Abstract
Background
PRE2DUP is a modeling method that generates drug use periods (ie, when drug use started and ended) from drug purchases recorded in dispensing-based register data. It is based on the evaluation of personal drug purchasing patterns and considers hospital stays, possible stockpiling of drugs, and package information.
Objective
The objective of this study was to investigate person-level agreement between self-reported drug use in the interview and drug use modeled from dispensing data with PRE2DUP method for various drug classes used by older persons.
Methods
Self-reported drug use was assessed from the GeMS Study including a random sample of persons aged ≥75 years from the city of Kuopio, Finland, in 2006. Drug purchases recorded in the Prescription register data of these persons were modeled to determine drug use periods with PRE2DUP modeling method. Agreement between self-reported drug use on the interview date and drug use calculated from register-based data was compared in order to find the frequently used drugs and drug classes, which was evaluated by Cohen’s kappa. Kappa values 0.61–0.80 were considered to represent good and 0.81–1.00 as very good agreement.
Results
Among 569 participants with mean age of 82 years, the agreement between interview and register data was very good for 75% and very good or good for 93% of the studied drugs or drug classes. Good or very good agreement was observed for drugs that are typically used on regular bases, whereas “as needed” drugs represented poorer results.
Conclusion
PRE2DUP modeling method validly describes regular drug use among older persons. For most of drug classes investigated, PRE2DUP-modeled register data described drug use as well as interview-based data which are more time-consuming to collect. Further studies should be conducted by comparing it with other methods and in different drug user populations.
Keywords: Prescription register, pharmacoepidemiology, drug utilization, validation studies
Introduction
Register-based data on drug use are increasingly utilized in various research questions.1 Interview-based drug use assessment is time-consuming and collection of such data may lead to a significant cost. Register-based drug data provide an alternative source of drug use information. Register-based purchase data are extensive and provide large and representative samples of the population. Drug utilization and associated beneficial and adverse outcomes may be accurately deduced from register-based data sources. On the contrary, interview-based drug exposure represents assessment of current drug use, and it cannot consider changes during long time periods. In addition, interview data are dependent on the actual way that drug use is questioned and is subject to recall bias.
Validity of Finnish Prescription register to describe psychotropic drug use has been previously demonstrated.2 Rikala et al compared self-reported drug use information collected in annual interviews to drugs purchased by the participants according to Prescription register data in Finland. They assessed which time windows captured self-reported drug use in the register data correctly. This validation study showed that register-based data are a valid source for psychotropic drug use among older persons and that reimbursement status of drugs had an impact on the sensitivity and specificity measures. Similar results were also reported by Haukka et al3 on psychotropic drug use by persons with schizophrenia.
Previous studies have determined the agreement between pharmacy records and drug use reported in the interview or home inventory of drugs to be mostly moderate or good.2,4–8 The comprehensiveness of the interview varied from survey to patient interview and to home drug inventory between the studies. Methods used for dispensing data varied between time windows (30, 60, or 90 days, or 4, 6, or 12 months), legend duration method assuming that duration for dispensing was the purchased amount divided by one defined daily dose per day (with or without any allowance for lower than perfect adherence) or divided by the prescribed dose and dispensing during the past 6 months for at least three times and at least once in the past month.
In this study, PRE2DUP method that models drug use longitudinally and assigns times when drug was used and when it was not used according to the regularity of drug purchases in pharmacy records was applied.9 PRE2DUP is a data-driven method that generates drug use periods from drug purchases recorded in register-based data. The method evaluates personal drug purchasing patterns in order to calculate an estimate of how long each drug purchase will last with the local estimated dosage and regularity of purchases. It considers hospital stays, possible stockpiling of drugs, and package information designed to control joining of the purchases. The method utilizes drug use pattern of the drug and package in the study population to determine the duration for single purchases. It uses recursive recalculation of the model and can be guided to more accurate results by adjusting drug-specific parameters. Validity of the PRE2DUP method was previously investigated by expert opinion-based evaluation on whether drug purchases recorded in the dispensing data were correctly placed in drug use periods and which purchases formed a continuous drug use period. The correctness of purchases included in the drug use period defined whether the duration of use was correctly estimated.
The objective of our study was to validate PRE2DUP method against interview-reported drug use data for various drug classes used by older persons. The agreement between interview and PRE2DUP-modeled data was tested in person level and considering discrepancies in both the data sources.
Materials and methods
The Geriatric Multidisciplinary Strategy for the Good Care of the Elderly (GeMS) Study was a randomized comparative study among persons aged ≥75 years.10–13 The study evaluated a model for geriatric assessment, care, and rehabilitation. A random sample of inhabitants of the city of Kuopio, Finland, were invited to participate and were randomized to intervention (N=500) and comparison (N=500) groups. Originally of these 1000 people, 781 provided written informed consent to participate, 162 refused participation, 2 relocated, and 55 died before the baseline examination. Altogether, 700 community-dwelling participants were included in the study at the baseline. The baseline examination was conducted in 2004, and annual examinations were conducted until 2007. The study was approved by the Research Ethics Committee of the Northern Savo Hospital District, Kuopio, Finland.
All the participants attended an interview in which their drug use, together with health status, and sociodemographic factors were thoroughly assessed by a trained nurse. Participants in both the groups continued to receive usual care during the study period. Participants’ self-reported drug use and diagnoses were verified from medical records from municipal health centers, home nursing service, local hospitals, and the Kuopio University Hospital. Drug use was thoroughly assessed, and the participants were also asked to bring their prescription forms and drug packages to the interview. If a participant had a prescription form, drug package, or medical record that suggested they took a drug that they did not self-report, then the nurse interviewer specifically asked about the use of this drug over the previous 2 weeks. Drug use was assessed on the basis of each participant’s actual pattern of use rather than the clinician’s prescribed or intended pattern of use. Drug use was also categorized as regular or as required use and was categorized according to Anatomical Therapeutic Chemical (ATC) coding system.14 Regularity of drug use was determined for each participant and drug according to their self-reported drug use by asking whether the drug is used regularly or on “as needed” basis and how often “as needed” drugs were actually used during the previous 2 weeks.
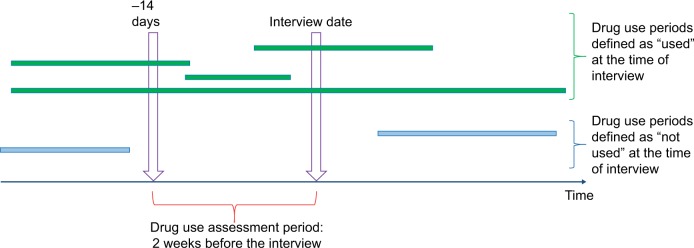
Data on drug purchases for the GeMS Study participants 2002–2007 were retrieved from Prescription register, which includes all reimbursed and prescribed drugs dispensed from pharmacies. Prescription register data are restricted to community-dwelling persons as drugs used in long-term care facilities are provided by the institution. When drug use started and ended, drug use periods were modeled from drug purchases with a PRE2DUP method.9 The modeling is based on sliding averages of daily dose (in defined daily doses)15 and was conducted separately with each ATC code for each person. The modeling aims to describe the actual pattern of use based on purchase regularity and considers hospitalizations, stockpiling of drugs, and changing dose. The method is described in more detail and drug use periods produced by PRE2DUP are validated against expert opinion by Tanskanen et al.9 As drug use in the interview was assessed as drugs used during previous 2 weeks, which drug use periods produced by PRE2DUP were ongoing during a 2-week period preceding the interview date (Figure 1) was assessed. Drug use periods might be ongoing the whole period or a part of it.
Figure 1.
Comparison of whether PRE2DUP modeled drug use periods were ongoing during the drug use assessment period which was 2 weeks preceding the interview date for each participant.
Note: Green bars indicate drug use periods defined as “used” at the interview and blue bars as “not used.”
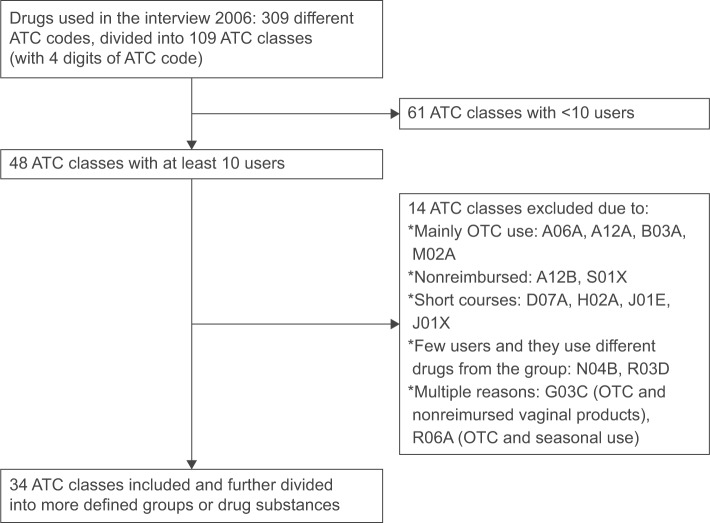
We compared self-reported and register-based data in several commonly used drugs or drug classes among the study participants. Drugs were also chosen on the basis of reimbursement status during the study period as our objective was to assess the validity of drug use periods produced by PRE2DUP and not the coverage of the Prescription register. Hence, whether drug use periods modeled with PRE2DUP were ongoing at the time of the interview for drugs that were reimbursed in 2006 were compared. Validation year 2006 was chosen as fixed co-payment was removed from reimbursement regulations at the beginning of 2006, and thus, register data from earlier years are less valid for inexpensive drugs. Drug classes with at least 10 users (reported in interview) were included; however drugs used mainly over the counter (OTC; antihistamines, laxatives, and vitamins) and on short courses (antibiotics) were excluded. Figure 2 describes the exclusion process for the selection of drugs or drug groups included in this study.
Figure 2.
Formation of the included drug groups (ATC classes) in the study.
Notes: A06A, drugs for constipation; A12A, calcium; B03A, iron preparations; M02A, topical products for joint and muscular pain; A12B, potassium; S01X, other ophthalmologicals; D07A, dermal corticosteroids; H02A, corticosteroids for systemic use; J01E, sulfonamides and trimethoprim; J01X, other antibacterials; N04B, dopaminergic agents; R03D, other systemic drugs for obstructive airway diseases; G03C, estrogens; R06A, antihistamines for systemic use.
Abbreviations: ATC, Anatomical Therapeutic Chemical; OTC, over the counter.
Benzodiazepines and related drugs (BZDRs) were defined to include both benzodiazepines (BZDs, ATC codes N05BA and N05CD) and benzodiazepine-related, so-called Z-drugs (ATC code N05CF). Triazolam and midazolam were excluded from BZDs because of their lack of reimbursement status. Codeine combinations (N02AA59) from opioids and acetylsalisylic acid (B01AC06) from antithrombotics were also excluded because they were not reimbursed in 2006. Glucosamine (M01AX05) was excluded from nonsteroidal anti-inflammatory drugs (NSAIDs).
In this study, community-dwelling persons were included and were interviewed in 2006, that is, alive and participated (loss of follow-up for the GeMS Study has been described elsewhere).11 Of 588 community-dwelling persons, 19 were excluded as their drug purchases were not recorded in the Prescription register data (indicative of living in long-term care facility providing drugs). After these exclusions, our study sample consisted of 569 persons.
Participants’ self-reported diagnoses were complemented with data obtained from the Finnish Special Reimbursement Registers, including cardiovascular diseases, diabetes, and history of stroke. Functional comorbidity index was calculated according to Groll et al16 and as in a previous study.12 Dementia was diagnosed as Alzheimer’s disease, vascular dementia or dementia due to other general medical conditions according to the Diagnostic and Statistical Manual for Mental Disorders (DSM)-IV criteria, and dementia with Lewy bodies according to the core criteria published by McKeith et al.17 Performance in Instrumental Activities of Daily Living was assessed using the eight-item scale developed by Lawton and Brody18 and was categorized as impaired Instrumental Activities of Daily Living (score 0–6) and normal function (score 7–8). Frailty was defined as frail, pre-frail, and robust as previously described.19 Mini-Mental State Examination20 was conducted by a trained nurse, and scores <25 were categorized as indicative of cognitive impairment.
Statistical analyses
Which drug use periods modeled by PRE2DUP method were ongoing during a 2-week period preceding the interview date were assessed. Agreement was tested in person level and in two different ways: 1) with self-reported drug use reported in the interview as a standard, which drugs were used by the participants according to register-based modeling were assessed, and 2) with drug use periods as a standard, whether drugs used according to register-based data were reported in the interview was assessed. The two-way agreement was tested because previous studies have indicated that also older persons under-report their drug use in interviews.8 This was conducted separately for all regular and “as needed” drugs together and separately for only regularly used drugs according to interview. Cohen’s kappa statistics were calculated by considering the differences in both ways. Interpretation of the kappa value was as previously suggested: poor (<0.20), fair (0.20–0.40), moderate (0.41–0.60), good (0.61–0.80), and very good (0.81–1.00).21
Results
Participants included in the study in 2006 were with a mean age of 82 years and 69% were women (Table 1). About half of the study population was pre-frail and 15% were frail, whereas dementia was diagnosed in 17% of the participants. The participants frequently used drugs and almost one-third of them used 10 or more drugs.
Table 1.
Characteristics of study participants (N=569) in 2006 interview
| Age, mean (SD) (years) | 82.4 (4.3) |
|---|---|
| Females, % (N) | 69% (391) |
| Intervention group, % (N) | 52% (295) |
| Functional status | |
| Frailty status, % (N)a | |
| Robust | 36% (195) |
| Pre-frail | 49% (263) |
| Frail | 15% (81) |
| IADL score, mean (SD) | 6.0 (2.3) |
| MMSE score, mean (SD) | 25.7 (5.4) |
| Comorbidities | |
| FCI, mean (SD) | 2.9 (1.7) |
| Cardiovascular disease, % (N) | 87% (492) |
| Diabetes, % (N) | 20% (115) |
| History of stroke, % (N) | 15% (85) |
| Dementia | 17% (274) |
| Drug use | |
| Mean number of regular drugs (SD) | 5.8 (3.0) |
| Excessive polypharmacy (≥10 drugs), % (N) | 28% (161) |
Note:
Missing data for 30 persons.
Abbreviations: FCI, functional comorbidity index; IADL, instrumental activities of daily living; MMSE, Mini-Mental State Examination; SD, standard deviation.
Agreement between interview and register data modeled with PRE2DUP was very good for 75% and very good or good for 93% of the studied drugs or drug classes (Table 2). Agreement was moderate for NSAIDs and poor for paracetamol.
Table 2.
Person-level agreement between self-report in interview and drug use calculated from prescription register data with PRE2DUP modeling method in the GeMS Study
| Drug class or drug name and ATC code | Interview as a standard
|
Register as standard
|
Kappa (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Number of users based on interview | Number of users in both | Proportion found in register data | Number of users based on register | Proportion found in the interview | |||
| Alimentary tract and metabolism | |||||||
| Proton pump inhibitors, A02BC | 50 | 40 | 80% | 50 | 80% | 0.78 (0.69–0.87) | |
| Antidiabetics, A10 | 63 | 61 | 97% | 61 | 100% | 0.98 (0.96–1.00) | |
| Insulins, A10A | 18 | 18 | 100% | 18 | 100% | 1.00 (1.00–1.00) | |
| Oral antidiabetics, A10B | 55 | 52 | 95% | 52 | 100% | 0.97 (0.93–1.00) | |
| Blood and blood forming organs | |||||||
| Antithrombotic drugs, B01A | 154 | 143 | 93% | 149 | 96% | 0.92 (0.89–0.96) | |
| Cardiovascular system | |||||||
| Digitalis, C01A | 37 | 35 | 95% | 38 | 92% | 0.93 (0.87–0.99) | |
| Organic nitrates, C01DA | 240 | 195 | 81% | 199 | 98% | 0.82 (0.77–0.87) | |
| Diuretics, C03 | 196 | 182 | 93% | 196 | 93% | 0.89 (0.85–0.93) | |
| Beta-blocking agents, C07 | 353 | 342 | 97% | 350 | 98% | 0.93 (0.90–0.96) | |
| Calcium channel blockers, C08 | 164 | 153 | 93% | 169 | 91% | 0.89 (0.84–0.93) | |
| Agents acting on the renin- angiotensin system, C09 | 227 | 223 | 98% | 240 | 93% | 0.92 (0.89–0.96) | |
| Lipid-modifying agents, C10 | 214 | 204 | 95% | 208 | 98% | 0.95 (0.92–0.98) | |
| Genitourinary system and sex hormones | |||||||
| Drugs for urinary incontinence, G04BD | 20 | 15 | 75% | 17 | 88% | 0.81 (0.66–0.95) | |
| Drugs for benign prostatic hypertrophy, G04C | 47 | 46 | 98% | 50 | 92% | 0.94 (0.90–0.99) | |
| Systemic hormonal preparations | |||||||
| Levothyroxine, H03AA01 | 60 | 56 | 93% | 56 | 100% | 0.96 (0.92–1.00) | |
| Musculoskeletal system | |||||||
| NSAIDs, M01A | 95 | 40 | 42% | 64 | 63% | 0.43 (0.32–0.53) | |
| Allopurinol, M04AA01 | 34 | 30 | 88% | 32 | 94% | 0.90 (0.83–0.98) | |
| Drugs for treatment of bone diseases/bisphosphonates, M05 | 62 | 57 | 92% | 63 | 91% | 0.90 (0.84–0.96) | |
| Nervous system | |||||||
| Opioids, N02A | 16 | 9 | 56% | 10 | 90% | 0.69 (0.48–0.89) | |
| Paracetamol, N02BE01 | 214 | 30 | 14% | 36 | 83% | 0.15 (0.10–0.21) | |
| Antipsychotics, N05A | 34 | 30 | 88% | 32 | 94% | 0.90 (0.83–0.98) | |
| Benzodiazepines and related drugs, N05BA, N05CD, N05CF | 189 | 134 | 71% | 150 | 89% | 0.70 (0.64–0.77) | |
| Antidepressants, N06A | 70 | 53 | 76% | 57 | 93% | 0.81 (0.74–0.89) | |
| Antidementia drugs, N06D | 65 | 59 | 91% | 59 | 100% | 0.95 (0.90–0.99) | |
| Respiratory system | |||||||
| Drugs for obstructive airway diseases, R03 | 68 | 55 | 81% | 61 | 90% | 0.83 (0.76–0.91) | |
| Adrenergics, inhalations, R03A | 55 | 35 | 64% | 41 | 85% | 0.71 (0.60–0.82) | |
| Other drugs for obstructive airway diseases, inhalations, R03B | 39 | 33 | 85% | 37 | 89% | 0.86 (0.77–0.95) | |
| Sensory organs | |||||||
| Antiglaucoma preparations, S01E | 44 | 43 | 98% | 50 | 86% | 0.91 (0.84–0.97) | |
Abbreviations: ATC, Anatomical Therapeutic Chemical; CI, confidence interval; NSAIDs, non-steroidal anti-inflammatory drugs.
While considering drugs reported to be used regularly in the interview, agreement between the interview and register data modeled with PRE2DUP was very good for 79% and very good or good for 89% of drug classes (Table 3). Moderate agreement was observed for regular opioid use and fair agreement for NSAIDs and paracetamol. Interview as standard showed higher agreement for many drugs or drug classes than results when register-based drug use was considered as a standard.
Table 3.
Person-level agreement between drugs self-reported being used on regular bases in the interview and drug use calculated from prescription register data with PRE2DUP modeling method in the GeMS Study
| Drug class or drug name and ATC code | Interview as a standard
|
Register as standard
|
Kappa (95% CI) | |||
|---|---|---|---|---|---|---|
| Number of regular users based on interview | Number of users in both | Proportion found in register data | Number of users based on register | Proportion found in the interview | ||
| Alimentary tract and metabolism | ||||||
| Proton pump inhibitors, A02BC | 40 | 34 | 85% | 50 | 68% | 0.74 (0.63–0.84) |
| Antidiabetics, A10 | 63 | 61 | 97% | 61 | 100% | 0.98 (0.96–1.00) |
| Insulins, A10A | 18 | 18 | 100% | 18 | 100% | 1.00 (1.00–1.00) |
| Oral antidiabetics, A10B | 55 | 52 | 95% | 52 | 100% | 0.97 (0.93–1.00) |
| Blood and blood forming organs | ||||||
| Antithrombotic drugs, B01A | 154 | 143 | 93% | 149 | 96% | 0.92 (0.89–0.96) |
| Cardiovascular system | ||||||
| Digitalis, C01A | 37 | 35 | 95% | 38 | 92% | 0.93 (0.87–0.99) |
| Organic nitrates, C01DA | 176 | 174 | 99% | 199 | 87% | 0.89 (0.85–0.93) |
| Diuretics, C03 | 195 | 182 | 93% | 196 | 93% | 0.90 (0.86–0.93) |
| Beta-blocking agents, C07 | 349 | 340 | 97% | 350 | 97% | 0.93 (0.90–0.96) |
| Calcium channel blockers, C08 | 162 | 153 | 94% | 169 | 91% | 0.89 (0.85–0.93) |
| Agents acting on the renin- angiotensin system, C09 | 227 | 223 | 98% | 240 | 93% | 0.93 (0.89–0.96) |
| Lipid-modifying agents, C10 | 214 | 204 | 95% | 208 | 98% | 0.95 (0.92–0.98) |
| Genitourinary system and sex hormones | ||||||
| Drugs for urinary incontinence, G04BD | 19 | 15 | 79% | 17 | 88% | 0.83 (0.69–0.96) |
| Drugs for benign prostatic hypertrophy, G04C | 47 | 46 | 98% | 50 | 92% | 0.94 (0.90–0.99) |
| Systemic hormonal preparations | ||||||
| Levothyroxine, H03AA01 | 60 | 56 | 93% | 56 | 100% | 0.96 (0.92–1.00) |
| Musculoskeletal system | ||||||
| NSAIDs, M01A | 21 | 17 | 81% | 64 | 27% | 0.37 (0.23–0.50) |
| Allopurinol, M04AA01 | 31 | 30 | 97% | 32 | 94% | 0.95 (0.89–1.00) |
| Drugs for treatment of bone diseases/bisphosphonates, M05 | 62 | 57 | 92% | 63 | 91% | 0.90 (0.84–0.96) |
| Nervous system | ||||||
| Opioids, N02A | 4 | 4 | 100% | 10 | 40% | 0.57 (0.26–0.88) |
| Paracetamol, N02BE01 | 50 | 13 | 25% | 36 | 36% | 0.25 (0.12–0.38) |
| Antipsychotics, N05A | 33 | 30 | 91% | 32 | 94% | 0.92 (0.85–0.99) |
| Benzodiazepines and related drugs, N05BA, N05CD, N05CF | 106 | 94 | 89% | 150 | 63% | 0.66 (0.59–0.73) |
| Antidepressants, N06A | 68 | 53 | 78% | 57 | 93% | 0.83 (0.76–0.90) |
| Antidementia drugs, N06D | 65 | 59 | 91% | 59 | 100% | 0.95 (0.90–0.99) |
| Respiratory system | ||||||
| Drugs for obstructive airway diseases, R03 | 54 | 49 | 91% | 61 | 80% | 0.84 (0.76–0.91) |
| Adrenergics, inhalations, R03A | 29 | 25 | 86% | 41 | 61% | 0.70 (0.57–0.82) |
| Other drugs for obstructive airway diseases, inhalations, R03B | 35 | 31 | 89% | 37 | 84% | 0.85 (0.76–0.94) |
| Sensory organs | ||||||
| Antiglaucoma preparations, S01E | 44 | 43 | 98% | 50 | 86% | 0.91 (0.84–0.97) |
Note: “As needed” drugs according to interview are not included.
Abbreviations: ATC, Anatomical Therapeutic Chemical; CI, confidence interval; NSAIDs, non-steroidal anti-inflammatory drugs.
Discussion
This is the first study to compare the validity of PRE2DUP with self-reported drug use as previous validations of the method have been expert opinion-based evaluation on the duration of drug use periods.9 The agreement between PRE2DUP and drug use reported on a particular day represent finer level agreement. The correctness of drug exposure status is especially important while assessing the impact of drug use on the beneficial or adverse outcomes. Misclassification of exposure status would lead to biased risk estimates.
Overall agreement
We found high agreement between self-reported drug use and drug use modeled with PRE2DUP method for the interview date. Agreement was very good or good for 93% of drugs and drug classes investigated while considering all drug use and 89% for regular drug use only. In this validation, 4 years of drug use history before the interview and 1 year after were examined, and hence, the estimates of current drug use were accurate. Data on drug purchases were also used, and hence, primary nonadherence problem found in data utilizing prescribed drugs was avoided. Using data on prescribed drugs instead of purchases could increase the number of users in the register-based results compared with the interview because drugs that have never been dispensed would appear in register data.
Comparison with previous studies
Kappa values describing agreement between interview and modeled register data in our study were high compared with previous studies that have compared interview or survey data with register-based data modeled with various methods.4,6–8 Only agreement in NSAIDs showed higher agreement in a previous study of French prescription data.7 For other comparable drug classes, our kappa values were similar (cardiovascular and respiratory drugs) or higher. Higher agreement in our study was found for antidiabetics (kappa values in previous studies were from 0.75 to 0.93, our study 0.98), lipid lowering drugs (previous studies from 0.73 to 0.85, our study 0.95), antipsychotics (previous studies from 0.54 to 0.76, our study 0.90), and drugs for the treatment of bone diseases (previous studies from 0.67 to 0.73, our study 0.90). All comparable drug classes in the results of Nielsen et al6 and Richardson et al8 showed poorer agreement between the data sources than our study.
OTC and “as needed” use
NSAIDs and paracetamol showed fairly poor agreement between PRE2DUP modeling and interview. This is because of several reasons. First, not all packages are reimbursed and smaller packages are available OTC. Second, these drugs are often used on “as needed” bases, that is, for symptomatic treatment. In the GeMS Study, the participants were not asked whether their drugs were prescribed by physician or bought OTC. To recall analgesic use during previous 2 weeks may be difficult without a diary especially for older people with several ongoing drug therapies. These factors may explain the under-reporting both from register and interview as standard. Poor agreement for “as needed” drugs has been found in previous studies comparing drug use between interview and dispensing data.7,8 Compared with the study by Noize et al,7 also the short time interval between purchases in France (1 month) compared with the Finnish dispensing regulations (3 months) makes modeling of many drug classes easier as dispensing intervals are shorter and better describe current use. The proportion of reimbursed NSAIDs in France may be larger than that in Finland,22 and the prevalence of OTC drug use may vary depending on the study population, age distribution, and accessibility of health care services. For analgesics, the actual timing of drug use is challenging to determine from register-based data with any method. Most likely the drug is used around the purchase date but the duration of use is somewhat speculative.
Agreement was less than very good also for BZDRs for which some smaller packages are not reimbursed. Furthermore, BZDRs should be used on “as needed” basis. The poorer agreement has been reported for benzodiazepines in previous studies.6–8 “As needed” use is challenging to model from register-based data, which does not indicate whether drug use is intended for regular or “as needed” use, and for drugs with addictive potential, even intention of use is different from actual use. It should be noted that in our study, “regular use” was recorded only in the interview, whereas register-based data includes all purchased drugs. Thus, register data also cover some purchases of “as needed” drugs compared to the interview, and this decreases kappa values for regular use. The lower agreement between register-based and interview-based BZDR use has also been reported in previous Finnish studies.2,3 The recommended use of these drugs is short-term and only “as needed.”23
Drugs in chronic use
High agreement was found in drugs that are used with fixed drug use patterns, such as statins, and drugs that are monitored, and doses are adjusted personally over the time such as antidiabetics. The higher agreement between interview and dispensing data for drugs used in the treatment of chronic diseases, such as cardiovascular diseases and diabetes, has been reported in all previous studies.4–8
Interview as golden standard
Although interview is often defined as golden standard for measuring drug use, it may also include some bias. In addition to unwillingness to report the use of particular drugs in the interview suggested in a previous study,8 the interviews, especially annual and repetitive interviews, may also pose changes in drug use behavior. When interview date is approaching, drugs and prescriptions are collected to recall drug use, and it is possible that this will lead to increased intensity of drug purchases. The interview itself may lead to re-starting of drug use that has been recently discontinued or forgotten for some time, especially in our study where data from the third repetitive interview was assessed and half of the group received a multidimensional health-related intervention which may have led to somewhat better adherence to drug therapy. Thus, even without an actual intervention such as medication review, an interview may pose an “intervention effect.” This may lead to overestimation of drug use compared with randomly selected time. However, when register data are used as a standard, it also includes drugs that are not actually used or have been recently discontinued. These drugs lower the kappa values when discrepancies are recorded in both ways (interview and register as standard).
Comparison to previous methods and implications for future
Our results are in line with results from previous studies on agreement between interview and dispensing data although methods used for dispensing data vary significantly between the studies.2,4–8 For some specific drug classes, our kappa statistics show better agreement (eg, antipsychotics), which may also be related to different drug use patterns (ie, regular versus “as needed” use) between the study populations. The main difference between time window methods and all other methods based on the particular date of drug use is assessed, and PRE2DUP is continuity of drug use. For cohort studies following up persons in time, time windows may not be accurate or practical to use as they do not consider the amount of drug dispensed each time. This is notable especially in countries where drugs may be purchased for treatment of ≥3 months such as in Nordic countries. The longer time windows may lead to biased estimates of drug use duration as noticed in previous studies that longer time windows increase the risk of false-positive classifications.4 PRE2DUP is modifiable in many ways, and it can also be adjusted for different dispensing regulations and practices such that drugs are dispensed for 1 month only instead of 3 months as in Nordic countries.
Strengths and limitations
The strength of the present study was comparison of register-based data to interview that included population-based sample of older persons, and their drug use was thoroughly assessed. Interview with prescription forms, drug packages, and medical records minimizes recall bias. The Prescription register data cover all community-dwelling residents of Finland. The register does not include drugs used in hospitals, but it includes drugs that have been prescribed by hospital physicians for the continuation of treatment at home. Hospitals are not allowed to dispense drugs for discharged patients, and patients may get drugs from hospital only for one night or for a short period of time when pharmacies are closed (such as public holidays). However, interviews were not conducted during public holidays, and hence, it is believed that this would not have a major impact on our results.
PRE2DUP was applied to register data with restriction parameters developed for the MEDALZ study including community dwelling older persons of similar age and drug use patterns.24–26 Limitations are related to limitations of the Prescription register data including only reimbursed drugs. Register-based data do not indicate whether drugs are intended for regular or “as needed” by the prescriber. It is also possible that participants might be prepared for the interview or interview encouraged them to purchase drugs more regularly, and hence, the agreement results may be better than in real life without an interview event. As the major change in drug reimbursement system impacting the inclusion of drugs in the Prescription register data happened before the chosen annual interview was conducted, the results are applicable to the current situation. However, the changing reimbursement status of specific drugs or drug products should always be considered while using register-based data.
Conclusion
PRE2DUP modeling resulted in highly reliable estimates of current drug use for most of the studied drugs. The agreement was higher for drugs used continuously and lower for “as needed” use and for also drugs available OTC. PRE2DUP modeling of register data showed similar or higher agreement compared with previous studies based on fixed time windows. Modeled register data can be used instead of interview data for most drug classes as reliable source of current drug use.
Footnotes
Disclosure
HT and AT have participated in research projects funded by Janssen with grants paid to the institution where they were employed. JT has served as a consultant in Lundbeck, Organon, Janssen-Cilag, Eli Lilly, AstraZeneca, F. Hoffman-La Roche, and Bristol-Myers Squibb. He has received fees for giving expert opinions to Bristol-Myers Squibb and Glaxo-SmithKline; lecture fees from Janssen-Cilag, Bristol-Myers Squibb, Eli Lilly, Pfizer, Lundbeck, GlaxoSmithKline, Astra-Zeneca, and Novartis; and grant from Stanley Foundation. He is a member of advisory board in AstraZeneca, Janssen-Cilag, and Otsuka. MK has received personal research grant from Oy H. Lundbeck Ab foundation outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Wettermark B, Zoega H, Furu K, et al. The Nordic prescription databases as a resource for pharmacoepidemiological research – a literature review. Pharmacoepidemiol Drug Saf. 2013;22(7):691–699. doi: 10.1002/pds.3457. [DOI] [PubMed] [Google Scholar]
- 2.Rikala M, Hartikainen S, Sulkava R, Korhonen MJ. Validity of the Finnish Prescription Register for measuring psychotropic drug exposures among elderly Finns. Drugs Aging. 2010;27(4):337–349. doi: 10.2165/11315960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Haukka J, Suvisaari J, Tuulio-Henriksson A, Lönnqvist J. High concordance between self-reported medication and official prescription database information. Eur J Clin Pharmacol. 2007;63(11):1069–1074. doi: 10.1007/s00228-007-0349-6. [DOI] [PubMed] [Google Scholar]
- 4.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–625. doi: 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 5.Sjahid SI, van der Linden P, Stricker BH. Agreement between the pharmacy medication history and patient interview for cardiovascular drugs: the Rotterdam elderly study. Br J Clin Pharmacol. 1998;45(6):591–595. doi: 10.1046/j.1365-2125.1998.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen MW, Søndergaard B, Kjøller M, Hansen EH. Agreement between self-reported data on medicine use and prescription records vary according to method of analysis and therapeutic group. J Clin Epidemiol. 2008;61(9):919–924. doi: 10.1016/j.jclinepi.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Noize P, Bazin F, Dufouil C, et al. Comparison of health insurance claims and patient interview in assessing drug use: data from the Three-City (3C) Study. Pharmacoepidemiol Drug Saf. 2009;18(4):310–319. doi: 10.1002/pds.1717. [DOI] [PubMed] [Google Scholar]
- 8.Richardson K, Kenny RA, Peklar J, Bennett K. Agreement between patient interview data on prescription medication use and pharmacy records in those aged older than 50 years varied by therapeutic group and reporting of indicated health conditions. J Clin Epidemiol. 2013;66(11):1308–1316. doi: 10.1016/j.jclinepi.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Tanskanen A, Taipale H, Koponen M, et al. From prescription drug purchases to drug use periods – a second generation method (PRE2DUP) BMC Med Inform Decis Mak. 2015;15:21. doi: 10.1186/s12911-015-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampela P, Hartikainen S, Sulkava R, Huupponen R. Adverse drug effects in elderly people – a disparity between clinical examination and adverse effects self-reported by the patient. Eur J Clin Pharmacol. 2007;63(5):509–515. doi: 10.1007/s00228-007-0283-7. [DOI] [PubMed] [Google Scholar]
- 11.Rikala M, Korhonen MJ, Sulkava R, Hartikainen S. The effects of medication assessment on psychotropic drug use in the community-dwelling elderly. Int Psychogeriatr. 2011;23(3):473–484. doi: 10.1017/S1041610210001547. [DOI] [PubMed] [Google Scholar]
- 12.Taipale HT, Bell JS, Gnjidic D, Sulkava R, Hartikainen S. Muscle strength and sedative load in community-dwelling people aged 75 years and older: a population-based study. J Gerontol A Biol Sci Med Sci. 2011;66(12):1384–1392. doi: 10.1093/gerona/glr170. [DOI] [PubMed] [Google Scholar]
- 13.Lihavainen K, Sipilä S, Rantanen T, Kauppinen M, Sulkava R, Hartikainen S. Effects of comprehensive geriatric assessment and targeted intervention on mobility in persons aged 75 years and over: a randomized controlled trial. Clin Rehabil. 2012;26(4):314–326. doi: 10.1177/0269215511423269. [DOI] [PubMed] [Google Scholar]
- 14.WHO Collaborating Centre for Drug Statistics Methodology The Anatomical Therapeutic Chemical Classification System. Structure and principles. [Accessed May 27, 2015]. Available from: http://www.whocc.no/atc/structure_and_principles/
- 15.WHO Collaborating Centre for Drug Statistics Methodology Defined daily dose. [Accessed May 27, 2015]. Available from: http://www.whocc.no/ddd/definition_and_general_considera/
- 16.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 17.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 19.Koponen M, Bell JS, Karttunen NM, Nykänen IA, Desplanter FA, Hartikainen SA. Analgesic use and frailty among community-dwelling older people: a population-based study. Drugs Aging. 2013;30(2):129–136. doi: 10.1007/s40266-012-0046-8. [DOI] [PubMed] [Google Scholar]
- 20.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and education level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33(2):363–374. [PubMed] [Google Scholar]
- 22.Duong M, Salvo F, Pariente A, et al. Usage patterns of ‘over-the-counter’ vs. prescription-strength nonsteroidal anti-inflammatory drugs in France. Br J Clin Pharmacol. 2014;77(5):887–895. doi: 10.1111/bcp.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finnish Medical Society Duodecim . Clinical care guideline on insomnia. Helsinki; 2015. [Accessed March 4, 2016]. (in Finnish with English summary). Available from: http:\\www.kaypahoito.fi. [Google Scholar]
- 24.Taipale H, Koponen M, Tanskanen A, Tolppanen AM, Tiihonen J, Hartikainen S. Antipsychotic polypharmacy among a nationwide sample of community-dwelling persons with Alzheimer’s disease. J Alzheimer Dis. 2014;41(4):1223–1228. doi: 10.3233/JAD-140282. [DOI] [PubMed] [Google Scholar]
- 25.Taipale H, Koponen M, Tanskanen A, Tolppanen AM, Tiihonen J, Hartikainen S. Long-term use of benzodiazepines and related drugs among community-dwelling individuals with and without Alzheimer’s disease. Int Clin Psychopharmacol. 2015;30(4):202–208. doi: 10.1097/YIC.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 26.Koponen M, Taipale H, Tanskanen A, et al. Long-term use of anti-psychotics among community-dwelling persons with Alzheimer’s disease: a nationwide register-based study. Eur Neuropsychopharmacol. 2015;25(10):1706–1713. doi: 10.1016/j.euroneuro.2015.07.008. [DOI] [PubMed] [Google Scholar]