Abstract
Breast cancer is the most frequently diagnosed malignancy amongst females worldwide. In recent years the management of this disease has transformed considerably, including the administration of chemotherapy in the neoadjuvant setting. Aside from increasing rates of breast conserving surgery and enabling surgery via tumour burden reduction, use of chemotherapy in the neoadjuvant setting allows monitoring of in vivo tumour response to chemotherapeutics. Currently, there is no effective means of identifying chemotherapeutic responders from non‐responders. Whilst some patients achieve complete pathological response (pCR) to chemotherapy, a good prognostic index, a proportion of patients derive little or no benefit, being exposed to the deleterious effects of systemic treatment without any knowledge of whether they will receive benefit. The identification of predictive and prognostic biomarkers could confer multiple benefits in this setting, specifically the individualization of breast cancer management and more effective administration of chemotherapeutics. In addition, biomarkers could potentially expedite the identification of novel chemotherapeutic agents or increase their efficacy. Micro‐RNAs (miRNAs) are small non‐coding RNA molecules. With their tissue‐specific expression, correlation with clinicopathological prognostic indices and known dysregulation in breast cancer, miRNAs have quickly become an important avenue in the search for novel breast cancer biomarkers. We provide a brief history of breast cancer chemotherapeutics and explore the emerging field of circulating (blood‐borne) miRNAs as breast cancer biomarkers for the neoadjuvant treatment of breast cancer. Established molecular markers of breast cancer are outlined, while the potential role of circulating miRNAs as chemotherapeutic response predictors, prognosticators or potential therapeutic targets is discussed.
Keywords: miRNA, micro‐RNA, neoadjuvant chemotherapy, breast cancer, circulating
Abbreviations
- ER
oestrogen receptor
- Her2
Erbb2 receptor
- miRNA
micro‐RNA
- NAC
neo‐adjuvant chemotherapy
- NICE
National Institute for Health and Care Excellence
- pCR
complete pathological response
- PR
progesterone receptor
Breast cancer is the most frequently diagnosed cancer among women worldwide, accounting for 23% of total cancer cases.1 In 2012, worldwide 1.7 million women were diagnosed with breast cancer, representing a >20% increase in incidence since 2008. Concurrently, worldwide more than 520,000 women died from breast cancer, representing a 14% increase in annual breast cancer related mortality and confirming breast cancer as the most common cause of cancer‐related deaths amongst women.1 Within developed countries the incidence of breast cancer continues to rise. This is likely due to the implementation of screening programs and improved imaging techniques, leading to many breast cancers being diagnosed at an earlier stage. Furthermore, our improved molecular understanding of breast cancer and the use of increasingly effective chemotherapeutics has resulted in improved patient outcomes, with mortality decreasing by 2 to 3% per year in developed countries.2
Historically, neoadjuvant chemotherapy (NAC) was reserved for locally advanced breast carcinoma, converting technically inoperable tumours into candidates for mastectomy. With the increasing trend toward breast conserving surgery, however, the use of primary systemic therapy was extended to include patients with invasive, early‐stage operable tumours. The adoption of NAC has led to increasing rates of breast conserving surgery and provides an opportunity to assess in vivo tumour responsiveness to chemotherapeutics.3 Although previous studies failed to identify any improvement in disease free and overall survival between neoadjuvant and adjuvant therapies, it has been established that patients achieving complete pathological response (pCR) to NAC therapy experience improved outcomes, while unresponsive patients or patients with progressive disease during NAC experience worse outcomes.4, 5, 6, 7 Supporting this, it has been shown that the early response to neoadjuvant treatment can predict pCR and therefore may serve as a predictor of long‐term outcome.8, 9
Unfortunately at present there is no reliable, clinically validated, method for predicting chemotherapeutic responders from non‐responders. While the likelihood of achieving pCR varies greatly by breast cancer subtype (from 7.5% in luminal cancers to 45% in HER2/Triple negative cancers10, 11), many patients are exposed to the potential morbidity and mortality associated with chemotherapy, without any certainty of benefit from treatment. This has resulted in global efforts to discover breast cancer biomarkers that can predict and detect response to neoadjuvant therapy. Such biomarkers could confer multiple benefits, including tailored patient‐care programs, reduced chemotherapy‐induced morbidity or mortality and potentially expedite the identification of effective new therapies for the treatment of breast carcinoma. At present, circulating micro‐RNAs (miRNAs) represent an important avenue in the search for a non‐invasive biomarker for Breast Cancer Response prediction and monitoring for neoadjuvant chemotherapy. The evidence for this is discussed further in the following sections.
Micro‐RNAs
Micro‐RNAs are a naturally‐occurring class of short, non‐coding RNA molecules ∼19 to 25 nucleotides in length. miRNAs have been demonstrated to regulate gene expression at the post‐transcriptional level, via binding primarily to 3’ or 5’ untranslated regions of target messenger RNAs (mRNA), leading to inhibition of translation or mRNA degradation.12 Interestingly in addition to their inhibitory role, miRNAs have recently been demonstrated to facilitate increases in transcript levels, under certain conditions.13, 14
Since their discovery in 1993, knowledge of the role of miRNAs in regulating gene expression across a spectrum of pathological processes has grown exponentially.15 It is now recognized that certain miRNAs are highly specific for tissue and developmental stages, exerting a regulatory effect on a myriad of cellular processes including cell development, differentiation, proliferation and apoptosis.12, 14, 16 Many miRNA are expressed in tissue‐ and disease‐specific patterns and are known to correlate with clinicopathological features and prognostic indices across a spectrum of pathologies.17, 18, 19, 20, 21, 22 However, to date few studies have investigated the effect of neoadjuvant chemotherapy on miRNA expression patterns in breast cancer.
miRNA biosynthesis and mechanisms of action
miRNA generation is a complex process that commences in the nucleus. miRNA genes are transcribed by RNA polymerase II/III as primary miRNAs (pri‐miRNAs). These pri‐miRNAs are processed by the Drosha‐DGCR8 complex, becoming pre‐miRNAs. Pre‐miRNAs are transported into the cell cytoplasm by the nuclear export protein Exportin 5, where they are cleaved by the RNase III enzyme Dicer, with either TRBP (Trans‐activator RNA‐binding protein) or PACT (protein activator of PKR), into a double stranded miRNA duplex. One strand of the duplex represents a mature miRNA and is incorporated into the RNA‐induced silencing complex (RISC), while the other strand is degraded. The miRNA:RISC complex (miRISC) then targets mRNA containing complementary sequences to the mature miRNA, inhibiting translation or inducing mRNA degradation (Fig. 1).12, 16, 23
Figure 1.
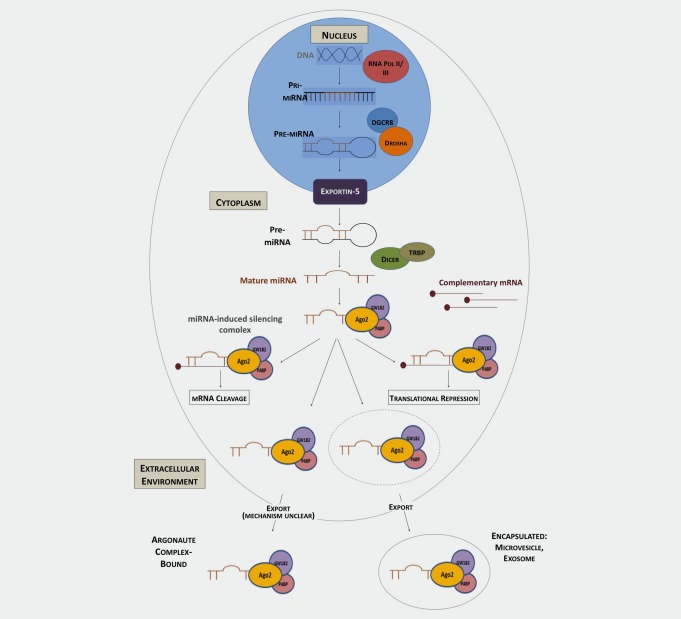
Model of miRNA biogenesis and cellular export.
Further to their intracellular function, it has been recognized that miRNAs function at an intercellular level, transmitting information from one cell population to another and inducing changes through this novel extracellular signaling mechanism.24, 25 Although small RNAs were detected in the circulation as early as 2004,26 it was in 2008 that the presence of miRNAs in the circulation was confirmed, with significant differences in expression patterns detectable between patients with cancer compared to controls.27, 28 These circulating miRNAs were found to be present with remarkable stability, indicating that they must be protected from the digestive action of circulatory RNases. Recent studies have confirmed that miRNAs are transported by a variety of mechanisms that shield them from this RNase degradation, including packaged into membrane‐derived vesicles, such as exosomes, bound to lipoproteins and as part of ribonucleoprotein complexes.24, 25, 29, 30, 31 While some mechanisms regulating the packaging and export of membrane‐bound miRNA are understood,32, 33, 34 the process of non‐membrane‐bound miRNA export from cells remains unclear.35, 36 The exact sources of all circulating miRNA remains unconfirmed. It appears there are two, complimentary, sources of circulating miRNA: (i) miRNA released passively into the bloodstream following tissue injury and cell death and (ii) miRNA actively exported from cells into the bloodstream. However, in both cases miRNA could be either protein bound “un‐encapsulated” miRNA or miRNA protected inside membrane coated vesicles (such as exosomes). Of note, it has been demonstrated that an estimated ≥90% of circulating miRNAs are bound to argonaute‐2 (Ago2) containing complexes, with only a minority being transported packaged in vesicles.30, 37 However, controversy regarding the proportions of free and membrane‐bound circulating miRNA remains, due to a lack of standardization of sample processing techniques, preventing data from individual studies from being directly compared.
Breast Cancer and Neoadjuvant Chemotherapy
The first successful chemotherapeutic regimen for operable breast cancer was described in 1976 by Bonadonna et al.38 The combination adjuvant therapy with cyclophosphamide, methotrexate and fluorouracil (CMF) was shown to significantly reduce postoperative recurrence rates. By the early 1990s, anthracycline‐containing regimens were introduced and are now recognized as superior to treatment with CMF alone.39 Importantly, it was during this time that administration of chemotherapy in the preoperative or neoadjuvant period began. The National Surgical Adjuvant Breast and Bowel Project B‐27 and the Aberdeen trial recognized the addition of a taxane to an anthracycline‐based chemotherapy further reduced the risk of recurrence, and in the neoadjuvant setting improved the rates of complete pathological remission and therefore overall outcome (Fig. 2).4, 40, 41
Figure 2.
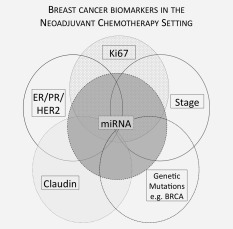
Breast cancer biomarkers in the neoadjuvant chemotherapy setting. Currently used clinical biomarkers (solid lines); new potentially clinically relevant biomarkers (dotted lines).
In recent years, it is recognized that breast cancer is a heterogeneous disease characterised by discrete breast cancer subtypes.42 While the exact number of subtypes remains to be elucidated, in a landmark paper, Sorlie et al.43 described Luminal A, Luminal B, Basal (also known as “triple negative” tumours) and HER2 over‐expressing (Table 1). More recently, 10 distinct breast cancer subtypes have been proposed, although this stratification is not yet applied clinically.45 Currently utilized breast cancer subtypes have known distinct clinical behaviors and responses to therapy and are stratified according to presence or absence of the oestrogen receptor (ER), progesterone receptor (PR) and the human epidermal growth factor receptor 2 (HER2) (discussed further in Current and Potential Molecular Markers Used to Guide the Administration of Chemotherapy section). While no gold‐standard chemotherapeutic regimen currently exists for breast cancer, it is generally accepted that an anthracycline‐based regimen be utilized, with the addition of a taxane.46, 47 For patients with breast cancer overexpressing the HER2 receptor, targeted therapy with the humanized monoclonal antibody Trastuzumab is recommended by NICE clinical guidelines48 (Fig. 2; Table 2).
Table 1.
Breast cancer subtypes with receptor status and prevalence44
| Breast cancer subtype | ER | PR | HER2 | Prevalence (%) |
|---|---|---|---|---|
| Luminal A | + | ± | − | 40 |
| Luminal B | + | ± | + | 20 |
| HER2 | − | − | + | 15–20 |
| Basal/triple negative | − | − | − | 10–15 |
This table outlines the prevalence and receptor status of current clinically utilized breast cancer subtypes.
Abbreviations: ER: oestrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2.
Table 2.
Chemotherapeutics used to treat breast cancer
| Drug class | Mechanism of action | Example | Reference |
|---|---|---|---|
| Anthracyclines |
Inhibition of DNA and RNA synthesis Disruption of DNA damage response Inhibition of Topoisomerase II |
Doxorubicin Epirubicin Mitoxantrone |
49 |
| Taxanes | Disruption of microtubule function |
Docetaxel Paclitaxel |
50 |
| Alkylating Agent: Nitrogen Mustard | Interference with DNA replication | Cyclophosphamide | 40 |
| Anti‐metabolites | Prevention of folate use for DNA generation |
Methotrexate Fluorouracil Capecitabine |
51 |
| Anti‐HER2/EGFR |
Tyrosine kinase inhibition Arrest of cell cycle Suppression of angiogenesis |
Trastuzumab Pertuzumab Lapatinib |
52 |
Current and Potential Molecular Markers Used to Guide the Administration of Chemotherapy
The current gold standard molecular markers for Breast Cancer Response prediction are the ER, PR and HER2 receptors and the proliferation marker Ki67. The American Society of Clinical Oncology guidelines mandate that the immunohistochemical markers ER, PR and HER2 be assessed in all cases of invasive breast carcinoma to guide management decisions, including choice of chemotherapeutic regimen.53 Further markers such as Claudin and specific miRNAs have been proposed and are presently undergoing further validation (Fig. 3).54
Figure 3.
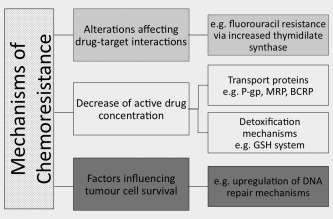
Mechanisms of chemoresistance.
ER/PR receptor
ER/PR expression, as identified by immunohistochemistry, provides an index for sensitivity to endocrine treatment and acts as a marker of chemosensitivity. Approximately two thirds of tumours display ER‐positivity, correlating with improved responsiveness to endocrine therapy and improved patient outcomes. PR expression is strongly dependent on ER expression, with <1% of breast cancers displaying sole PR‐positivity. In this instance, limited benefits from endocrine therapy have been described.55 Associating chemotherapeutic treatments and ER/PR status, ER positivity correlates with poor tumour response.56 However, ER‐negative tumours are more likely to achieve complete pathological remission and thus experience improved outcomes with chemotherapy.57
HER2 (ERBB2)
HER2 is a membrane tyrosine kinase receptor that upon activation affects cell proliferation and survival.58 It is located on chromosome 17q12 and is an oncogene, amplified in ∼15 to 20% of breast cancer cases. Initially identified as a prognostic marker, HER2 overexpression is associated with increased relapse rates, increased incidence of metastases and worse overall survival.59, 60 However, the development of therapies specifically targeting HER2 has resulted in significant improvements in outcomes for patients with HER2‐positive breast cancer.61 Risk of relapse and death are reduced by approximately 50% and 30% respectively, improving disease‐free and overall survival.62 In 2013 the addition of Trastuzumab to neoadjuvant chemotherapy in patients with HER2‐positive tumours was found to double complete pathological remission rates (compared to chemotherapy alone) and was associated with a longer event free survival.63 Most recently, use of neoadjuvant followed by adjuvant HER2 has demonstrated sustained benefit in event‐free survival and a strong association with complete pathological remission.64
Basal/triple negative breast cancers
“Basal” and “triple negative (TNBC)” breast cancer subtypes overlap greatly in terms of their immunophenotype (ER, PR & HER2 negative), aggressive clinical behavior and increased prevalence in younger, African‐American patients. However, they are not synonymous, as not all basal cancers determined by gene expression analysis lack ER, PR and HER2 and not all triple‐negative cancers show a basal phenotype by expression array analysis.65 Due to a more aggressive clinical pathology, both of these subtypes are associated with a higher risk of mortality. Both subtypes lack all known effective biomarkers and therefore targeted therapies. However, both subtypes are highly sensitive to neoadjuvant chemotherapy.66, 67, 68 The highest rates of complete pathological remission have been achieved in TNBC tumours utilising a neoadjuvant regimen of docetaxel, doxorubicin and cyclophosphamide, while the addition of bevacizumab is expected to increase this rate further.63
Ki67
Ki67 is a nuclear non‐histone protein utilized as a marker of proliferation, as it is absent in quiescent cells, yet universally expressed among proliferating cells. Immunohistochemical staining of, Ki67 expression levels is associated with the percentage of tumour cell nuclei positively stained and are used to determine a Ki67 score. In early and advanced breast cancer the Ki67 score can predict the response to chemotherapy.69 A high pre‐treatment score is associated with a good chance of complete pathological remission to therapy and therefore improved long‐term outcome.70 Lee et al.71 describe a significant decrease in Ki67 index following neoadjuvant chemotherapy, a finding that is recognized as a strong predictor of recurrence‐free and overall survival. Of concern regarding the use of Ki67 is the lack of standardization of analytical practice, with laboratories utilising differing cut‐off points to differentiate between “high” and “low” Ki67. Further to this, as with all of the above described markers, determination of expression levels requires tumour tissue, mandating invasive sampling techniques, which further emphasizes the need for a non‐invasive breast cancer biomarker.
Claudins
More recently, a potential fifth subtype of breast cancer has been described, classified as “Claudin‐low.”72, 73 Claudins are a family of proteins that function in tight‐junctions and cell‐cell adhesion, including Claudin 3, 4, 7 and E‐cadherin. A new subtype of breast cancer, “Claudin‐low” has recently been described, characterised by lack of expression of the Claudin proteins.72, 74 Claudin‐low tumours are typically triple‐negative and display high expression of epithelial‐to‐mesenchymal transition (EMT) markers.75 The expression of EMT markers has known associations with resistance to therapeutics and higher metastatic potential.76 Claudin‐low cancers display lower complete pathological remission rates following NAC. Overall Claudin‐low cancers were found to have an intermediate prognosis, worse than basal‐like breast cancer, but better than luminal cancer.72 However, the “Claudin‐low” breast cancer subtype remains poorly described.77 Further definitive characterization is required before it is fully accepted into clinical practice.
miRNA
In 2005, genome‐wide miRNA expression analysis enabled identification of miRNAs that were differentially expressed in breast cancer tissue.78 The panel of 29 miRNAs identified differentiated tumours from normal tissues with an accuracy of 100%. Importantly, miRNA expression correlated with distinct tumour phenotypes, ER and PR expression and tumour stage. More recently, a miRNA expression pattern of 31 miRNAs evaluated in 93 tumour samples was found to predict hormone receptor status, and thus classify tumours by genetic subtype.79 These findings were independently corroborated by Lowery et al.20 who profiled 453 miRNAs in 29 early‐stage breast cancer specimens, identifying a distinct panel of miRNAs corresponding to expression of ER, PR and HER2.
Translating these tissue findings into the circulation, it was found that miR‐195 expression was significantly elevated in breast cancer patients (n = 148) compared to controls (n = 44), and that these levels reduced postoperatively.80 Furthermore, high levels of circulating miR‐21 and miR‐10b were found to be associated with ER negativity, thus a poorer prognosis.80 In a further study, elevated miR‐155 expression was associated with PR positivity.81
The ability of miRNA expression profiles to classify breast tumours by biopathologic variables currently utilized to determine responsiveness to neoadjuvant chemotherapy highlights the potential of miRNA signatures as novel predictive and prognostic biomarkers that could allow individualization of breast cancer treatment and improved selection of patients for neoadjuvant chemotherapy (Fig. 3).
miRNA as Novel Biomarkers of NAC Response
The role of miRNA in neoadjuvant chemotherapeutic response prediction and monitoring has been investigated across a variety of pathologies. In colorectal carcinoma an association between miRNAs and tumour response to neoadjuvant chemoradiotherapy was proposed.82 This association was confirmed by identification of a distinct miRNA expression signature that could effectively predict colorectal cancer response to neoadjuvant chemoradiotherapy.83 In human gastric cancer, decreased let‐7i expression was found to have a significant association with a poorer response to chemotherapy and shorter overall survival.84 In breast cancer the ability of a panel of miRNAs to predict response of triple negative breast carcinoma to neoadjuvant chemotherapy was investigated.85 Although study numbers were limited (11 patients), results indicated higher miR‐200b‐3p and miR‐190a expression and lower miR‐512‐5p expression was associated with a better pathologic response to chemotherapy.
While all the above findings involved miRNA analysis from tumour samples, some peri‐neoadjuvant studies of circulating miRNA have also been conducted. One study investigated miRNA extracted from the sera of stage II‐III locally advanced and inflammatory breast carcinoma patients preneoadjuvant chemotherapy.86 A two‐gene signature of miR‐375 and miR‐122 was identified with the ability to predict metastatic disease relapse with a sensitivity of 80% and specificity of 100%. Patients relapsing following neoadjuvant chemotherapy were found to have significantly up‐regulated expression of miR‐122 while patients with higher circulating miR‐375 experienced a good clinical outcome. Fluctuation within a panel of miRNA was also found in patients with primary operable or locally advanced breast cancer receiving neoadjuvant chemotherapy.87 Of a panel of eight miRNAs, miR‐221, miR‐195 and miR‐21 were noted to decrease most significantly with the administration of chemotherapeutics, although correlation with response to systemic therapy was not conducted. A further study recognized expression of two particular miRNAs to be induced by treatment with chemotherapeutics, namely miR‐34a and miR‐122. Elevated expression of these miRNAs was detected both in tumour tissue and serum, and was particularly associated with anthracycline‐based regimens in patients achieving partial response to neoadjuvant chemotherapy.88
Some interesting in vitro findings that show promise for translation to the clinical setting have been conducted. Investigating targeted therapies for triple negative breast cancers, the overexpression of miR‐181a/b was found to associate with more aggressive breast cancer subtypes.89 Utilizing a range of cell lines (MDA‐MB‐231, HEK 293GP, MDA‐MB‐468, SUM159PT, OVCAR, HT29, PANC1 and Sk‐Br‐3) the overexpression of miR‐181a/b was found to dampen the DNA damage response, thus increasing the sensitivity of the triple negative cells in which it was expressed to poly (ADP‐Ribose) polymerase‐1 (PARP‐1) inhibition. It was proposed that profiling miR‐181a/b expression in patients with triple negative breast cancer could identify patients for PARP‐1 inhibition or platinum‐based chemotherapy.
miRNA and chemoresistance
It is recognized that specific miRNA expression signatures are associated with resistance to all forms of breast cancer treatment, including chemotherapy, anti‐endocrine therapy and radiotherapy.90, 91, 92, 93, 94 Regarding chemotherapeutic resistance, several recent reports have revealed the key regulatory role of miRNAs affecting drug resistance proteins and targeting proteins involved in apoptosis (Table 3, Fig. 3).
Table 3.
miRNAs with a validated involvement in chemotherapeutic resistance in breast cancer
| miRNA | Expression | Target(s) | Drug Assn | Source (# patient samples) | Reference |
|---|---|---|---|---|---|
| miR‐7 | Down‐regulation | MDR‐1 | Cisplatin | Cell line | 95 |
| miR‐19 | Up‐regulation | MDR‐1, MRP‐1 and BCRP via PTEN |
Paclitaxel Mitoxantrone VP‐16 |
Cell Line | 96 |
| miR‐21 |
Up‐regulation Up‐regulation |
PTEN PTEN |
Doxorubicin Trastuzumab |
Cell Line | 97, 98 |
| miR‐25 | Up‐regulation | Inhibits autophagic cell death | Epirubicin | Cell Line | 99 |
| miR‐30c |
Down‐regulation Down‐regulation |
YWHAZ TWF1, IL‐11 |
Doxorubicin Paclitaxel Doxorubicin |
Cell Line Human breast tissue (n = 51) Cell Lines |
100 101 |
| miR‐34a |
Up‐regulation Down‐regulation Down‐regulation |
BCL‐2, Cyclin D1 Notch‐1 E2F3, PXR |
Docetaxel Adriamycin Doxorubicin |
Cell Line Cell Line Cell Line |
102 103 104 |
| miR‐125b |
Up‐regulation Up‐regulation |
E2F3 Bak 1 |
5‐Fluorouracil Paclitaxel |
Blood Serum Cell Line |
105 106 |
| miR‐137 | Down‐regulation | P‐glycoprotein, via YB‐1 |
Vincristine Doxorubicin Paclitaxel |
Cell Line | 107 |
| miR‐149 | Down‐regulation | NDST1 | Adriamycin | Cell Line | 108 |
| miR‐155 | Up‐regulation | FOXO3a |
Doxorubicin VP‐16 Paclitaxel |
Human Breast Tissue (n = 126) | 109 |
| miR‐200c |
Down‐regulation Down‐regulation |
P‐glycoprotein MDR mRNA TrkB, Bmi1 |
Doxorubicin Doxorubicin |
Human Breast Tissue (n = 39) Cell Line Cell Line |
110 111 |
| miR‐210 | Up‐regulation | Not studied | Trastuzumab | Blood Serum (n = 43) Cell Lines | 91 |
| miR‐221 | Up‐regulation | Not studied | Adriamycin | Blood Plasma (n = 125) | 112 |
| miR‐288 | Down‐regulation | MDR‐1, P‐glycoprotein | Doxorubicin | Cell Line | 113 |
| miR‐320a | Down‐regulation | TRPC5, NFATC3 |
Adriamycin Paclitaxel |
Cell Line | 114 |
| miR‐345 | Down‐regulation | MDR‐1 | Cisplatin | Cell Line | 95 |
| miR‐451 | Down‐regulation | P‐glycoprotein, MDR‐1 | Doxorubicin | Cell Line | 92 |
| miR‐489 | Down‐regulation | Smad3 | Adriamycin | Cell Line | 115 |
| miR‐663 | Up‐regulation | HSPG2 | Adriamycin | Cell Line | 116 |
This table presents an overview of miRNAs that have a validated role in chemotherapeutic resistance, referencing the drug and source examined and the identified miRNA targets.
Abbreviations: HSPG2: heparin sulfate proteoglycan 2; Smad3: mothers against decapentaplegic homolog 3; NDST1: GlcNAc N‐deacetylase/N‐sulfotransferase‐1; TRPC5: transient receptor potential channel C5; NFATC3: nuclear factor of activated T‐cells isoform C3; YWHAZ: tyrosine 3‐monooxygenase/tryptophan 5‐monooxygenase activation protein zeta; PXR: pregnane X receptor; BCL‐2: B‐cell lymphoma 2; YB‐1: Y‐box binding protein‐1; MRP‐1: multidrug resistance‐associated protein‐1; BCRP: breast cancer resistance protein; TWF1: Twinfilin 1; IL‐1: interleukin‐1; TrkB: tyrosine receptor kinase type 2; Bmi1: B‐cell‐specific Moloney murine leukemia virus integration site 1; Bak 1: Bcl‐2 antagonist killer 1.
In the circulation, an association between serum miR‐125b levels from patients with invasive ductal breast carcinoma receiving NAC and chemoresistance has been described.105 Increased expression of miR‐125b was found to have a significant association (p = 0.008) with non‐response to chemotherapy. Further to this, forced miR‐125b overexpression in breast cancer cells in vitro increased chemotherapeutic resistance, with subsequent reduction in miR‐125b levels sensitizing the cells to chemotherapy once more. Similar results were observed regarding miR‐210, where increased plasma miR‐210 levels correlated with a reduced sensitivity of HER2 breast carcinoma to Trastuzumab therapy. High pre‐treatment circulating mir‐210 was found to be associated with lower pCR rates and lymph node metastasis. Recently the radiological and clinical response of breast cancer to either neoadjuvant chemotherapy or hormonal therapy was assessed using a low density miRNA array. Significantly increased Let‐7a was found in the plasma of patients achieving a radiological response following neoadjuvant chemotherapy, but not hormonal therapy.117
In breast cancer tissue, down‐regulation of miR‐200c was found in patients who were non‐responsive to NAC.110 Subsequently, up‐regulation of miR‐200c in human breast cancer cell lines enhanced chemosensitivity and decreased expression of multi‐drug resistance (MDR) proteins P‐glycoprotein (P‐gp) and MDR‐associated protein (MRP‐1).
Utilizing breast cancer cell lines, differential miRNA expression has been noted to correlate with resistance to chemotherapeutics. Using MCF‐7/MX100 cell miR‐328 was identified as a negative regulator of breast cancer‐resistance protein (BCRP), with higher miR‐328 levels facilitating an improved Mitoxantrone response.118 Up‐regulated miR‐19 (in MCF‐7/TX200, MCF‐7/VP‐17, MCF‐7/MX100 and MCF‐7/WT cell lines) correlated with overexpression of three MDR‐related transport proteins (MDR‐1, MRP‐1 and BCRP). Importantly, miR‐19 inhibitors decreased the expression of these MDR proteins.96 MiR‐451 was also found to regulate expression of MRP‐1, with up‐regulation of miR‐451 in doxorubicin‐resistant MCF‐7 cells returning chemotherapeutic sensitivity.92 Mir‐326 exhibited the same effect on MRP‐1, inducing sensitivity to doxorubicin in MDR MCF‐7 cells.119 Further miRNAs noted to target MRP‐1 include miR‐345 and miR‐7, which were found to decrease cellular levels of MRP‐1.95
In BT474, SKBR3, and MDA‐MB‐453 breast cancer cell lines miR‐21 conferred resistance to Trastuzumab, via down‐regulation of its target PTEN.97 Subsequently, this pathway was also found to modulate resistance to doxorubicin in doxorubicin‐resistant MCR‐7 cell lines.98 In addition, miR‐137 was found to be down‐regulated in MDR MCF‐7 cells.107
Cells lines can provide conflicting data however. MCF‐7 and MDA‐MB‐231 cells with acquired docetaxel resistance, showed increased expression of miR‐34a, with miR‐34a inhibition enhancing chemotherapeutic response in docetaxel‐resistant MCF‐7 cell lines.102 However, decreased levels of miR‐34a were described in the Adriamycin‐resistant MCF‐7 cell line,104 with forced overexpression of miR‐34a found to increase chemotherapeutic sensitivity of Adriamycin‐resistant MCF‐7 cell lines.103 The exact role of miR‐34a in breast cancer chemotherapeutic response is not fully understood and requires further investigation. This seemingly contradictory data highlights the complex nature of miRNAs and their role in chemoresistance, particularly in relation to taxane resistance.120
miRNAs as Targeted Therapies in the Neoadjuvant Setting
The use of miRNAs in the treatment of breast cancer includes using miRNAs as therapeutic treatments and manipulating miRNA expression to enhance existing treatments. As miRNAs function as oncomirs and tumour suppressors, two therapeutic potentials exist: overexpression of targeted miRNAs (miRNA replacement therapy) or down‐regulation (silencing) of miRNAs.
Oncomir inhibition
Antisense‐inhibition of miRNA activity can be achieved by using miRNA antagonist oligonucleotides (anti‐miRs), locked‐nucleic acids (LNA), or targeted miRNA silencing (antagomiRs).121 The efficacy of this targeted therapy has been demonstrated by several studies. MCF‐7 cells transfected with anti‐miR‐21 oligonucleotides were grown in vitro and in a xenograft murine model. Anti‐miR‐21 suppressed both cell growth in vitro and tumour growth in the mouse model. In addition, cell growth inhibition was associated with increased apoptosis and decreased cell proliferation.122
In the chemotherapeutic setting, down‐regulation of mir‐21 was seen to increase sensitivity of MCF‐7 cells to taxol therapy,123 with miR‐203 knockdown increasing cisplatin sensitivity.124 Increased sensitivity in response to miRNA knockdown has also been demonstrated, in HS578T cells whereby by miR‐155 down‐regulation (using antisense‐miR‐155 oligonucleotides) increased apoptosis in response to treatment with Paclitaxel, Doxorubicin and VP‐16.109
miRNA replacement therapy
This strategy involves the reintroduction of function of a tumour suppressing miRNA.
In NOD/SCID (nonobese diabetic/severe combined immunodeficient) mice, SK‐3rd cells over‐expressing Let‐7 or miR‐30 displayed significantly reduced tumourigenicity and lung and liver metastasis.125, 126 In a further study in chemoresistant MDA‐MB‐231 and BT‐549 cells, overexpression of miR‐200c was found to restore chemosensitivity to microtubule‐directed agents.127
Although rapid and continual advancements are being made regarding the manipulation of miRNAs for the treatment of breast cancer, this area of research remains in its infancy, with many obstacles to overcome prior to mainstream implementation in breast cancer therapeutics. The majority of studies conducted to date are in vitro, examining miRNAs and their effects in various cell lines. To validate these studies, large clinical trials are required to support these preliminary findings. Present obstacles to overcome include the identification of optimal delivery methods and the prevention of off‐target effects and safety optimization.
Discussion
The management of breast cancer, in terms of diagnosis, chemotherapeutics and surgical intervention, continues to adapt in line with translational research and evidence. Whilst the use of chemotherapy in the neoadjuvant setting is now acceptable for any patient considered a candidate for adjuvant therapy,128 there currently exists no clinically validated means of differentiating chemotherapeutic responders from non‐responders. While miRNA expression analysis holds significant promise in this setting, further studies investigating miRNAs in the neoadjuvant breast cancer setting are undoubtedly warranted. Presently, as per ClinicalTrials.gov, two clinical trials are currently recruiting breast cancer patients undergoing neoadjuvant chemotherapy for miRNA profiling and analysis.
A further challenge affecting the use of circulating miRNA as breast cancer biomarkers is the lack of accepted standardized protocols for sample collection, handling and processing. As a consequence, many previous studies cannot be easily compared. This is due to intrinsic differences in: Patient cohorts (treatment regimes, timing of sample collection), Bio‐fluid collected and analyzed (whole blood, plasma, serum), Collection methods (EDTA, Paxgene tubes, sample handling), Processing/extraction techniques (diverse extraction kits, timing of extraction), Investigation of miRNA pool (total, free or membrane bound), Use of multiple non‐standardized endogenous controls and Detection methods utilized [arrays (which are constantly being updated) or different RQ‐PCR platforms].129, 130, 131, 132 The development and adoption of a standardized set of operating and technical protocols would facilitate study comparison, improved reproducibility and the development of improved targeted future studies.
The use of miRNAs as targeted therapies, although in its infancy, holds immense promise, although further evaluation and validation of current findings are required. The identification of a biomarker that could predict or potentially monitor tumour response to neoadjuvant chemotherapy could revolutionize the manner in which chemotherapeutics are administered, bringing us ever closer to personalized breast cancer management.
Future directions
In addition to miRNAs, dysregulation of miRNA machinery can play a crucial role in cancer initiation and progression.133 miRNA‐binding proteins mediate miRNA‐dependent cleavage or degradation of target mRNAs, with all miRNAs studied to date assembling into miRNA‐silencing complexes. Studies have shown that genes involved in miRNA biogenesis are dysregulated in various breast cancer subtypes.79 Down‐regulation of Drosha and Dicer, two key elements of the miRNA machinery, has been associated with more aggressive breast cancer subtypes.134, 135 Furthermore, increased expression of exportin‐5, a pre‐miRNA transporting nuclear receptor, has been associated with increased breast cancer susceptibility.136 In addition, expression of the key miRNA‐binding protein Argonaute‐2 protein (Ago2), required for miRNA extracellular transport, is elevated in basal‐like breast cancer subtypes, with elevated Ago2 levels producing enhanced proliferation, reduced cell‐cell adhesion and increased migratory ability,137 implicating Ago2 in more aggressive breast cancer subtypes. Further to this, single‐nucleotide polymorphisms of Ago2 have been associated with disease free and overall survival in breast cancer.138
The use of miRNA machinery genes as breast cancer biomarkers is still in its infancy, however, with further investigation required to fully elucidate mechanisms of miRNA maturation, miRNA‐machinery gene regulation and the cancer‐specific functions of these miRNA machinery genes and their resultant proteins.
Conclusion
Whilst breast cancer management continues to improve the requirement of a breast cancer biomarker that is both predictive and prognostic remains. Investigation of the potential for miRNAs to fulfill this role holds much promise, although further clinical studies are required, particularly in the neoadjuvant chemotherapeutic setting.
Acknowledgement
We thank all the members of Prof Kerins’ group for stimulating discussions.
This article was published online on 30 January 2016. An error was subsequently identified. This notice is included in the online and print versions to indicate that both have been corrected on 4 February 2016.
References
- 1. International Agency for Research on Cancer . Latest world cancer statistics: Global cancer burden rises to 14.1 million new cases in 2012: Marked increase in breast cancers must be addressed 2013. Lyon, France: World Health Organization. [Google Scholar]
- 2. Levi F, Bosetti C, Lucchini F, et al. Monitoring the decrease in breast cancer mortality in Europe. Eur J Cancer Prevent 2005; 14:497–502. [DOI] [PubMed] [Google Scholar]
- 3. Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast‐conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg 2014; 260:608–14; discussion 614‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B‐18 and B‐27. J Clin Oncol 2008; 26:778–85. [DOI] [PubMed] [Google Scholar]
- 5. Mauri D, Pavlidis N, Ioannidis JP., Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta‐analysis. J Natl Cancer Inst 2005; 97:188–94. [DOI] [PubMed] [Google Scholar]
- 6. van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001; 19:4224–37. [DOI] [PubMed] [Google Scholar]
- 7. Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local‐regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B‐18. J Clin Oncol 1997; 15:2483–93. [DOI] [PubMed] [Google Scholar]
- 8. von Minckwitz G, Blohmer JU, Raab G, et al. In vivo chemosensitivity‐adapted preoperative chemotherapy in patients with early‐stage breast cancer: the GEPARTRIO pilot study. Ann Oncol 2005; 16:56–63. [DOI] [PubMed] [Google Scholar]
- 9. von Minckwitz G, Kummel S, Vogel P, et al. Intensified neoadjuvant chemotherapy in early‐responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst 2008; 100:552–62. [DOI] [PubMed] [Google Scholar]
- 10. Zambetti M, Mansutti M, Gomez P, et al. Pathological complete response rates following different neoadjuvant chemotherapy regimens for operable breast cancer according to ER status, in two parallel, randomized phase II trials with an adaptive study design (ECTO II). Breast Cancer Res Treat 2012; 132:843–51. [DOI] [PubMed] [Google Scholar]
- 11. Bonnefoi H, Litiere S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two‐step approach analyses from the EORTC 10994/BIG 1‐00 phase III trial. Ann Oncol 2014; 25:1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartel DP. Micro‐RNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281–97. [DOI] [PubMed] [Google Scholar]
- 13. Vasudevan S, Tong Y, Steitz JA., Switching from repression to activation: micro‐RNAs can up‐regulate translation. Science 2007; 318:1931–4. [DOI] [PubMed] [Google Scholar]
- 14. Place RF, Li LC, Pookot D, et al. Micro‐RNA‐373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci USA 2008; 105:1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casey MC, Kerin MJ, Brown JA, et al. Evolution of a research field‐a micro (RNA) example. PeerJ 2015; 3:e829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Filipowicz W, Bhattacharyya SN, Sonenberg N., Mechanisms of post‐transcriptional regulation by micro‐RNAs: are the answers in sight? Nat Rev Genet 2008; 9:102–14. [DOI] [PubMed] [Google Scholar]
- 17. Lagos‐Quintana M, Rauhut R, Yalcin A, et al. Identification of tissue‐specific micro‐RNAs from mouse. Curr Biol 2002; 12:735–9. [DOI] [PubMed] [Google Scholar]
- 18. Liang Y, Ridzon D, Wong L, et al. Characterization of micro‐RNA expression profiles in normal human tissues. BMC Genomics 2007. Jun 12; 8:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yanaihara N, Caplen N, Bowman E, et al. Unique micro‐RNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006; 9:189–98. [DOI] [PubMed] [Google Scholar]
- 20. Lowery AJ, Miller N, Devaney A, et al. Micro‐RNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res 2009; 11:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schetter AJ, Okayama H, Harris CC. The role of micro‐RNAs in colorectal cancer. Cancer J 2012; 18:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dvinge H, Git A, Graf S, et al. The shaping and functional consequences of the micro‐RNA landscape in breast cancer. Nature 2013; 497:378–82. [DOI] [PubMed] [Google Scholar]
- 23. He L, Hannon GJ., Micro‐RNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004; 5:522–31. [DOI] [PubMed] [Google Scholar]
- 24. Valadi H, Ekstrom K, Bossios A, et al. Exosome‐mediated transfer of mRNAs and micro‐RNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654–9. [DOI] [PubMed] [Google Scholar]
- 25. Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating micro‐RNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 2011; 108:5003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El‐Hefnawy T, Raja S, Kelly L, et al. Characterization of amplifiable, circulating RNA in plasma and its potential as a tool for cancer diagnostics. Clin Chem 2004; 50:564–73. [DOI] [PubMed] [Google Scholar]
- 27. Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour‐associated micro‐RNAs in serum of patients with diffuse large B‐cell lymphoma. Br J Haematol 2008; 141:672–5. [DOI] [PubMed] [Google Scholar]
- 28. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating micro‐RNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci USA 2008; 105:10513–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vickers KC, Palmisano BT, Shoucri BM, et al. Micro‐RNAs are transported in plasma and delivered to recipient cells by high‐density lipoproteins. Nat Cell Biol 2011; 13:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turchinovich A, Weiz L, Langheinz A, et al. Characterization of extracellular circulating micro‐RNA. Nucleic Acids Res 2011; 39:7223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caby MP, Lankar D, Vincendeau‐Scherrer C, et al. Exosomal‐like vesicles are present in human blood plasma. Int Immunol 2005; 17:879–87. [DOI] [PubMed] [Google Scholar]
- 32. Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 2011; 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weilner S, Schraml E, Redl H, et al. Secretion of microvesicular miRNAs in cellular and organismal aging. Exp Gerontol 2013; 48:626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 2010; 107:1047–57. [DOI] [PubMed] [Google Scholar]
- 35. Creemers EE, Tijsen AJ, Pinto YM. Circulating micro‐RNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 2012; 110:483–95. [DOI] [PubMed] [Google Scholar]
- 36. Turchinovich A, Samatov TR, Tonevitsky AG, et al. Circulating miRNAs: cell‐cell communication function? Front Genet 2013; 4:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu H, Fan GC., Extracellular/circulating micro‐RNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis 2011; 1:138–49. [PMC free article] [PubMed] [Google Scholar]
- 38. Bonadonna G, Brusamolino E, Valagussa P, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med 1976; 294:405–10. [DOI] [PubMed] [Google Scholar]
- 39. Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin‐cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive‐node breast cancer patients with tamoxifen‐nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B‐15. J Clin Oncol 1990; 8:1483–96. [DOI] [PubMed] [Google Scholar]
- 40. Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B‐27. J Clin Oncol 2003; 21:4165–74. [DOI] [PubMed] [Google Scholar]
- 41. Heys SD, Hutcheon AW, Sarkar TK, et al. Neoadjuvant docetaxel in breast cancer: 3‐year survival results from the Aberdeen trial. Clin Breast Cancer 2002; 3:S69–74. [DOI] [PubMed] [Google Scholar]
- 42. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000; 406:747–52. [DOI] [PubMed] [Google Scholar]
- 43. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98:10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. American Cancer Society Breast Cancer Facts & Figures 2013–2014. Atlanta: American Cancer Society, Inc; 2013. [Google Scholar]
- 45. Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verrill M. Chemotherapy for early‐stage breast cancer: a brief history. Br J Cancer 2009; 101:S2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levine MN, Pritchard KI, Bramwell VH, et al. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node‐positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol 2005; 23:5166–70. [DOI] [PubMed] [Google Scholar]
- 48.Early and Locally Advanced Breast Cancer Diagnosis and Treatment. NICE Clinical Guidelines, No. 80 2009. Cardiff, UK: National Collaborating Centre for Cancer (UK). Feb. ISBN-13: 978-0-9558265-2-8. [PubMed]
- 49. Minotti G, Menna P, Salvatorelli E, et al. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 2004; 56:185–229. [DOI] [PubMed] [Google Scholar]
- 50. McGrogan BT, Gilmartin B, Carney DN, et al. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta 2008; 1785:96–132. [DOI] [PubMed] [Google Scholar]
- 51. Kaye SB. New antimetabolites in cancer chemotherapy and their clinical impact. Br J Cancer 1998; 78:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roy V, Perez EA., Beyond trastuzumab: small molecule tyrosine kinase inhibitors in HER‐2‐positive breast cancer. Oncologist 2009; 14:1061–9. [DOI] [PubMed] [Google Scholar]
- 53. Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007; 25(33):5287–312. [DOI] [PubMed] [Google Scholar]
- 54. Blumenthal RD. Chemosensitivity: vol. II: in vivo models, imaging, and molecular regulators. In: Blumenthal RD, editor. Methods in molecular medicine, vol. III, vol. II Totowa, NJ: Humana Press, 2005. 127–144. [Google Scholar]
- 55. Weigel MT, Dowsett M., Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer 2010; 17:R245–62. [DOI] [PubMed] [Google Scholar]
- 56. Ring AE, Smith IE, Ashley S, et al. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer 2004; 91:2012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Colleoni M, Minchella I, Mazzarol G, et al. Response to primary chemotherapy in breast cancer patients with tumors not expressing estrogen and progesterone receptors. Ann Oncol 2000; 11:1057–9. [DOI] [PubMed] [Google Scholar]
- 58. Park JW, Neve RM, Szollosi J, et al. Unraveling the biologic and clinical complexities of HER2. Clin Breast Cancer 2008; 8:392–401. [DOI] [PubMed] [Google Scholar]
- 59. Slamon DJ, Clark GM, Wong SG, et al. Human‐breast cancer ‐ correlation of relapse and survival with amplification of the Her‐2 Neu oncogene. Science 1987; 235:177–82. [DOI] [PubMed] [Google Scholar]
- 60. Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 2004; 5:63–9. [DOI] [PubMed] [Google Scholar]
- 61. Perez EA, Reinholz MM, Hillm W, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol 2010; 28:4307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith I, Procter M, Gelber RD, et al. 2‐year follow‐up of trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer: a randomised controlled trial. Lancet 2007; 369:29–36. [DOI] [PubMed] [Google Scholar]
- 63. von Minckwitz G, Fontanella C., Selecting the neoadjuvant treatment by molecular subtype: how to maximize the benefit? Breast 2013; 22:S149–51. [DOI] [PubMed] [Google Scholar]
- 64. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2‐positive locally advanced breast cancer (NOAH): follow‐up of a randomised controlled superiority trial with a parallel HER2‐negative cohort. Lancet Oncol 2014; 15:640–7. [DOI] [PubMed] [Google Scholar]
- 65. Bertucci F, Finetti P, Cervera N, et al. How basal are triple‐negative breast cancers? Int J Cancer 2008; 123:236–40. [DOI] [PubMed] [Google Scholar]
- 66. Moreno‐Aspitia A, Perez EA., Treatment options for breast cancer resistant to anthracycline and taxane. Mayo Clin Proc 2009; 84:533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 2007; 13:2329–34. [DOI] [PubMed] [Google Scholar]
- 68. Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005; 11:5678–85. [DOI] [PubMed] [Google Scholar]
- 69. Faneyte IF, Schrama JG, Peterse JL, et al. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer 2003; 88:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jones RL, Salter J, A'Hern R, et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat 2009; 116:53–68. [DOI] [PubMed] [Google Scholar]
- 71. Lee HC, Ko H, Seol H, et al. Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response. J Breast Cancer 2013; 16:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin‐low intrinsic subtype of breast cancer. Breast Cancer Res 2010; 12:R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Perou CM. Molecular stratification of triple‐negative breast cancers. Oncologist 2010; 15:39–48. [DOI] [PubMed] [Google Scholar]
- 74. Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol 2007; 8(5):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Peddi PF, Ellis MJ, Ma C., Molecular basis of triple negative breast cancer and implications for therapy. Int J Breast Cancer 2012; 2012:217185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor‐initiating features. Proc Natl Acad Sci USA 2008; 106:13820–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sabatier R, Finetti P, Guille A, et al. Claudin‐low breast cancers: clinical, pathological, molecular and prognostic characterization. Mol Cancer 2014; 13:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Iorio MV, Ferracin M, Liu CG, et al. Micro‐RNA gene expression deregulation in human breast cancer. Cancer Res 2005; 65:7065–70. [DOI] [PubMed] [Google Scholar]
- 79. Blenkiron C, Goldstein LD, Thorne NP, et al. Micro‐RNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol 2007; 8:R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Heneghan HM, Miller N, Lowery AJ, et al. Circulating micro‐RNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg 2010; 251:499–505. [DOI] [PubMed] [Google Scholar]
- 81. Zhu W, Qin W, Atasoy U, et al. Circulating micro‐RNAs in breast cancer and healthy subjects. BMC Res Notes 2009; 2:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Svoboda M, Sana J, Fabian P, et al. Micro‐RNA expression profile associated with response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Radiat Oncol 2012; 7:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kheirelseid EA, Miller N, Chang KH, et al. miRNA expressions in rectal cancer as predictors of response to neoadjuvant chemoradiation therapy. Int J Colorectal Dis 2013; 28:247–60. [DOI] [PubMed] [Google Scholar]
- 84. Liu K, Qian T, Tang L, et al. Decreased expression of micro‐RNA let‐7i and its association with chemotherapeutic response in human gastric cancer. World J Surg Oncol 2012; 10:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kolacinska A, Morawiec J, Fendler W, et al. Association of micro‐RNAs and pathologic response to preoperative chemotherapy in triple negative breast cancer: preliminary report. Mol Biol Rep 2014; 41:2851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wu X, Somlo G, Yu Y, et al. De novo sequencing of circulating miRNAs identifies novel markers predicting clinical outcome of locally advanced breast cancer. J Transl Med 2012; 10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gezer U, Keskin S, Igci A, et al. Abundant circulating micro‐RNAs in breast cancer patients fluctuate considerably during neoadjuvant chemotherapy. Oncol Lett 2014; 8:845–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Freres P, Josse C, Bovy N, et al. Neoadjuvant chemotherapy in breast cancer patients induces miR‐34a and miR‐122 expression. J Cell Physiol 2014; 230(2):473–81. [DOI] [PubMed] [Google Scholar]
- 89. Bisso A, Faleschini M, Zampa F, et al. Oncogenic miR‐181a/b affect the DNA damage response in aggressive breast cancer. Cell Cycle 2013; 12:1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li H, Yang BB., Friend or foe: the role of micro‐RNA in chemotherapy resistance. Acta Pharmacol Sin 2013; 34:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jung EJ, Santarpia L, Kim J, et al. Plasma miR‐210 levels correlate with sensitivity to Trastuzumab and tumour presence in breast cancer patients. Cancer 2012; 118:2603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kovalchuk O, Filkowski J, Meservy J, et al. Involvement of micro‐RNA‐451 in resistance of the MCF‐7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther 2008; 7:2152–9. [DOI] [PubMed] [Google Scholar]
- 93. Ward A, Shukla K, Balwierz A, et al. Micro‐RNA‐519a is a novel oncomir conferring tamoxifen resistance by targeting a network of tumour‐suppressor genes in ER plus breast cancer. J Pathol 2014; 233:368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Anastasov N, Hofig I, Vasconcellos IG, et al. Radiation resistance due to high expression of miR‐21 and G2/M checkpoint arrest in breast cancer cells. Radiat Oncol 2012; 7:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pogribny IP, Filkowski JN, Tryndyak VP, et al. Alterations of micro‐RNAs and their targets are associated with acquired resistance of MCF‐7 breast cancer cells to cisplatin. Int J Cancer 2010; 127:1785–94. [DOI] [PubMed] [Google Scholar]
- 96. Liang Z, Li Y, Huang K, et al. Regulation of miR‐19 to breast cancer chemoresistance through targeting PTEN. Pharm Res 2011; 28:3091–100. [DOI] [PubMed] [Google Scholar]
- 97. Gong C, Yao Y, Wang Y, et al. Up‐regulation of miR‐21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem 2011; 286:19127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang Z, Lu BB, Wang H, et al. Micro‐RNA‐21modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch Med Res 2011; 42:281–90. [DOI] [PubMed] [Google Scholar]
- 99. Wang Z, Wang N, Liu P, et al. Micro‐RNA‐25 regulates chemoresistance‐associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014; 5:7013–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fang Y, Shen H, Cao Y, et al. Involvement of miR‐30c in resistance to doxorubicin by regulating YWHAZ in breast cancer cells. Braz J Med Biol Res 2014; 47:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bockhorn J, Dalton R, Nwachukwu C, et al. Micro‐RNA‐30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL‐11. Nat Commun 2013; 4:1393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kastl L, Brown I, Schofield AC. miRNA‐34a is associated with docetaxel resistance in human breast cancer cells. Breast Cancer Res Treat 2012; 131:445–54. [DOI] [PubMed] [Google Scholar]
- 103. Li XJ, Ren ZJ, Tang JH. Micro‐RNA‐34a: a potential therapeutic target in human cancer. Cell Death Dis 2014; 5:e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen GQ, Zhao ZW, Zhou HY, et al. Systematic analysis of micro‐RNA involved in resistance of the MCF‐7 human breast cancer cell to doxorubicin. Med Oncol 2010; 27:406–15. [DOI] [PubMed] [Google Scholar]
- 105. Wang HJ, Tan G, Dong L, et al. Circulating MiR‐125b as a Marker Predicting Chemoresistance in Breast Cancer. PLos One 2012; 7(4):e34210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhou M, Liu ZX, Zhao YH, et al. Micro‐RNA‐125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro‐apoptotic Bcl‐2 antagonist killer 1 (Bak1) expression. J Biol Chem 2010; 285:21496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhu X, Li Y, Shen H, et al. miR‐137 restoration sensitizes multidrug‐resistant MCF‐7/ADM cells to anticancer agents by targeting YB‐1. Acta Biochim Biophys Sin (Shanghai) 2013; 45:80–6. [DOI] [PubMed] [Google Scholar]
- 108. He DX, Gu XT, Li YR, et al. Methylation‐regulated miR‐149 modulates chemoresistance by targeting GlcNAc N‐deacetylase/N‐sulfotransferase‐1 in human breast cancer. Febs J 2014; 281:4718–30. [DOI] [PubMed] [Google Scholar]
- 109. Kong W, He LL, Coppola M, et al. Micro‐RNA‐155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem 2010; 285:17869–79. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110. Chen JQ, Tian W, Cai HK, et al. Down‐regulation of micro‐RNA‐200c is associated with drug resistance in human breast cancer. Med Oncol 2012; 29:2527–34. [DOI] [PubMed] [Google Scholar]
- 111. Kopp F, Oak PS, Wagner E, et al. miR‐200c Sensitizes Breast Cancer Cells to Doxorubicin Treatment by Decreasing TrkB and Bmi1 Expression. PLos One 2012; 7(11):e50469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhao RH, Wu JN, Jia WJ, et al. Plasma miR‐221 as a predictive biomarker for chemoresistance in breast cancer patients who previously received neoadjuvant chemotherapy. Onkologie 2011; 34:675–80. [DOI] [PubMed] [Google Scholar]
- 113. Bao LL, Hazari S, Mehra S, et al. Increased expression of P‐glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR‐298. Am J Pathol 2012; 180:2490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. He DX, Gu XT, Jiang L, et al. A methylation‐based regulatory network for micro‐RNA 320a in chemoresistant breast cancer. Mol Pharmacol 2014; 86:536–47. [DOI] [PubMed] [Google Scholar]
- 115. Jiang L, He DX, Yang DT, et al. MiR‐489 regulates chemoresistance in breast cancer via epithelial mesenchymal transition pathway. FEBS Lett 2014; 588:2009–15. [DOI] [PubMed] [Google Scholar]
- 116. Hu HY, Li SQ, Cui XY, et al. The overexpression of hypomethylated miR‐663 induces chemotherapy resistance in human breast cancer cells by targeting heparin sulfate proteoglycan 2 (HSPG2). J Biol Chem 2013; 288:10973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Palmieri C, Cleator S, Kilburn LS, et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER‐rich postmenopausal primary breast cancer. Breast Cancer Res Treat 2014; 148:581–90. [DOI] [PubMed] [Google Scholar]
- 118. Pan YZ, Morris ME, Yu AM., Micro‐RNA‐328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol 2009; 75:1374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Liang ZX, Wu H, Xia J, et al. Involvement of miR‐326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance‐associated protein 1. Biochem Pharmacol 2010; 79:817–24. [DOI] [PubMed] [Google Scholar]
- 120. Cui SY, Wang R, Chen LB. Micro‐RNAs: key players of taxane resistance and their therapeutic potential in human cancers. J Cell Mol Med 2013; 17:1207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Langer C, Rucker FG, Buske C, et al. Targeted therapies through micro‐RNAs: pulp or fiction? Ther Adv Hematol 2012; 3:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Si ML, Zhu S, Wu H, et al. miR‐21‐mediated tumor growth. Oncogene 2007; 26:2799–803. [DOI] [PubMed] [Google Scholar]
- 123. Mei M, Ren Y, Zhou X, et al. Downregulation of miR‐21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol Cancer Res Treat 2010; 9:77–86. [DOI] [PubMed] [Google Scholar]
- 124. Ru P, Steele R, Hsueh EC, et al. Anti‐miR‐203 upregulates SOCS3 expression in breast cancer cells and enhances cisplatin chemosensitivity. Genes Cancer 2011; 2:720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yu F, Deng H, Yao H, et al. Mir‐30 reduction maintains self‐renewal and inhibits apoptosis in breast tumor‐initiating cells. Oncogene 2010; 29:4194–204. [DOI] [PubMed] [Google Scholar]
- 126. Yu F, Yao H, Zhu P, et al. let‐7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007; 131:1109–23. [DOI] [PubMed] [Google Scholar]
- 127. Cochrane DR, Spoelstra NS, Howe EN, et al. Micro‐RNA‐200c mitigates invasiveness and restores sensitivity to microtubule‐targeting chemotherapeutic agents. Mol Cancer Therap 2009; 8:1055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 2012; 19:1508–16. [DOI] [PubMed] [Google Scholar]
- 129. Zampetaki A, Mayr M. Analytical challenges and technical limitations in assessing circulating miRNAs. Thromb Haemost 2012; 108:592–8. [DOI] [PubMed] [Google Scholar]
- 130. Nelson PT, Wang WX, Wilfred BR, et al. Technical variables in high‐throughput miRNA expression profiling: much work remains to be done. Biochim Biophys Acta 2008; 1779:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Koshiol J, Wang E, Zhao Y, et al. Strengths and limitations of laboratory procedures for micro‐RNA detection. Cancer Epidemiol Biomarkers Prev 2010; 19:907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sourvinou IS, Markou A, Lianidou ES., Quantification of circulating miRNAs in plasma: effect of preanalytical and analytical parameters on their isolation and stability. J Mol Diagn 2013; 15:827–34. [DOI] [PubMed] [Google Scholar]
- 133. Huang JT, Wang J, Srivastava V, et al. Micro‐RNA machinery genes as novel biomarkers for cancer. Front Oncol 2014; 4:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dedes KJ, Natrajan R, Lambros MB, et al. Down‐regulation of the miRNA master regulators Drosha and Dicer is associated with specific subgroups of breast cancer. Eur J Cancer 2011; 47:138–50. [DOI] [PubMed] [Google Scholar]
- 135. Avery‐Kiejda KA, Braye SG, Forbes JF, et al. The expression of Dicer and Drosha in matched normal tissues, tumours and lymph node metastases in triple negative breast cancer. BMC Cancer 2014; 14:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Leaderer D, Hoffman AE, Zheng T, et al. Genetic and epigenetic association studies suggest a role of micro‐RNA biogenesis gene exportin‐5 (XPO5) in breast tumorigenesis. Int J Mol Epidemiol Genet 2011; 2:9–18. [PMC free article] [PubMed] [Google Scholar]
- 137. Adams BD, Claffey KP, White BA. Argonaute‐2 expression is regulated by epidermal growth factor receptor and mitogen‐activated protein kinase signaling and correlates with a transformed phenotype in breast cancer cells. Endocrinology 2009; 150:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sung H, Jeon S, Lee KM, et al. Common genetic polymorphisms of micro‐RNA biogenesis pathway genes and breast cancer survival. BMC Cancer 2012; 12:195. [DOI] [PMC free article] [PubMed] [Google Scholar]


