Scholar]
- 23. Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 5 CD007891. [DOI] [PubMed] [Google Scholar]
- 24. Keating GM. Tiotropium bromide inhalation powder: a review of its use in the management of chronic obstructive pulmonary disease. Drugs 2012; 72(2): 273–300. [DOI] [PubMed] [Google Scholar]
- 25. de Luise C, Lanes SF, Jacobsen J, et al. Cardiovascular and respiratory hospitalizations and mortality among users of tiotropium in Denmark. Eur J Epidemiol 2007; 22(4): 267–272. [DOI] [PubMed] [Google Scholar]
- 26. Gershon AS, Wang L, To T, et al. Survival with tiotropium compared to long‐acting beta‐2‐agonists in chronic obstructive pulmonary disease. COPD 2008; 5(4): 229–234. [DOI] [PubMed] [Google Scholar]
- 27. Jara M, Lanes SF, Wentworth C, 3rd , et al. Comparative safety of long‐acting inhaled bronchodilators: a cohort study using the UK THIN primary care database. Drug Saf 2007; 30(12): 1151–1160. [DOI] [PubMed] [Google Scholar]
- 28. Jara M, Wentworth C, 3rd , Lanes S. A new user cohort study comparing the safety of long‐acting inhaled bronchodilators in COPD. BMJ Open 2012; 2(3): e000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gershon A, Croxford R, To T, et al. Comparison of inhaled long‐acting β‐agonist and anticholinergic effectiveness in older patients with chronic obstructive pulmonary disease: a cohort study. Ann Intern Med 2011; 154(9): 583–592. [DOI] [PubMed] [Google Scholar]
- 30. Di Martino M, Agabiti N, Bauleo L, et al. Use patterns of long‐acting bronchodilators in routine COPD care: the OUTPUL study. COPD 2014; 11(4): 414–423. [DOI] [PubMed] [Google Scholar]
- 31. Fano V, D'Ovidio M, del Zio K, et al. The role of the quality of hospital discharge records on the comparative evaluation of outcomes: the example of chronic obstructive pulmonary disease (COPD). Epidemiol Prev 2012; 36(3‐4): 172–179. [PubMed] [Google Scholar]
- 32. Weitzen S, Lapane KL, Toledano AY, et al. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf 2004; 13: 841–853. [DOI] [PubMed] [Google Scholar]
- 33. Di Martino M, Agabiti N, Cascini S, et al. The effect on total mortality of adding inhaled corticosteroids to long‐acting bronchodilators for COPD: a real practice analysis in Italy. COPD 2015: 1–12. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


