Abstract
Background and Objective
Transcranial laser stimulation of the brain with near‐infrared light is a novel form of non‐invasive photobiomodulation or low‐level laser therapy (LLLT) that has shown therapeutic potential in a variety of neurological and psychological conditions. Understanding of its neurophysiological effects is essential for mechanistic study and treatment evaluation. This study investigated how transcranial laser stimulation influences cerebral hemodynamics and oxygenation in the human brain in vivo using functional near‐infrared spectroscopy (fNIRS).
Materials and Methods
Two separate experiments were conducted in which 1,064‐nm laser stimulation was administered at (1) the center and (2) the right side of the forehead, respectively. The laser emitted at a power of 3.4 W and in an area of 13.6 cm2, corresponding to 0.25 W/cm2 irradiance. Stimulation duration was 10 minutes. Nine healthy male and female human participants of any ethnic background, in an age range of 18–40 years old were included in each experiment.
Results
In both experiments, transcranial laser stimulation induced an increase of oxygenated hemoglobin concentration (Δ[HbO2]) and a decrease of deoxygenated hemoglobin concentration (Δ[Hb]) in both cerebral hemispheres. Improvements in cerebral oxygenation were indicated by a significant increase of differential hemoglobin concentration (Δ[HbD] = Δ[HbO2] − Δ[Hb]). These effects increased in a dose‐dependent manner over time during laser stimulation (10 minutes) and persisted after laser stimulation (6 minutes). The total hemoglobin concentration (Δ[HbT] = Δ[HbO2] + Δ[Hb]) remained nearly unchanged in most cases.
Conclusion
Near‐infrared laser stimulation applied to the forehead can transcranially improve cerebral oxygenation in healthy humans. Lasers Surg. Med. 48:343–349, 2016. © 2016 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: functional near‐infrared spectroscopy (fNIRS), low‐level laser therapy (LLLT), photobiomodulation, brain tissue oxygenation
INTRODUCTION
Photobiomodulation uses low‐power, high‐fluence light from lasers or LEDs in the red to near‐infrared range (620–1,100 nm) to modulate mitochondrial respiration in a nondestructive and non‐thermal manner 1, 2. Transcranial laser stimulation of the brain with near‐infrared light is a novel form of photobiomodulation, also known as low‐level light (laser) therapy (LLLT) when applied to patients 1, 2, 3. In recent years, transcranial laser stimulation has gained attention for its therapeutic potential in a variety of neurological and psychological conditions. It has been shown to be effective for treating ischemic stroke patients in a few controlled clinical trials 3, 4. Two studies by Naeser et al. reported that daily use of near‐infrared light to the forehead improved cognitive functions in patients with chronic traumatic brain injuries 5, 6. Schiffer et al. also found that a single near‐infrared light treatment to the forehead using LEDs could have psychological benefits in ten patients with major depression and anxiety 7. Stimulating with the same 0.25 W/cm2 irradiance as Schiffer et al. 7, but using a laser with a longer wavelength (1,064 nm), Barrett and Gonzalez‐Lima conducted the first controlled study in 40 healthy human participants and demonstrated that transcranial laser stimulation improves cognitive and emotional functions 8. A subsequent controlled study by Blanco et al. 9 also demonstrated that transcranial laser stimulation with 0.25 W/cm2 irradiance and 1,064‐nm laser improves executive functions in healthy human participants.
The mechanism of action of near‐infrared light rests on photon absorption by cytochrome oxidase 10, which is the terminal enzyme in the mitochondrial respiratory chain that plays a key role in cerebral oxygen utilization for energy metabolism 1, 2. The more the activity of cytochrome oxidase increases, the more oxygen consumption and metabolic energy is produced via mitochondrial oxidative phosphorylation 11. This photonics‐bioenergetics mechanism results in metabolic and hemodynamic alterations in the brain that facilitate both neuroprotection and cognitive enhancement 12, 13. In 2012, Rojas et al. 14 were the first to report that near‐infrared light increased oxygen consumption in the rat prefrontal cortex in vivo. However, most of the human studies have evaluated the effects of low‐level laser therapy by observing the changes in behavioral and psychological measures and postulating the underlying neurophysiological mechanism that causes them. To date, only the study by Schiffer et al. 7 has looked at the effects of near‐infrared LEDs on human cerebral hemodynamics by measuring the total hemoglobin changes with a cerebral oximeter.
Functional near‐infrared spectroscopy (fNIRS) 15, 16 is an emerging neuroimaging technology that measures the changes in cerebral hemodynamics and oxygenation related to neuronal activities. Because both fNIRS and transcranial laser stimulation use light in the near‐infrared range, they share similar optical pathways through the tissues. Thus, fNIRS is a suitable tool for in vivo mechanistic study of transcranial laser stimulation. Furthermore, both transcranial laser stimulation and fNIRS are safe, compact and easy to implement. A combination of these two non‐invasive, near‐infrared technologies can potentially provide an effective treatment‐with‐imaging approach for neurological and psychological applications.
The present study used fNIRS to quantitatively assess the neurophysiological effects of a single transcranial laser stimulation session on healthy human participants. The irradiance of the 1,064‐nm laser was 0.25 W/cm2, same as that used by Schiffer et al. 7, Barrett and Gonzalez‐Lima 8 and Blanco et al. 9. A portable fNIRS system was used to measure the changes in hemoglobin concentrations at the bilateral prefrontal cortices during and shortly after the laser stimulation.
MATERIALS AND METHODS
Participants
Healthy human participants of either sex, any ethnic background and in an age range of 18–40 years old were recruited from the local community of the University of Texas at Arlington. Interested individuals were screened by one of the investigators to determine whether they were eligible for the study. The exclusion criteria included: (i) being diagnosed with a psychiatric disorder, (ii) having history of a neurological condition, history of severe brain injury, history of violent behavior, (iii) ever being institutionalized/imprisoned, (iv) currently taking any medicine or currently pregnant. Eligible participants were scheduled for two separate experiments, which would be at least two weeks apart to reduce the chance of carryover effects from experiment I to experiment II. Informed consent was obtained prior to each experiment. The experimental protocol was approved by the University of Texas at Arlington's Institutional Review Board (IRB). All of the guidelines and regulations were followed during the experiments.
Instruments
Single‐session transcranial laser stimulation was administered with a continuous‐wave, 1,064‐nm laser (Model CG‐5000 Laser, Cell Gen Therapeutics LLC, Dallas, TX). This laser is FDA‐cleared for various uses on humans such as relief of muscle and joint pain. It has a hand‐held aperture with a button on the handle to open and shut the laser beam. The diameter of the aperture to deliver the laser beam is 4.16 cm. The laser was operated at a constant power of 3.4 W. Thus, the irradiance (or power density) in the 13.6‐cm2 beam area was 3.4 W/13.6 cm2 = 0.25 W/cm2, which was the same as that used in previous studies 8, 9. At this irradiance, the laser caused negligible heat and no physical damage. Exposure to the laser was not deemed harmful to the tissues.
A portable, continuous‐wave fNIRS system (CW‐2, TechEn Inc., Milford, MA) was used to measure the cerebral hemodynamic changes induced by the laser stimulation. This system has two pairs of lasers as light sources, each pair having 690 nm and 830 nm light, and four avalanche photo‐diodes (APDs) as detectors. The lasers are amplitude‐modulated at the kHz range to reduce optical interference from ambient light. The data sampling rate is 200 Hz. Low‐weight optical fibers were used to transmit the light between the instrument and the participants' heads. The four fibers from the light sources were merged into two source optodes for this study.
Experiments
Two experiments were conducted separately to study the cerebral hemodynamic responses to the laser stimulation, by following the same stimulation paradigm 8, 9. Laser stimulation was applied to the center of the forehead, aimed at the medial frontal lobes bilaterally (Fig. 1A) in the first experiment. Laser stimulation was applied to the right side of the forehead, aimed at the right lateral frontal lobe (Fig. 1B) in the second experiment, as previously done to improve prefrontal functions 8. In each experiment, an fNIRS probe was placed bilaterally and symmetrically on the participants' foreheads (Figs. 1A and B) and was held in place with Velcro strips. The probe consisted of two source optodes and two detector optodes, which provided two measurement channels, one channel per cerebral hemisphere (Figs. 1A and B). The source‐detector separation was 3.5 cm for each channel. After the probe was in place, the participants were instructed to sit stably on a chair. After 1‐minute baseline readings of fNIRS were taken, the transcranial laser stimulation was administered. The stimulation session was divided into 10 one‐minute cycles, 55‐second stimulation laser on and 5‐second stimulation laser off per cycle (Fig. 2). Hence radiant exposure (or energy density) was 0.25 W/cm2 × 55 sec = 13.75 J/cm2 per cycle of stimulation. During each cycle, an experimenter held the CG‐5000 aperture closely towards the participants' foreheads and pressed the button to shine the laser beam. Then a timer of 55 seconds on the frontal panel of the CG‐5000 apparatus started to count down and the laser beam was automatically shut at the end. A beep was also given by the apparatus when the laser beam was shut. Then the experimenter waited for 5 seconds before starting the next cycle. After all of the 10 stimulation cycles were completed, the participants were asked to keep still for another 6 minutes while the fNIRS readings were continuously taken. Thus, the total data acquisition time of fNIRS was 17 minutes by including the 1‐minute baseline, 10‐minute transcranial laser stimulation, and 6‐minute recovery (Fig. 2).
Figure 1.
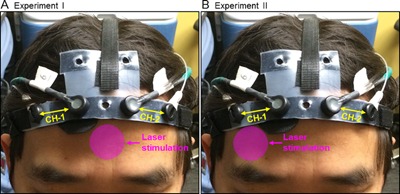
Positions of the transcranial laser stimulation and configuration of the functional near‐infrared spectroscopy (fNIRS) probe in (A) Experiment I, and (B) Experiment II. In each graph, the pink circle indicates the approximate position where the treatment laser (in 4.16‐cm diameter) was shined on. The fNIRS probe consisted of two measurement channels (CH‐1 and CH‐2), one channel over each cerebral hemisphere.
Figure 2.
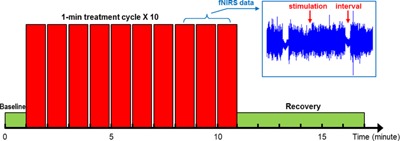
Schematic diagram of the transcranial laser stimulation and functional near‐infrared spectroscopy (fNIRS) data acquisition paradigm. The laser stimulation session was divided into 10 one‐minute cycles, 55‐second stimulation laser on and 5‐second stimulation laser off per cycle. The fNIRS data acquisition included 1‐minute baseline, 10‐minute laser stimulation, and 6‐minute recovery.
Both experiments were conducted in a locked room with no reflective surfaces. A warning sign was hung on the outer door, indicating that the laser was in use. During the experiments, 1,064‐nm protective eyewear was worn by all individuals present in the room. In addition, the participants were instructed to keep their eyes closed during laser stimulation.
Data Processing
All of the fNIRS data were processed in MATLAB. The raw data from each participant was first visually inspected for significant discontinuities and interference. Because the stimulation laser from the CG‐5000 had a much higher power (=3.4 W) than the light sources in CW‐2 (∼10 mW), the data acquired during the 55‐second stimulation periods suffered from significant light interference (see the subgraph of Fig. 2) and had to be discarded. Alternatively, the data during each 5‐second intercycle interval was selected and averaged to represent the corresponding stimulation cycle. The data during the initial baseline and post‐stimulation recovery were also averaged at a step of 1 minute. In this way, a total of 17 averaged data points were derived from the raw data of each participant. These data points were then used to calculate the changes of oxygenated hemoglobin concentration (Δ[HbO2]) and deoxygenated hemoglobin concentration (Δ[Hb]) relative to the initial baseline based on the modified Beer–Lambert Law 17. In this step, the differential pathlength factor (DPF) was 6.8 at 690 nm and 5.8 at 830 nm 18. Furthermore, changes in total hemoglobin concentration (Δ[HbT] = Δ[HbO2] + Δ[Hb]) and differential hemoglobin concentration (Δ[HbD] = Δ[HbO2] − Δ[Hb]) were also calculated. While Δ[HbT] represents the changes of total blood volume in the tissues, Δ[HbD] is an indicator of changes in tissue oxygen saturation (StO2). In particular, Δ[HbD] is proportional to ΔStO2 when total hemoglobin concentration maintains constant, that is, Δ[HbD] = 2[HbT]constant · ΔStO2.
Statistical Analysis
For each experiment, the statistical analysis of results was conducted in two ways. First, one‐sample t‐tests were used to examine the significance of hemodynamic changes from each channel. The results from these tests would show if the single‐session transcranial laser stimulation induced any type of cerebral hemodynamic change as compared to the baseline (zero). Second, paired t‐tests were used to examine the significance of differences between two measurement channels. The results from these tests would show if there was any regional difference (right vs. left hemisphere) in laser‐stimulated cerebral hemodynamic changes. A significance level of P < 0.01 was applied in both analyses.
RESULTS
Nine participants (five males and four females, age = 24.7 ± 5.3 years) were successfully measured in the first experiment. Nine participants (five males and four females, age = 24.6 ± 5.6 years) were successfully measured in the second experiment; six of them had participated in the first experiment previously. For those who participated in both experiments, the two experiments were spaced at least two weeks apart. There was no significant difference in gender and age between the two groups of participants.
In both experiments, transcranial laser stimulation induced an increase of oxygenated hemoglobin concentration and a decrease of deoxygenated hemoglobin concentration from the baseline in both cerebral hemispheres (Figs. 3 and 4). The total hemoglobin concentration remained nearly unchanged in most cases (Figs. 3 and 4). The differential hemoglobin concentration demonstrated a robust increase associated with the laser stimulation (Figs. 3 and 4), which was statistically significant in all the cases (Table 1). Notably, all of the hemodynamic changes were sustained during the 6‐minute recovery, indicating the effects of transcranial laser stimulation persisted even after the laser was turned off.
Figure 3.
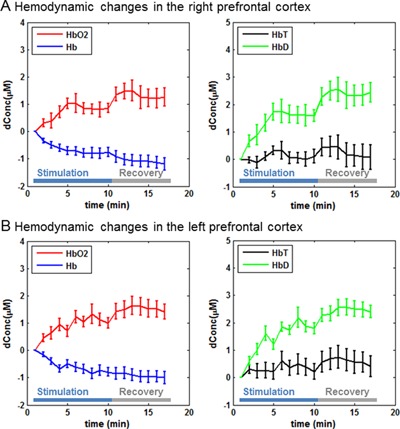
Prefrontal hemodynamic responses (mean ± SE) in the first experiment: (A) hemodynamic changes in the right prefrontal cortex and (B) hemodynamic changes in the left prefrontal cortex.
Figure 4.
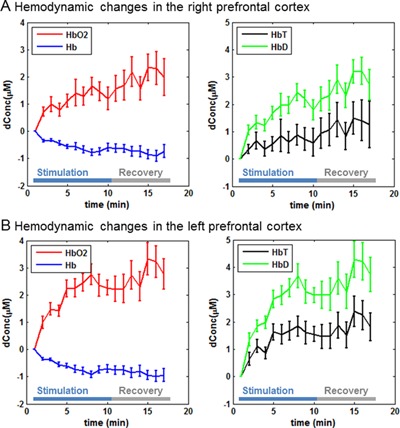
Prefrontal hemodynamic responses (mean ± SE) in the second experiment: (A) hemodynamic changes in the right prefrontal cortex and (B) hemodynamic changes in the left prefrontal cortex.
Table 1.
Cerebral Hemodynamic Changes Induced by Transcranial Laser Stimulation at the Group Level (Mean ± SD)
| Experiment I (N = 9) | Experiment II (N = 9) | |||
|---|---|---|---|---|
| CH‐1 | CH‐2 | CH‐1 | CH‐2 | |
| HbO2 | 1.01 ± 0.97 | 1.19 ± 0.82 * | 1.52 ± 1.63 | 2.32 ± 1.61 * |
| Hb | −0.84 ± 0.73 * | −0.74 ± 0.52 * | −0.64 ± 0.56 | −0.74 ± 0.42 * |
| HbT | 0.16 ± 1.12 | 0.45 ± 1.25 | 0.88 ± 1.99 | 1.58 ± 1.56 |
| HbD | 1.85 ± 1.30 * | 1.94 ± 0.58 * | 2.16 ± 1.41 * | 3.06 ± 1.76 * |
Indicate statistically non‐zero changes at the group level (one‐sample t‐test, P < 0.01). For each participant and hemoglobin species, the data were averaged across all of the 16 non‐baseline time points.
In both experiments, the right and left prefrontal cortices showed approximately the same amplitudes in hemodynamic changes. Particularly for the second experiment, although the laser stimulation was administered on the right side, the hemodynamic changes induced by transcranial laser stimulation did not show any significant difference between the right and left hemispheres.
DISCUSSION AND CONCLUSION
Previous human studies have reported that near‐infrared light benefited the neuropsychological status of patients with stroke 3, 4, depression 7, and brain trauma 4, 10. In particular, placebo‐controlled studies of transcranial laser stimulation, with the same laser device and stimulation parameters as those used in this study, improved cognitive and executive brain functions in healthy human participants 8, 9. Despite these promising case studies and clinical trials, however, there was still a lack of understanding of the neurophysiological mechanism in vivo of transcranial laser stimulation. In this study, we aimed to determine if transcranial laser stimulation alters the cerebral hemodynamic status in healthy human adults. We used fNIRS as a measurement tool because it has similar optical pathways through the tissues to transcranial laser stimulation. Four variables, namely the changes in oxygenated, deoxygenated, total and differential hemoglobin concentrations, were quantified to comprehensively describe the cerebral hemodynamics changes during and shortly after a single transcranial laser stimulation session that was aimed at two different locations of the frontal lobe.
Our results showed that the transcranial laser stimulation induced reliable hemodynamic changes in the bilateral prefrontal cortices. We observed a slight and non‐significant increase of total hemoglobin concentration. This observation is consistent with a previous study that used the same irradiance of stimulation light 7. We also observed an increase of oxygenated hemoglobin concentration and a decrease of deoxygenated hemoglobin concentration, which together led to a significant increase of differential hemoglobin concentration in all the cases (two stimulation locations × two cerebral hemispheres). Because the total hemoglobin concentration was relatively unchanged in general, we believe the significant increase of differential hemoglobin concentration indicates that transcranial laser stimulation elevated the tissue oxygenation saturation in the bilateral prefrontal cortices. This conclusion is consistent with a previous animal study showing a direct increase in oxygen consumption measured invasively with an oxygen probe inside the rat prefrontal cortex during near‐infrared light stimulation 14. This light‐stimulated oxygen consumption also led to upregulation of cytochrome oxidase activity in the rat prefrontal cortex and improved prefrontal cortex‐based memory function 14.
Furthermore, a few studies on animal models 19 and human infants 20, 21 have shown that the differential hemoglobin concentration correlated tightly with the change in regional cerebral blood flow (rCBF). Since a significant increase of differential hemoglobin concentration was consistently observed in this study, it is very likely that the single‐session transcranial laser stimulation caused an increase of rCBF to the bilateral prefrontal cortices. In the future this conjecture needs to be verified using techniques that measure rCBF directly, such as diffuse correlation spectroscopy (DCS) 22.
Previous fNIRS studies of human brain functions have demonstrated that neuronal activations can induce an increase of oxygenated hemoglobin concentration and a decrease of deoxygenated hemoglobin concentration 23, 24, 25 through neurovascular coupling 26, 27, which describes the tight relationship between local neuronal activations and subsequent changes in rCBF to supply oxygen and nutrients to meet energy demand. Therefore the similar increase of oxygenated hemoglobin concentration and decrease of deoxygenated hemoglobin concentration observed in our results may suggest that this single transcranial laser stimulation session improves neuronal activities in the bilateral prefrontal cortices as well. This scenario agrees with two previous studies 8, 9 that reported the same laser irradiance to the forehead was able to improve prefrontal cortex‐based cognitive and executive functions among healthy human participants.
In both experiments, the right and left prefrontal cortices showed approximately the same amplitudes in hemodynamic changes. Particularly for the second experiment, although the laser stimulation was administered on the right side, the evoked hemodynamic changes did not show any significant difference between the two sides. A possible interpretation of this phenomenon relies on the fact that interhemispheric cortical regions are functionally connected by commissural axons in the corpus callosum 28, 29, 30. Therefore, laser‐stimulated neuronal activations in one side of the prefrontal cortices could automatically trigger the neuronal activities on the other side, which subsequently resulted in the same amplitudes of hemodynamic changes on both sides.
Another important matter for evaluating whether transcranial laser stimulation may serve as an effective neuro‐enhancement or clinical treatment is the duration of its effects. If the effects of transcranial laser stimulation were short‐lasting, it would prove its limited use. In this study we used continuous‐wave fNIRS to measure the relative changes of cerebral hemoglobin concentrations from an initial baseline; the participants were instructed to keep their bodies stable during the measurements to ensure the fNIRS data were reliable. For this reason we recorded for only a 6‐minute recovery following the treatment as it would be difficult for the participants to hold on for longer time. The results showed that the effects of the treatment were persistent 6 minutes after the laser was turned off, much longer than the hemodynamic effects of transcranial magnetic stimulation that lasted for about only 1 minute according to our previous study 31. This may be because while the transcranial electrical or magnetic interventions alter the excitability of neurons directly, the action of transcranial laser stimulation may rest on photobiomodulation of intracellular metabolism and hemodynamic processes that increase brain tissue oxygenation. These processes are slower than the excitability of neurons and linked to metabolic cascades, making the effects of transcranial laser stimulation sustained even after the laser was off.
Due to the limitation in continuous‐wave fNIRS, the long‐term duration of effects of transcranial laser stimulation remains unknown. Some previous studies have suggested the benefits could last for several weeks. For example, Barrett and Gonzalez‐Lima 8 found a significant benefit as compared to the placebo group in positive and negative affective states in healthy volunteers two weeks after a single 8‐minute laser stimulation as described here. Schiffer et al. 7 reported psychological benefits at 2 and 4 weeks after a single treatment in patients with anxiety and depression. Light power density (0.25 W/cm2) and energy density (60 J/cm2) used in these two studies were the same, but Schiffer et al. 7 used 810‐nm LEDs instead of 1,064‐nm laser. Naeser et al. 6 used similar LEDs in patients with mild traumatic brain injury for 18 treatments (three treatments per week for 6 weeks), and measured cognitive performance after one week, and 1 and 2 months after the 18th treatment. They found a significant linear trend for the effect of LED treatment over time for various cognitive tests. While these pioneering studies are promising, there are no placebo‐controlled human studies investigating long‐term neuronal or cognitive effects after single or repeated transcranial laser treatments.
In conclusion, near‐infrared laser stimulation applied to the forehead can transcranially improve cerebral oxygenation in healthy humans.
ACKNOWLEDGMENTS
The authors thank Douglas W. Barrett, PhD, Ginikachi C. Ojinnaka, MS, Ashima D. Sherekar, MS, and Mr. Eduardo Velasco for their assistance in human participant recruitment and data collection. FGL gratefully acknowledges support from an institutional research fellowship from the College of Liberal Arts of the University of Texas at Austin. FGL holds the George I. Sanchez Centennial Endowed Professorship in Liberal Arts and Sciences.
Conflict of Interest Disclosures: Dr. Gonzalez‐Lima reported receiving support from an institutional research fellowship from the College of Liberal Arts of the University of Texas at Austin. Dr. Gonzalez‐Lima holds the George I. Sanchez Centennial Endowed Professorship in Liberal Arts and Sciences. Dr. Tian, M.S. Hase, and Dr. Liu had no disclosures to report.
REFERENCES
- 1. Eells JT, Wong‐Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near‐infrared light therapy. Mitochondrion 2004; 4:559–567. [DOI] [PubMed] [Google Scholar]
- 2. Wong‐Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem 2005; 280:4761–4771. [DOI] [PubMed] [Google Scholar]
- 3. Lampl Y, Zivin J A, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial‐1 (NEST‐1). Stroke 2007; 38:1843–1849. [DOI] [PubMed] [Google Scholar]
- 4. Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J, for the NEST‐2 Investigators. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke 2009; 40:1359–1364. [DOI] [PubMed] [Google Scholar]
- 5. Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light‐emitting diode treatments in chronic, traumatic brain injury: Two case reports. Photomed Laser Surg 2011; 29:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, Knight JA, Meehan WP 3rd, Baker EH. Significant improvements in cognitive performance post‐transcranial, red/near‐infrared light‐emitting diode treatments in chronic, mild traumatic brain injury: Open‐protocol study. J Neurotrauma 2014; 31:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: A pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 2009; 5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barrett DW, Gonzalez‐Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013; 230:13–23. [DOI] [PubMed] [Google Scholar]
- 9. Blanco NJ, Maddox WT, Gonzalez‐Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol 2015. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pastore D, Greco M, Passarella S. Specific helium‐neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol 2000; 76:863–870. [DOI] [PubMed] [Google Scholar]
- 11. Rojas JC, Gonzalez‐Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol 2013; 86:447–457. [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez‐Lima F, Barrett DW. Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci 2014; 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez‐Lima F, Auchter A. Protection against neurodegeneration with low‐dose methylene blue and near‐infrared light. Front Cell Neurosci 2015; 9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rojas JC, Bruchey AK, Gonzalez‐Lima F. Low‐level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis 2012; 32:741–752. [DOI] [PubMed] [Google Scholar]
- 15. Hoshi Y. Functional near‐infrared spectroscopy: current status and future prospects. J Biomed Opt 2007; 12:062106. [DOI] [PubMed] [Google Scholar]
- 16. Ferrari M, Quaresima V. A brief review on the history of human functional near‐infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012; 63:921–935. [DOI] [PubMed] [Google Scholar]
- 17. Cope M, Delpy DT, Reynolds EO, Wray S, Wyatt J, van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Adv Exp Med Biol 1988; 222:183–189. [DOI] [PubMed] [Google Scholar]
- 18. Essenpreis M, Elwell CE, Cope M, van der Zee P, Arridge SR, Delpy DT. Spectral dependence of temporal point spread functions in human tissues. Appl Opt 1993; 32:418–425. [DOI] [PubMed] [Google Scholar]
- 19. Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res 1998; 44:591–595. [DOI] [PubMed] [Google Scholar]
- 20. Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, Volpe JJ. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 2000; 106:625–632. [DOI] [PubMed] [Google Scholar]
- 21. Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, du Plessis AJ. Fluctuating pressure‐passivity is common in the cerebral circulation of sick premature infants. Pediatr Res 2007; 61:467–473. [DOI] [PubMed] [Google Scholar]
- 22. Durduran T, Yodh AG. Diffuse correlation spectroscopy for non‐invasive, micro‐vascular cerebral blood flow measurement. Neuroimage 2014; 85:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage 2006; 29:368–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hassanpour MS, White BR, Eggebrecht AT, Ferradal SL, Snyder AZ, Culver JP. Statistical analysis of high density diffuse optical tomography. Neuroimage 2014; 85:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tian F, Delgado MR, Dhamne SC, Khan B, Alexandrakis G, Romero MI, Smith L, Reid D, Clegg NJ, Liu H. Quantification of functional near infrared spectroscopy to assess cortical reorganization in children with cerebral palsy. Opt Express 2010; 18:25973–25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006; 100:328–335. [DOI] [PubMed] [Google Scholar]
- 27. Ou W, Nissila I, Radhakrishnan H, Boas DA, Hamalainen MS, Franceschini MA. Study of neurovascular coupling in humans via simultaneous magnetoencephalography and diffuse optical imaging acquisition. Neuroimage 2009; 46:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sporns O, Tononi G, Kotter R. The human connectome: A structural description of the human brain. PLoS Comput Biol 2005; 1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sporns O. The human connectome: A complex network. Ann N Y Acad Sci 2011; 1224:109–125. [DOI] [PubMed] [Google Scholar]
- 30. Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, SeidlerRD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci USA 2010; 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kozel FA, Tian F, Dhamne S, Croarkin PE, McClintock SM, Elliott A, Mapes KS, Husain MM, Liu H. Using simultaneous repetitive Transcranial Magnetic Stimulation/functional Near Infrared Spectroscopy (rTMS/fNIRS) to measure brain activation and connectivity. Neuroimage 2009; 47:1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]


