Abstract
Aims
To evaluate the efficacy and safety of two insulin intensification strategies for patients with type 2 diabetes previously treated with basal insulin – insulin degludec (IDeg) and insulin aspart (IAsp) – administered as a co‐formulation (IDegAsp) or as a basal‐bolus regimen (IDeg and IAsp in separate injections).
Methods
This 26‐week, open‐label, treat‐to‐target, phase IIIb, non‐inferiority trial randomized patients (1 : 1) to IDegAsp twice daily with main meals (n = 138; IDegAsp group) or IDeg once daily and IAsp 2–4 times daily (n = 136; IDeg+IAsp group).
Results
After 26 weeks, the mean glycated haemoglobin (HbA1c) level was 7.0% (53 mmol/mol) for the IDegAsp group and 6.8% (51 mmol/mol) for the IDeg+IAsp group (Δ%HbA1c from baseline −1.31 and −1.50%, respectively). The non‐inferiority of IDegAsp versus IDeg+IAsp was not confirmed for mean change in HbA1c [estimated treatment difference (ETD) 0.18, 95% confidence interval (CI) −0.04, 0.41; p = non‐significant]. No significant differences were observed in the proportion of patients achieving HbA1c <7.0% (56.5 and 59.6%, respectively). IDegAsp treatment resulted in a significantly lower total daily insulin dose, a smaller change in body weight, numerically lower rates of confirmed hypoglycaemia (self‐reported plasma glucose <3.1 mmol/l; rate ratio 0.81; p = non‐significant), and nocturnal confirmed hypoglycaemic episodes (rate ratio 0.80; p = non‐significant) versus IDeg+IAsp. Patient‐reported outcome scores for social functioning were significantly higher for IDegAsp versus IDeg+IAsp (ETD 2.2; 95% CI 0.3, 4.1; p < 0.05).
Conclusions
Both intensification strategies effectively improved glycaemic control. Although non‐inferiority was not confirmed, there were no significant differences between the groups that could affect clinical utility.
Keywords: efficacy, insulin aspart, insulin degludec, insulin intensification, type 2 diabetes
Introduction
Recent guidelines highlight the need to tailor treatment strategies and/or targets to individual patient characteristics, including age, existing comorbidities and the duration of diabetes 1, 2. Of special consideration are the patient's attitude to and expectations of treatment efforts and the support available to them to meet treatment goals successfully 1, 2, 3. Optimum care should be aimed at facilitating patient adherence to treatment 1, 2, taking into consideration that simpler, less frequent dosing regimens can result in better compliance 4.
Many patients with type 2 diabetes requiring insulin therapy can be successfully treated, at least initially, with basal insulin alone 1, 5, 6. A basal‐only insulin regimen has the benefit of being well tolerated, with a low injection frequency and a low risk of hypoglycaemia 7, thus facilitating patient acceptance of insulin therapy 8; however, because of the progressive loss of the prandial insulin response in type 2 diabetes and the contribution of both fasting and postprandial hyperglycaemia to glycated haemoglobin (HbA1c) 9, 10, many patients require intensification beyond a basal‐only insulin regimen in order to reach glycaemic targets 7. Current options for treatment intensification include the addition of incretin‐based therapies to basal insulin or preprandial addition of a rapid‐acting insulin analogue 7. Regarding the latter, treatment guidelines recommend either sequential addition of rapid‐acting insulin injections at one or more meal times (basal‐bolus regimen) – the so‐called stepwise intensification therapy – or a switch to a twice‐daily premixed insulin regimen 1. The stepwise intensification approach, where up to three prandial insulin boluses are added sequentially according to individual needs, has been shown to provide glycaemic control that is non‐inferior to full basal‐bolus insulin therapy, with significantly lower risk of hypoglycaemia and better patient satisfaction 11, 12.
Basal‐bolus therapy offers a tailored approach to treatment but usually involves multiple daily injections to reach the desired glycaemic target 1, 3. Many patients perceive the basal‐bolus regimen as being inconvenient because of its complexity, both in the number of daily injections required and the need to titrate and administer two separate types of insulin 1, 3. An alternative intensification option is the use of premixed insulins, containing a rapid‐ and a long‐acting insulin in a single preparation 1, 3, thereby permitting fewer injections than basal‐bolus therapy regimens 13. Premixed insulin regimens may be preferred for patients who have a regularly scheduled diet and physical activity, who do not require stringent glycaemic targets or who have difficulty complying with basal‐bolus regimens 3. Currently available premixed insulins cannot provide 24‐h glucose control 14, are associated with excess weight gain compared with basal insulin therapy 3, and require patients to re‐suspend the insulin 1, 15, 16. Accordingly, if glucose levels are inadequately controlled with a premixed insulin regimen, treatment guidelines often recommend a switch from a twice‐daily premixed insulin regimen to a basal‐bolus regimen 1, 2, 17.
Insulin degludec (IDeg) is a new‐generation basal insulin with a duration of action that exceeds 42 h 18. A reduced risk of hypoglycaemia has been shown in patients with type 2 diabetes treated with IDeg versus insulin glargine (IGlar) 16, 19. Insulin degludec/insulin aspart (IDegAsp) is the first soluble coformulation of a long‐acting insulin analogue (IDeg: 70%) with a short‐acting insulin analogue (IAsp: 30%) 20. It preserves the glucose‐lowering effects of both the basal and the prandial component in solution and post‐injection 21, 22, and does not require re‐suspension before use. Compared with premixed insulins such as biphasic insulin aspart 30 (BIAsp 30), IDegAsp provides a simple insulin therapy regimen comprising both basal and meal‐time coverage 23.
The present randomized, open‐label, treat‐to‐target, phase IIIb study compared the efficacy and safety of intensification with IDegAsp administered twice daily (IDegAsp group) and basal‐bolus therapy with IDeg once daily administered with IAsp 2–4 times daily (IDeg + IAsp group) in patients with type 2 diabetes previously treated with basal insulin with/without oral antidiabetic drugs (OADs).
Materials and Methods
Study Design
Forty‐eight sites in five countries (Algeria, Austria, France, Norway and the USA) participated in this 26‐week trial (ClinicalTrials.gov identifier: NCT01713530), which was performed in accordance with the Declaration of Helsinki and good clinical practice as defined by the International Conference on Harmonisation. All patients provided written informed consent before trial initiation.
Trial Population
Inclusion criteria were diagnosis ≥6 months before screening, age ≥18 years, body mass index ≤40 kg/m2, HbA1c 7.0–10.0% (53–86 mmol/mol), and previous treatment with basal insulin (insulin detemir, IGlar, or neutral protamine Hagedorn) with or without OADs (metformin, sulphonylureas/glinides, dipeptidyl peptidase‐4 inhibitors, α‐glucosidase inhibitors) for ≥3 months before trial initiation.
Key exclusion criteria were: treatment with a glucose‐lowering agent other than those stated in the inclusion criteria; a history of stroke, decompensated heart failure or myocardial infarction within 26 weeks of screening; presence of recurrent severe hypoglycaemia (>1 event within the previous 12 months); or hypoglycaemic unawareness, as evaluated by the investigator.
Study Procedures
Eligible patients were randomized 1 : 1 to receive IDegAsp twice daily ± OADs (IDegAsp group) or IDeg + IAsp 2–4 times daily ± OADs (IDeg + IAsp group; Figure S1). Sulphonylureas were discontinued at randomization, but no other changes to pre‐randomization OAD therapy were permitted (although the dose could be reduced during the study period if requested by the investigator).
At the investigators' discretion, the daily dose of IDegAsp was split into two doses, ensuring that the dose of the short‐acting component was appropriate to the intended meal size. IDegAsp was always administered immediately before the evening meal and with either breakfast or lunch, as the patient wished. Patients previously receiving a twice‐daily (or more frequent) basal insulin regimen were switched to IDeg + IAsp as follows: IDeg dose was reduced by 20% compared with the previous total insulin dose, and IAsp was administered at a 4 U starting dose 2–4 times daily, in accordance with local labelling. IDegAsp and IDeg (both at 100 U/ml) were delivered via subcutaneous (s.c.) injection in the abdomen, upper arm (deltoid area) or lateral thigh. IAsp 100 U/ml was administered by s.c. injection, usually in the abdomen.
Insulin Dosing
Insulin dose was titrated weekly to a self‐monitored pre‐breakfast/pre‐evening meal plasma glucose target of 4–5 mmol/l (71–90 mg/dl). Patients were required to provide a four‐point self‐monitored plasma glucose (SMPG) profile, with measurements before breakfast, lunch, evening meal and bedtime, on three consecutive days before each scheduled visit. This allowed physicians to adjust or maintain the insulin dose, as described in the titration algorithm (Table S1), to provide optimum glycaemic control. IDegAsp doses were increased based on the mean of three prebreakfast and three pre‐evening meal SMPG values, respectively, measured on the 3 days preceding titration. Similarly, the IDeg dose was increased based on the mean of three prebreakfast SMPG values measured on the 3rd day before titration. The breakfast, lunch and evening IAsp doses were titrated according to the mean prelunch, pre‐evening meal and bedtime SMPG values, respectively. A fourth IAsp dose was allowed, if necessary (Table S1).
Study Endpoints
The primary endpoint was non‐inferiority of IDegAsp versus IDeg + IAsp in change in HbA1c levels from baseline to week 26. Non‐inferiority was considered to be confirmed if the upper bound of the two‐sided 95% confidence interval (CI) for the treatment difference (IDegAsp minus IDeg + IAsp) for the mean change in HbA1c was ≤0.4% (−19.1 mmol/ml), as per US Food and Drug Administration guidance 24.
Secondary endpoints included change from baseline to week 26 in fasting plasma glucose (FPG), the proportion of patients achieving HbA1c <7.0% (53 mmol/mol), change in eight‐point SMPG profile, change in mean total daily insulin dose, and changes in patient‐reported outcomes.
Safety assessments included changes in body weight from baseline to week 26, the incidence and rates of overall confirmed hypoglycaemia (defined by SMPG levels <3.1 mmol/l or requiring assistance from another person), nocturnal confirmed hypoglycaemia (i.e. onset between 00 : 01 and 05 : 59 h) and severe hypoglycaemia (requiring assistance from another person). Safety was also assessed by systematic reporting of adverse events (AEs). The 36‐item Short‐Form version 2 (SF‐36 v2) health survey was used to examine general health and well‐being.
Sample Size
Sample size was determined using a t‐statistic, assuming a one‐sided test of 2.5% and a zero mean treatment difference. It was anticipated that 15% of patients in the full analysis set (FAS; all randomized patients) would be excluded from the per‐protocol (PP) analysis set; therefore, a total of 270 patients needed to be randomized to demonstrate non‐inferiority at a 0.4% margin, with 85% power in the PP analysis set.
Statistical Methods
The primary endpoint was analysed using analysis of variance (anova), with treatment, antidiabetic therapy at screening, sex and geographic region as fixed factors, and age and baseline HbA1c as covariates. Changes from baseline in FPG and body weight were similarly analysed using anova, with age and endpoint values as covariates. Responder analyses were carried out using a logistic regression model using the same factors and covariates employed for the primary analysis.
The eight‐point SMPG profile included measurements made immediately before and 90 min after the start of breakfast, lunch and the main evening meal, before bedtime, and before breakfast the following day. A mixed‐effects model was fitted to these data and included treatment, time of day category, interaction between treatment and time of day, antidiabetic therapy at screening, sex and geographic region as fixed factors, age and baseline HbA1c as covariates, and subject as random effect. From this model, mean profile by treatment and relevant treatment differences were estimated.
The number of hypoglycaemic episodes was analysed using a negative binomial regression model. The model included treatment, antidiabetic therapy at screening, sex and geographic region as fixed factors, and age as a covariate. Other AEs, laboratory variables, physical examination, electrocardiography, fundoscopy/fundus photography, vital signs and insulin dose were summarized using descriptive statistics.
Formal statistical analyses (including safety analyses regarding hypoglycaemic episodes, body weight, and lipids) were performed using the FAS. Other safety endpoints were summarized and analysed using the safety analysis set, which included all patients who received at least one dose of IDegAsp, IDeg or IAsp.
Results
Patient Characteristics
Of 391 patients screened, 274 were randomized to receive IDegAsp (n = 138) or IDeg + IAsp (n = 136). Of those receiving IDegAsp, 82% (n = 113) completed the study, and 86% (n = 117) completed the study in the IDeg + IAsp group (Figure S2). Patient demographics and baseline characteristics were similar in the two groups, although there was a slightly larger proportion of female patients and longer duration of diabetes in the IDegAsp group (Table 1).
Table 1.
Patient demographics and baseline characteristics.
| Characteristic | IDegAsp | IDeg + IAsp |
|---|---|---|
| FAS, n | 138 | 136 |
| Female/male, % | 47.1/52.9 | 36.8/63.2 |
| Race: White/Black/Asian/Other, % | 92.0/6.5/0.0/1.4 | 92.6/5.1/2.2/0.0 |
| Ethnicity: Hispanic or Latin American, % | 13.8 | 12.5 |
| Age, years | 59.6 (8.3) | 59.6 (9.2) |
| Weight, kg | 91.2 (17.7) | 93.3 (15.2) |
| BMI, kg/m2 | 32.2 (4.7) | 32.0 (4.5) |
| Duration of diabetes, years | 13.5 (7.2) | 11.7 (7.2) |
| HbA1c, % | 8.3 (0.9) | 8.3 (0.7) |
| HbA1c, mmol/mol* | 67.2 | 67.2 |
| FPG, mmol/l | 9.0 (3.0) | 8.8 (2.9) |
| FPG, mg/dl | 162.4 (54.0) | 159.2 (52.7) |
| With OAD at screening, n | 130 | 122 |
BMI, body mass index; FAS, full analysis set; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; OAD, oral antidiabetic drug.
FAS: values are mean (standard deviation) unless otherwise stated.
Calculated, not measured.
Glycaemic Control
Glycaemic control was achieved by week 14 and was maintained through week 26 (Figure 1A). After 26 weeks, HbA1c levels were reduced from 8.3% (67.2 mmol/mol) to 7.0% (53 mmol/mol) with IDegAsp and from 8.3% (67.2 mmol/mol) to 6.8% (51 mmol/mol) with IDeg + IAsp (Figure 1A). The mean HbA1c values decreased by 1.31% in the IDegAsp group and by 1.50% in the IDeg + IAsp group. After 26 weeks, the estimated treatment difference (ETD) between groups (IDegAsp – IDeg + IAsp) was 0.18% (95% CI −0.04, 0.41; p = non‐significant); therefore, the non‐inferiority of IDegAsp versus IDeg + IAsp was not confirmed. All sensitivity analyses showed an upper confidence limit below 0.4 (ETD 0.16, 95% CI −0.06, 0.38 for the PP analysis set; and 0.14, 95% CI −0.08, 0.36 for the completers). Results from the FAS were 0.16% (95% CI −0.06, 0.39) using the simple model and 0.16% (95% CI −0.06, 0.38) using the repeated‐measures model.
Figure 1.
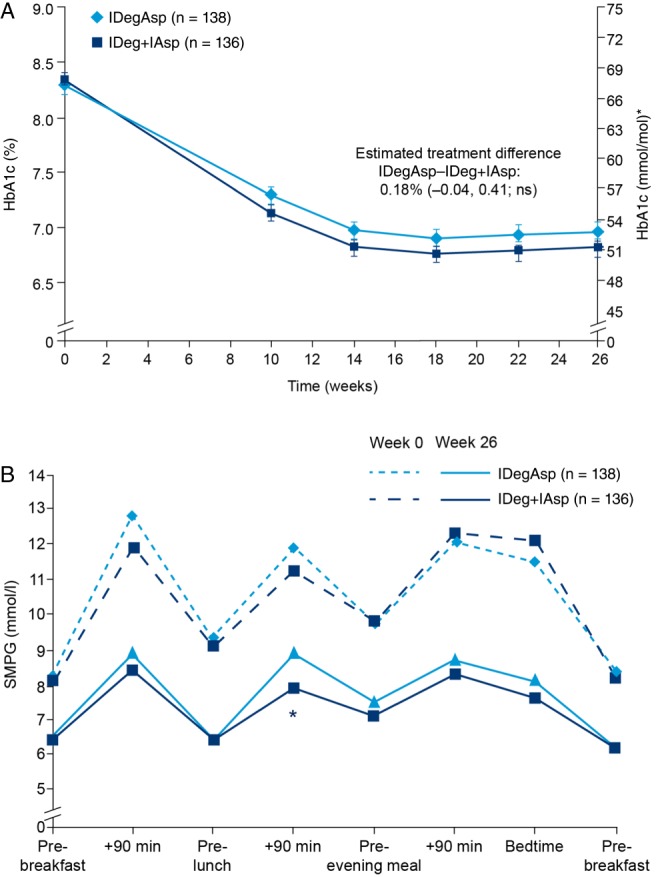
(A) Mean glycated haemoglobin (HbA1c) over time to 26 weeks for insulin degludec/insulin aspart (IDegAsp) twice daily versus insulin degludec once daily + insulin aspart (IDeg + IAsp). Data are mean (standard error of the mean) in the full analysis set (LOCF). Comparisons: estimates adjusted for multiple covariates. *Calculated, not measured. Non‐inferiority margin: upper bound of the two‐sided 95% confidence interval for the mean HbA1c treatment difference was ≤0.4%. ns, not significant; (B) Eight‐point self‐monitored plasma glucose profiles at baseline and week 26. *Estimated treatment difference: 1.01 mmol/l (95% CI 0.30, 1.73); p < 0.05. Treatment differences are derived from a least squares means‐based model. SMPG, self‐monitored plasma glucose.
The proportion of patients achieving HbA1c <7.0% (53 mmol/mol) after 26 weeks was similar for IDegAsp and IDeg + IAsp (56.5 and 59.6%, respectively). Based on the estimated odds ratio for IDegAsp versus IDeg + IAsp, the difference in the proportion of patients achieving HbA1c <7.0% (53 mmol/mol) after 26 weeks was not significant (0.83; 95% CI 0.50, 1.38).
After 26 weeks, FPG levels were reduced to similar levels in the IDegAsp and IDeg + IAsp groups (6.8 and 7.1 mmol/l, respectively; ETD −0.31 mmol/l; 95% CI −0.97, +0.34; p = non‐significant). SMPG profiles were similar between regimens at baseline (Figure 1B), as well as at week 26 for all time points except for 90 min after lunch, when SMPG levels were significantly higher for IDegAsp compared with IDeg + IAsp [ETD +1.01 mmol/l; 95% CI +0.30, +1.73; p < 0.05 (Figure 1B)].
Insulin Dose
The mean daily insulin dose is shown in Table 2. After 26 weeks, the mean total daily dose was significantly lower for IDegAsp versus IDeg + IAsp [107 U (1.11 U/kg) vs 131 U (1.34 U/kg); p < 0.05]. In the IDeg + IAsp group, the insulin dose ratio IDeg/IAsp changed systematically from 80%/20% at baseline to 55%/45% at week 26.
Table 2.
Mean (standard deviation) total daily insulin doses.
| U/kg (U) | ||
|---|---|---|
| IDegAsp (n = 136) | IDeg + IAsp (n = 135) | |
| Baseline* | 0.47 (44) | 0.59 (55) |
| End of trial† | 1.11 (107) | 1.34 (131) |
IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; U, units.
Baseline dose reflects the first dose of study treatment and therefore dose size was mandated by the study protocol.
p < 0.05. Safety analysis set. Comparisons: estimates adjusted for multiple covariates.
During the trial, patients had the option of choosing between 2, 3 or 4 injections of IAsp based on the number of meals they would have on a given day. Patients in the IDeg + IAsp group administered, on average, 3.6 injections/day of IDeg + IAsp (Table S2). This study allowed flexibility in the timing of the IDegAsp morning injection. The majority of patients opted to administer IDegAsp with breakfast and the main evening meal rather than with lunch and the main evening meal (80% at week 1; 69% at week 26).
Body Weight
The mean [standard deviation (s.d.)] increase in body weight from baseline to week 26 was significantly lower for IDegAsp versus IDeg + IAsp [2.8 (3.4) and 3.8 (4.5) kg, respectively; ETD −1.04 kg; 95% CI −1.99, −0.10; p < 0.05).
Hypoglycaemic Events
Confirmed hypoglycaemic episodes were reported by 72.1% (n = 98) of patients in the IDegAsp group and 80.7% (n = 109) in the IDeg + IAsp group. The rate of overall confirmed hypoglycaemic events [events per patient‐years of exposure (PYE)] was numerically lower with IDegAsp than with IDeg + IAsp [11.6 vs 13.6 events/PYE; rate ratio 0.81; 95% CI 0.61, 1.07; p = non‐significant (Figure 2A)].
Figure 2.
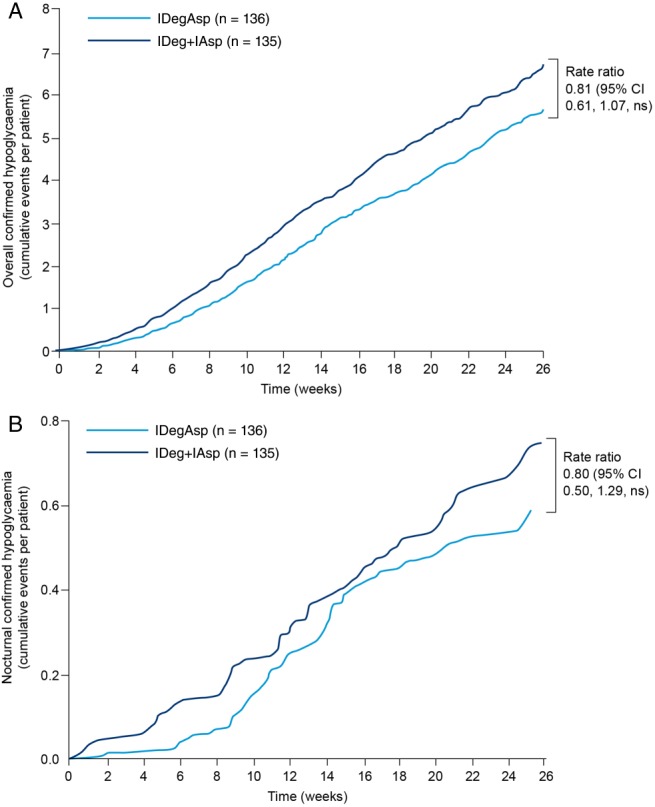
(A) Overall confirmed hypoglycaemia. (B) Nocturnal confirmed hypoglycaemia. Comparisons: estimates adjusted for multiple covariates. Nocturnal events defined as occurring between 00 : 01 and 05 : 59 h. CI, confidence interval; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; ns, not significant.
Nocturnal confirmed hypoglycaemic events were reported in 31.6% (n = 43) and 28.9% (n = 39) of patients in the IDegAsp and IDeg + IAsp groups. The rate of nocturnal events was numerically lower for IDegAsp versus IDeg + IAsp [1.2 vs 1.6 events/PYE; rate ratio 0.80; 95% CI 0.50, 1.29; p = non‐significant (Figure 2B)]. Eight patients from the IDegAsp group (5.9%; 0.47 episodes/PYE) and nine patients from the IDeg + IAsp group (6.7%; 0.24 episodes/PYE) reported severe hypoglycaemic events.
Patient‐Reported Outcomes: SF‐36v2 Scores
There were no significant between‐group differences in the overall physical scores on the SF‐36v2. A higher overall change from baseline in the mental score was seen with IDegAsp versus IDeg + IAsp. The social function domain questions used for this study evaluated the extent of health‐related problems and the frequency with which they interfered with normal social activities. Change from baseline in the social functioning scale was significantly higher for IDegAsp versus IDeg + IAsp (ETD 2.2; 95% CI 0.3, 4.1; p < 0.05).
Adverse Events
The percentage of patients who reported ≥1 AE (other than hypoglycaemia) was similar in the IDegAsp and IDeg + IAsp groups (62.5 and 68.9%, respectively). Serious AEs were reported by 5.1% (n = 7) and 9.6% (n = 13) of patients, respectively. For more details on AEs and serious AEs see Table S3.
Discussion
The study results showed that treatment with IDeg and IAsp, administered either as a soluble co‐formulation or as separate injections, effectively improved glycaemic control in patients with type 2 diabetes who had previously been treated with basal insulin. The non‐inferiority of IDegAsp compared with IDeg + IAsp was not confirmed at week 26; however, there was no significant difference in HbA1c achieved for the two treatment groups and patients in both groups achieved target HbA1c levels by week 14. Because the primary analysis (change in HbA1c) did not show a statistically significant difference between treatment arms, the secondary endpoints should be interpreted in this context.
Consistent with HbA1c results, FPG reductions were similar with IDegAsp and IDeg + IAsp at week 26. Overall, the mean plasma glucose level at week 26, as assessed by the eight‐point SMPG profiles, was similar between both regimens except after lunch, when significantly lower SMPG levels were observed for IDeg + IAsp versus IDegAsp.
The majority of patients administered IDegAsp before breakfast and the main evening meal (80% at week 1; 69% at week 26). This degree of flexibility represents an important advance with the new co‐formulation. Moreover, the SMPG levels post‐lunch did not exceed the 9 mmol/l target recommended by the International Diabetes Federation guidelines for postprandial glucose 25, 26.
The mean total daily insulin dose increased during the trial period, as expected with a treat‐to‐target regimen. The difference in mean daily dose between the two regimens remained relatively constant throughout the trial, with patients in the IDegAsp group ending the trial with a significantly lower mean daily dose than patients in the IDeg + IAsp group. Because the basal insulin dose was similar in both groups at week 26, the difference in total insulin dose was primarily attributable to the difference in bolus insulin dose. This difference might explain the improved postprandial glycaemic control (especially after lunch) achieved with IDeg + IAsp versus IDegAsp. The significantly lower weight gain observed with IDegAsp could be attributable, in part, to the significantly lower mean total daily insulin dose used with IDegAsp versus IDeg + IAsp.
Numerically lower rates of confirmed hypoglycaemia (overall and nocturnal) were observed in the IDegAsp group versus the IDeg + IAsp group throughout the entire study period 27. Although patients in both treatment groups had similar FPG and eight‐point SMPG profiles, the end‐of‐treatment HbA1c values were numerically lower with IDeg + IAsp compared with IDegAsp. The lower end‐of‐treatment HbA1c with IDeg + IAsp may explain the numerically higher rates of confirmed and nocturnal hypoglycaemia observed with IDeg + IAsp compared with IDegAsp.
The results from the patient‐reported outcomes questionnaire showed a significant increase in social functioning for the IDegAsp versus IDeg + IAsp group, suggesting that IDegAsp resulted in less interference with day‐to‐day social activities. This is probably attributable to the reduced burden of injections with IDegAsp. Improved patient‐reported outcome scales and reduced regimen complexity have been associated with improved adherence, which in turn has been linked to improvement in glycaemic control in the real‐world setting 28, 29.
Few treatment‐associated AEs were reported, and no unusual patterns were observed between regimens.
A recent study assessing the efficacy and safety of IDegAsp twice daily versus BIAsp 30 twice daily in Asian adults with type 2 diabetes, inadequately controlled on basal insulin, showed that IDegAsp was non‐inferior to BIAsp 30 in the mean change in HbA1c. Treatment with IDegAsp twice daily led to significantly lower FPG level and final mean daily insulin dose compared with BIAsp 30 30. When intensification from basal insulin is necessary, it is beneficial to have several options available to allow treatment to be tailored to individual patient needs and for optimum glycaemic control to be achieved. In a clinical setting, the choice of preferred intensification regimen should take into account a variety of patient characteristics, such as age, weight, comorbidities, duration of diabetes and racial background, as well as patient's attitudes and expectations towards insulin treatment and glycaemic control. A limitation of the present study was that the study population was largely white, and therefore factors affecting insulin therapy that are dependent on race, such as diet and lifestyle, may not have been fully explored here.
Until recently, premixed insulins have been the preferred alternative in patients who needed intensification but did not want to follow a full basal‐bolus regimen 25. Compared with premixed insulins, IDegAsp twice daily represents an alternative to basal‐bolus regimens for patients who require bolus insulin but need a simplified insulin regimen to overcome the commonly cited issues of complexity and inconvenience as barriers to basal‐bolus regimens 28. In addition to its simplicity, IDegAsp offers 24‐h basal coverage when used with additional bolus insulin doses. In clinical trials comparing BIAsp 30 and IDegAsp, patients taking either treatment were able to achieve glycaemic targets similar to those recommended by current guidelines 30, 31.
The present study shows that the transition of patients from basal insulin to IDeg and IAsp, either co‐formulated IDegAsp or as separate injections IDeg + IAsp, can be conducted effectively and safely, with desired HbA1c targets being achieved within 26 weeks. The IDegAsp twice‐daily regimen may provide an effective solution to the need for insulin intensification in many patients for whom adherence to more complex and demanding regimens is challenging.
Conflict of Interest
H. W. R. has served on advisory boards for AstraZeneca Pharmaceuticals LP, Biodel, Inc., Bristol‐Myers Squibb, Boehringer Ingelheim, Janssen Pharmaceuticals, Lilly, Merck, Novo Nordisk A/S and Sanofi, has provided consultancy for Biodel Inc., Takeda Pharmaceuticals U.S.A. Inc. and Merck, has received research support from AstraZeneca Pharmaceuticals LP, Biodel Inc., Boehringer Ingelheim Pharmaceuticals Inc., Eli Lilly and Company, Merck, Novo Nordisk A/S, Roche Pharmaceuticals, Sanofi, GlaxoSmithKline, Halozyme, Hamni and Janssen Pharmaceuticals, and has attended speakers' bureaux for AstraZeneca Pharmaceuticals LP, Bristol‐Myers Squibb, Boehringer Ingelheim Pharmaceuticals, Inc., Janssen Pharmaceuticals, Eli Lilly and Company, Merck, Novo Nordisk A/S, and Takeda Pharmaceuticals U.S.A. Inc. H. W. R.'s spouse has provided consultancy for Abbott Diabetes Care, Halozyme and Sanofi. B. C. has received research funds from Sanofi and is a consultant or on an advisory panel for Amgen, AstraZeneca, DebioPharm, Janssen, Eli Lilly, Genfit, Novo Nordisk, Sanofi‐Regeneron and Takeda. T. R. P. has served on advisory boards for Novo Nordisk, Eli Lilly, Bristol‐Myers Squibb and Roche Diagnostics, has received research support from Novo Nordisk A/S and has taken part in speakers' bureaux for Novo Nordisk and AstraZeneca. L. A. E. and J. Z. are employees and shareholders of Novo Nordisk. J. G. C. has received research support from Novo Nordisk, Merck, AstraZeneca, Sanofi, Boehringer Ingelheim and Lilly, and has taken part in speakers' bureaux for most companies involved in diabetes.
H. W. R. takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. H. W. R. performed the clinical research and authored the manuscript. B. C. performed the research and contributed to the interpretation of the results and reviewed the manuscript. T. R. P. performed the research and analysed the data. L. A. E. designed the research, performed the research and contributed to interpretation of the results. J. Z. co‐authored the manuscript, designed the research, performed the research and analysed the data. J. G. C. co‐authored the manuscript and performed the research.
Supporting information
Figure S1. Study design. [IDegAsp] IDegAsp administered BID; [IDeg+IAsp] basal‐bolus therapy with IDeg once daily administered with IAsp 2–4 times daily. Abbreviations: IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; OAD, oral antidiabetic drug.
Figure S2. Study flow diagram. Abbreviations: BID, twice daily; FAS, full analysis set; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; SAS, safety analysis set.
Table S1. Titration algorithms for [IDegAsp], IDeg OD and IAsp. Footnote: *Mean pre‐breakfast/pre‐evening meal plasma glucose measurements (adjustment: evening meal dose was increased based on the mean of three pre‐breakfast SMPG values measured on the 3 days before titration; breakfast or lunch doses were increased based on the mean of three pre‐evening meal SMPG values measured on the 3 days before titration). †Mean pre‐breakfast plasma glucose measurements (adjustment: dose was increased based on the mean of 3 consecutive days' measurements). ‡Lowest pre‐breakfast/pre‐evening meal plasma glucose measurement used to determine dose decreases. §Adjustment: mean of 3 consecutive days' measurements; breakfast IAsp dose titrated according to the mean pre‐lunch PG; lunch IAsp dose titrated according to the mean pre‐evening meal PG; evening meal IAsp dose titrated according to the mean bedtime PG. A fourth IAsp dose was allowed if necessary. BID, twice daily; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; OD, once daily; PG, plasma glucose; SMPG, self‐monitored plasma glucose; U, units.
Table S2. Number of daily IAsp injections for subjects in the [IDeg+IAsp] basal‐bolus treatment group during the study. Abbreviations and footnotes: IAsp, insulin aspart; IDeg, insulin degludec. n, number.
Table S3. Adverse events and serious adverse events. Abbreviations: AE, adverse event; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart.
Acknowledgements
Medical writing support was provided by apothecom scopemedical ltd and funded by Novo Nordisk A/S. This study was funded by Novo Nordisk A/S.
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 3. Mosenzon O, Raz I. Intensification of insulin therapy for type 2 diabetic patients in primary care: basal–bolus regimen versus premix insulin analogs when and for whom? Diabetes Care 2013; 36(Suppl. 2): S212–S218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23: 1296–1310. [DOI] [PubMed] [Google Scholar]
- 5. Gough SCL, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal RM. Low‐volume insulin degludec 200 units/ml once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin‐naive patients with type 2 diabetes: a 26‐week, randomized, controlled, multinational, treat‐to‐target trial: the BEGIN LOW VOLUME trial. Diabetes Care 2013; 36: 2536–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zinman B, Philis‐Tsimikas A, Cariou B et al. Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moghissi E, King AB. Individualizing insulin therapy in the management of type 2 diabetes. Am J Med 2014; 127(Suppl. 10): S3–S10. [DOI] [PubMed] [Google Scholar]
- 8. Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care 2009; 32(Suppl. 2): S253–S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients variations with increasing levels of HbA1c. Diabetes Care 2003; 26: 881–885. [DOI] [PubMed] [Google Scholar]
- 10. Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011; 34: 2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodbard HW, Visco VE, Andersen H, Hiort LC, Shu DHW. Treatment intensification with stepwise addition of prandial insulin aspart boluses compared with full basal–bolus therapy (FullSTEP Study): a randomised, treat‐to‐target clinical trial. Lancet Diabetes Endocrinol 2014; 2: 30–37. [DOI] [PubMed] [Google Scholar]
- 12. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management–exploring the concept of the basal‐plus approach in clinical practice. Diabetes Med J Br Diabetes Assoc 2013; 30: 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garber AJ, Ligthelm R, Christiansen JS, Liebl A. Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes Obes Metab 2007; 9: 630–639. [DOI] [PubMed] [Google Scholar]
- 14. Heise T, Nosek L, Klein O, Coester H, Svendsen AL, Haahr H. Insulin degludec/insulin aspart produces a dose‐proportional glucose‐lowering effect in subjects with type 1 diabetes mellitus. Diabetes Obes Metab 2015; 17: 659–664. [DOI] [PubMed] [Google Scholar]
- 15. Garber AJ. Insulin intensification strategies in type 2 diabetes: when one injection is no longer sufficient. Diabetes Obes Metab 2009; 11(Suppl. 5): 14–18. [DOI] [PubMed] [Google Scholar]
- 16. Rodbard HW, Gough S, Lane W, Korsholm L, Bretler D‐M, Handelsman Y. Reduced risk of hypoglycemia with insulin degludec versus insulin glargine in patients with type 2 diabetes requiring high doses of Basal insulin: a meta‐analysis of 5 randomized BEGIN trials. Endocr Pract 2014; 20: 285–292. [DOI] [PubMed] [Google Scholar]
- 17. Edelman SV, Liu R, Johnson J, Glass LC. Response to Comment on Edelman et al. AUTONOMY: the first randomized trial comparing two patient‐driven approaches to initiate and titrate prandial insulin lispro in type 2 diabetes. Diabetes Care 2014; 37: 2132–2140. Diabetes Care 2014; 37: e263–e264. [DOI] [PubMed] [Google Scholar]
- 18. Haahr H, Heise T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin Pharmacokinet 2014; 53: 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ratner RE, Gough SCL, Mathieu C et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab 2013; 15: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havelund S. Insulin degludec (IDeg) and insulin aspart (IAsp) can be coformulated such that the formation of IDeg multi‐hexamers and IAsp monomers is retained upon subcutaneous injection. Poster presented at the American Diabetes Association, Chicago, 21–25 June 2013. (Abstract 945‐P).
- 21. Dardano A, Bianchi C, Del Prato S, Miccoli R. Insulin degludec/insulin aspart combination for the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manag 2014; 10: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. European Medicines Agency . Ryzodeg summary of product characteristics. 2014. Available from URL: http://ec.europa.eu/health/documents/community‐register/2013/20130121124986/anx_124986_en.pdf. Accessed August 2015.
- 23. Heise T, Nosek L, Roepstorff C, Chenji S, Klein O, Haahr H. Distinct prandial and basal glucose‐lowering effects of insulin degludec/insulin aspart (IDegAsp) at steady state in subjects with type 1 diabetes mellitus. Diabetes Ther 2014; 5: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FDA . Guidance for industry diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention. 2008. Available from URL: http://www.fda.gov/downloads/Drugs/…/Guidances/ucm071624.pdf. Accessed August 2015.
- 25. International Diabetes Federation . Global guideline for type 2 diabetes. 2012. Available from URL: http://www.idf.org/publications/global‐guideline‐type‐2‐diabetes. Accessed August 2015.
- 26. International Diabetes Federation Guideline Development Group . Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014; 104: 1–52. [DOI] [PubMed] [Google Scholar]
- 27. Unnikrishnan AG, Singh AK, Modi KD, Saboo B, Garcha SC, Rao PV. Review of clinical profile of IDegAsp. 2009. Available from URL: http://www.japi.org/may_2015_special_issue/003_review_of_clinical.pdf. Accessed August 2015. [PubMed]
- 28. Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief report: the burden of diabetes therapy: implications for the design of effective patient‐centered treatment regimens. J Gen Intern Med 2005; 20: 479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care 2010; 33: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaneko S, Chow F, Choi DS et al. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre‐/self‐mixed insulin: a 26‐week, randomised, treat‐to‐target trial. Diabetes Res Clin Pract 2015; 107: 139–147. [DOI] [PubMed] [Google Scholar]
- 31. Fulcher GR, Christiansen JS, Bantwal G et al. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin‐treated type 2 diabetes: a phase 3a, randomized, treat‐to‐target trial. Diabetes Care 2014; 37: 2084–2090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study design. [IDegAsp] IDegAsp administered BID; [IDeg+IAsp] basal‐bolus therapy with IDeg once daily administered with IAsp 2–4 times daily. Abbreviations: IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; OAD, oral antidiabetic drug.
Figure S2. Study flow diagram. Abbreviations: BID, twice daily; FAS, full analysis set; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; SAS, safety analysis set.
Table S1. Titration algorithms for [IDegAsp], IDeg OD and IAsp. Footnote: *Mean pre‐breakfast/pre‐evening meal plasma glucose measurements (adjustment: evening meal dose was increased based on the mean of three pre‐breakfast SMPG values measured on the 3 days before titration; breakfast or lunch doses were increased based on the mean of three pre‐evening meal SMPG values measured on the 3 days before titration). †Mean pre‐breakfast plasma glucose measurements (adjustment: dose was increased based on the mean of 3 consecutive days' measurements). ‡Lowest pre‐breakfast/pre‐evening meal plasma glucose measurement used to determine dose decreases. §Adjustment: mean of 3 consecutive days' measurements; breakfast IAsp dose titrated according to the mean pre‐lunch PG; lunch IAsp dose titrated according to the mean pre‐evening meal PG; evening meal IAsp dose titrated according to the mean bedtime PG. A fourth IAsp dose was allowed if necessary. BID, twice daily; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart; OD, once daily; PG, plasma glucose; SMPG, self‐monitored plasma glucose; U, units.
Table S2. Number of daily IAsp injections for subjects in the [IDeg+IAsp] basal‐bolus treatment group during the study. Abbreviations and footnotes: IAsp, insulin aspart; IDeg, insulin degludec. n, number.
Table S3. Adverse events and serious adverse events. Abbreviations: AE, adverse event; IAsp, insulin aspart; IDeg, insulin degludec; IDegAsp, insulin degludec/insulin aspart.


