ABSTRACT
Most research on human fear conditioning and its generalization has focused on adults whereas only little is known about these processes in children. Direct comparisons between child and adult populations are needed to determine developmental risk markers of fear and anxiety. We compared 267 children and 285 adults in a differential fear conditioning paradigm and generalization test. Skin conductance responses (SCR) and ratings of valence and arousal were obtained to indicate fear learning. Both groups displayed robust and similar differential conditioning on subjective and physiological levels. However, children showed heightened fear generalization compared to adults as indexed by higher arousal ratings and SCR to the generalization stimuli. Results indicate overgeneralization of conditioned fear as a developmental correlate of fear learning. The developmental change from a shallow to a steeper generalization gradient is likely related to the maturation of brain structures that modulate efficient discrimination between danger and (ambiguous) safety cues. © 2016 The Authors. Developmental Psychobiology Published by Wiley Periodicals, Inc. Dev Psychobiol 58: 471–481, 2016.
Keywords: fear conditioning, fear generalization, development, skin conductance, maturation
INTRODUCTION
Fear conditioning is a central learning mechanism in the pathogenesis of anxiety disorders (Hofmann, Alpers, & Pauli, 2008; Lissek et al., 2005). It refers to the process by which an initially neutral stimulus (conditioned stimulus, CS) comes to elicit fear after being repeatedly paired with an aversive event (unconditioned stimulus, UCS). Differential fear conditioning refers to learning that one conditioned stimulus, the CS+, is reinforced by the UCS while another stimulus, the CS−, is never followed by the UCS. In fear generalization, the conditioned fear response is transferred to stimuli (generalization stimuli, GS) that are similar but not identical to the reinforced stimulus and have never co‐occurred with the UCS. Typically, response strength to the GSs diminishes with decreasing similarity to the reinforced stimulus. The degree of stimulus generalization can be described by a gradient, the shape of which is determined by the strength of the conditioned responses across stimuli with different degrees of similarity (Ghirlanda & Enquist, 2003). A shallow generalization gradient is indicative of a greater degree of fear generalization, while steeper downward slopes indicate limited generalization to the GSs as similarity to the CS+ decreases.
A comparison of generalization gradients between healthy controls and patients with various anxiety disorders revealed overgeneralization of conditioned fear, that is, more shallow generalization gradients, in panic disorder (Lissek et al., 2010), post‐traumatic stress disorder (Lissek & Grillon, 2012), and generalized anxiety disorder patients (Lissek et al., 2014; but see Tinoco‐González et al., 2015). Additionally, enhanced generalization of conditioned fear has been found to predict anxiety levels in healthy adults six months later (Lenaert et al., 2014). Thus, overgeneralization of conditioned fear seems to be a characteristic of anxiety disorders and likely constitutes a risk factor in their pathogenesis.
Most research on human fear conditioning and its generalization has been conducted in adults whereas little is known about fear conditioning processes in children. Given the implication of fear generalization in the etiology of anxiety disorders and the fact that anxiety disorders typically first emerge during childhood (Beesdo, Knappe, & Pine, 2009), understanding the developmental trajectory of fear learning and generalization seems pivotal. So far only one study (Glenn et al., 2012) investigated the generalization of conditioned fear in children (N = 40, 8–13 years). This study on the basis of fear‐potentiated startle and fear rating data found that while all children were able to differentiate between the CS+ and CS−, only older children showed a decline in response strength from the CS+ over the GS to the CS− reminiscent of fear generalization patterns in adults. By contrast, younger children were characterized by larger startle responses and fear ratings to both the CS+ and CS− relative to the GS. However, these results should be interpreted cautiously because group sizes were small, and only one intermediate generalization stimulus was used. Commonly fear generalization gradients rely on several intermediate GSs (Lissek et al., 2010) which are needed to detect subtle generalization effects and are crucial to identify when gradients start to diverge between groups. Animal research using one or more GSs also supports the conclusion of better discrimination with advancing age (Campbell & Haroutunian, 1983; Rudy & Pugh, 1996).
An issue complicating fear conditioning research in underage populations pertains to the choice of an effective UCS. In adult samples, a mildly painful electric stimulus is a commonly used UCS that leads to reliable and potent fear responses. However, the use of such a UCS in underage populations is problematic for ethical reasons. Since the strength of the conditioned response depends upon the potency of the UCS, suitable alternatives of comparable strength are required. Recently, a promising alternative has been successfully used in children and adolescents. Lau et al. (2008) introduced a conditioning paradigm using two pictures of female faces with neutral expressions as the CS+ and CS−, respectively, while a scream presented simultaneously with a fearful face constituted the UCS. Although an electric stimulus as the UCS is perceived as more aversive, the combination of a fearful facial expression with a scream can be considered similarly effective in fear learning (Glenn, Lieberman, & Hajcak, 2012).
In sum, there is a considerable knowledge gap concerning developmental effects on fear conditioning and generalization. Direct comparisons between child and adult populations in particular are necessary to identify developmental vulnerability markers of fear and anxiety. Different paradigms, varying experimental conditions and small sample sizes make it difficult to generalize from previous findings and limit the ability to compare fear learning across different ages. In the present study, we aim to address this gap in the literature by comparing large samples1 of healthy children and adults using the same paradigm under the same experimental conditions in order to elucidate age‐related differences in the acquisition and generalization of conditioned fear. To this end, all participants were exposed to a differential fear conditioning paradigm followed by a generalization test, measuring subjective and physiological indicators of fear learning. While we expected both age groups to show differential conditioning, we predicted heightened fear generalization in children.
METHODS
Sample
A total of 285 adults and 267 children were recruited from the community and primary schools, respectively, in the greater region of Würzburg within the context of the collaborative research center SFB‐TRR‐58 subproject Z02. Seven adults and 19 children were excluded from analysis due to technical errors with physiological recordings. Nine children did not complete the experiment, thus resulting in a final sample of 278 adults (189 female) between the ages of 18 and 50 (mean age: 25.56, SD: 6.193) and 239 children (119 female) between 8 and 10 years (mean age: 9.00, SD: .812). All potential participants were screened for general inclusion criteria with a telephone interview, and only participants meeting the inclusion/exclusion criteria were invited for participation. Inclusion criteria were Caucasian descent, right‐handedness, and fluency in German. Exclusion criteria were manifest or lifetime DSM‐IV axis I disorder, severe medical conditions, and intake of psychoactive medication. Additional exclusion criteria for adults were excessive consumption of alcohol (>15 units/week), nicotine (>20 cigarettes/day), caffeine (>4 cups/day), drug use, and pregnancy. An IQ <85 as ascertained by the German version of the Culture Fair Intelligence Test 2 (Weiss, 2006) was defined as an additional exclusion criterion for children. Absence of DSM‐IV axis I disorder was ascertained using the German versions of the Mini International Psychiatric Interview (Sheehan et al., 1998) in adults and the Diagnostic Interview for Mental Disorders for Children and Adolescents (Kinder‐DIPS; Schneider, Unnewehr, & Margraf, 2009) in children.
Stimuli
Stimuli and procedures are shown in Figure 1. Pictures of two actresses with neutral facial expression (NimStim Face Stimulus Set; Tottenham et al., 2009) served as either the CS+ or CS−, with one of the two faces being randomly selected as the CS+ for each participant. The UCS was a 95 dB female scream (International Affective Digital Sounds system) presented simultaneously with a fearful facial expression of the same actress assigned as the CS+. Four generalization stimuli depicting gradual morphs from CS+ to CS− in 20%‐steps (GS1‐4) were created using the graphics software Sqirlz Morph Version 2.1 (Xiberpix, Solihull, UK). Stimulus presentation was controlled using Presentation software version 17.2 (Neurobehavioral Systems, Inc., Albany, CA). CSs and GSs were presented for 6 s each. The UCS was presented immediately following CS+ offset for 1.5 s. Inter‐trial intervals varied from 9 to 12 s, during which a white fixation cross was displayed centrally on the screen. Stimulus order was pseudo‐randomized so that the same stimulus could not appear more than twice in a row.
Figure 1.
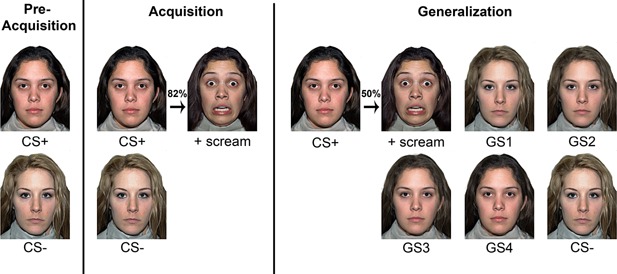
Schematic overview of the fear conditioning and generalization paradigm.
Task
The paradigm was based on Lau et al. (2008). The experiment was divided into three consecutive phases: pre‐acquisition, acquisition, and generalization. Pre‐acquisition consisted of four CS+ and four CS−; no UCS was presented. During acquisition, 12 CS+ and 12 CS− were presented. The CS+ was paired with the UCS on 10 trials. The generalization phase consisted of 12 CS+, 12 CS−, and 12 of each of the four GSs. Half the CS+ trials were followed by the UCS to prevent premature extinction. CS− and all GSs were never paired with the UCS. Participants were not informed of the CS‐UCS contingencies. Acquisition and generalization trials were separated into two phases, each containing half the trials per phase, that is, 6 presentations per stimulus category.
Procedure
Written informed consent/assent was obtained from all participants and additionally in the case of minors, their parents. Participants were instructed to passively view pictures of two female faces, and that an unpleasant sound would be heard occasionally. They were told that it would be possible to become startled and/or frightened and that participation could be discontinued at any time. Participants were offered an extinction session to ensure that no permanent conditioning would occur. For children, the extinction session was always included. Participants received 50€ remuneration. The study was approved by the ethical committee of the Medical Faculty of the University of Würzburg and complied with the latest version of the declaration of Helsinki.
Ratings
Following pre‐acquisition and each acquisition and generalization phase, participants rated each stimulus on arousal, valence, and UCS expectancy. Arousal and valence were indicated on 9‐point Likert scales, ranging from “very calm” (1) to “very arousing” (9), and “very unpleasant” (1) to “very pleasant” (9), respectively. UCS expectancy was recorded in percent on a scale from 1 to 100 in 10% increments as the probability of an aversive noise following each stimulus.
Contingency Awareness
Participants were considered aware of the CS‐UCS relationship if contingency ratings were higher for the CS+ than the CS−, and if UCS expectancy for the CS− was no higher than 50%. Contingency awareness after acquisition and generalization was determined using the ratings after the second acquisition and generalization phase, respectively.
Physiological Recordings and Data Reduction
Throughout the experiment, skin conductance responses (SCR) were recorded continuously using Brainproducts V‐Amp‐16 and Vision Recorder software (Brainproducts, Gilching, Germany) at a sampling rate of 1000Hz and analyzed offline using Vision Analyzer 2 software (Brainproducts, Gilching, Germany). SCR was recorded from the thenar and hypothenar eminences of the left hand using two Ag/AgCl electrodes. The amplifier delivered a constant current of 0.5V. The SCR signal was filtered offline with a high cutoff filter of 1Hz and a notch filter of 50 Hz. SCR was defined as the base‐to‐peak difference (in μS) between response onset (900–4000 ms after stimulus onset) and peak (2000–6000 ms after stimulus onset). A minimum response criterion of 0.02 μS was applied, with lower responses scored as 0. SCR data was normalized following an approach described by Dunsmoor, Prince, Murty, Kragel, & LaBar (2011), that is, by computing generalization gradients for each phase and block as a function of the response to one stimulus type relative to the sum of responses to all stimuli. That is, for each of the pre‐acquisition, acquisition and generalization phases, the sum of SCRs to each stimulus was divided by the sum of responses to all stimuli, resulting in an index for each stimulus type that allows for the direct comparison of generalization patterns between groups.
Statistical Analyses
All statistical tests were carried out using SPSS version 22 (SPSS Inc., Chicago, IL). Ratings were analyzed using repeated‐measures ANOVAs with the between‐subject factor group (adults, children) and the within‐subject factor stimulus type (CS+/CS− at acquisition, CS+/GS−/GS1‐4 at generalization). For analysis of acquisition and generalization blocks, two additional factors were included: the within‐subject factor phase (1, 2) to detect possible reaction changes between phases, and the between‐subject factor awareness (unaware, aware), since awareness of the CS‐UCS relationship may influence the conditioned responses (Lovibond & Shanks, 2002). Since these analyses did not reveal any effect of awareness on SCR, awareness was omitted as a group factor for the reported analysis of SCR data. For the other dependent variables, awareness effects were found, however, no awareness × group interactions. Therefore, we report awareness effects in a separate section. Finally, we repeated the main analyses in aware participants only, mainly to reveal that the group effects observed in the complete samples are similar in the subsamples of aware participants.
ANOVAs were followed by t‐tests where necessary. Alpha was set at .05 and Bonferroni correction was applied where necessary. Greenhouse‐Geisser corrections for non‐sphericity were performed where indicated, though uncorrected degrees of freedom are reported for the sake of better readability. Corrected P‐values, Greenhouse‐Geisser ϵ (GG‐ϵ), and partial η 2 are reported. Changes in contingency awareness between blocks were tested using Chi square (χ 2) tests, and phi coefficients (φ) are reported as measures of the corresponding effect sizes.
RESULTS
Contingency Awareness
Following acquisition, 247 (89%) of the 278 adults and 114 (48%) of the 239 children were considered aware of the CS‐UCS relationship. At generalization, 263 adults (95%) and 145 children (61%) fulfilled criteria for contingency awareness. Awareness was higher in adults compared to children both at acquisition (χ2 (1, N = 517) = 103.29, p < .001, φ = −.45) and generalization (χ2 (1, N = 517) = 88.95, p < .001, φ = −.42). In both groups, awareness increased from acquisition to generalization (χ2 Adults (1, N = 278) = 61.88, p < .001, φ = .47; χ2 Children (1, N = 239) = 13.46, p < .001, φ = .24). The criteria for awareness both at acquisition and generalization were fulfilled by 243 adults, 11 remained unaware throughout the experiment and 20 adults became aware during generalization only. In children, 83 were aware of the stimulus contingency at both assessment times, 63 children remained unaware throughout the experiment and 62 became aware at generalization only. Unexpectedly, four adults and 31 children were aware at acquisition but became unaware at generalization. Since we continued CS+ reinforcement during generalization to prevent premature extinction effects we suspect that this unexpected loss in awareness was due to inattentiveness or inadvertence (e.g., typing mistakes) and reflects unsystematic errors. Therefore, these participants were excluded from all analyses.
Acquisition and Generalization Effects
Results for valence and arousal ratings as well as SCR at pre‐acquisition and acquisition are depicted in Figure 2. Differences between children and adults regarding generalization are depicted in Figure 3A–C.
Figure 2.
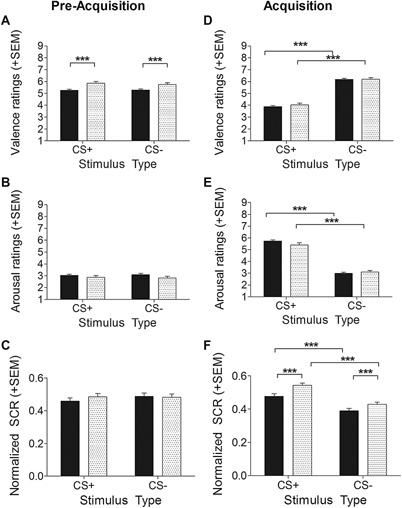
Children and adults displayed robust differential conditioning to the CS+ in valence ratings (top), arousal ratings (center), and normalized skin conductance response (bottom) at acquisition (D–F), but not at pre‐acquisition (A–C). ***p < .001; **p < .01; *p < .05.
Figure 3.
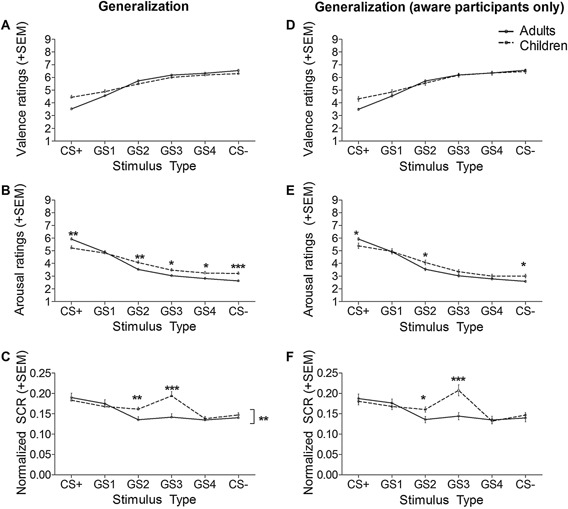
Greater fear generalization of arousal ratings and SCR was observed in children compared to adults (A–C). The observed generalization differences remained when only participants explicitly aware of the CS‐UCS relationship were compared (263 adults, 145 children; D–F). Thus, group differences cannot be explained by the greater proportion of unaware children relative to adults. ***p < .001; **p < .01; *p < .05, #p < .10.
Pre‐acquisition
As depicted in Figure 2 (A–C), no differences were found between the CS+ and the CS− prior to conditioning in both children and adults for valence and arousal ratings (ps ≥ .410) and for SCR (ps ≥ .451). However children rated both the CS+ and the CS− as more pleasant than adults prior to conditioning (F(1,480) = 15.26, p < .001, η 2 = .03).
Acquisition
Valence ratings (Fig. 2D) revealed that after conditioning, both groups rated the CS+ as more unpleasant than the CS−, as indicated by a significant main effect of stimulus type (F(1,478) = 200.45, p < .001, η 2= .30) and a non‐significant interaction of stimulus type × group (p = .138). Additionally, ratings differed according to phase (main effect phase: F(1,478) = 4.93, p = .027, η 2=.01; interaction stimulus type × phase: F(1,478) = 5.41, p = .020, η 2 =.01), with more pleasant valence ratings to the CS− in phase 2 than in phase 1 (M CS−_Phase1= 6.04, SDCS−_Phase1 = 1.93 vs. M CS−_Phase2 = 6.34, SDCS−_Phase2 = 2.02; t(481) = −3.34, p = .001).
Arousal ratings (Fig. 2E) were higher for the CS+ relative to the CS− in both groups (F(1,478) = 252.33, p < .001, η 2 = .35). Again, no stimulus type × group interaction was observed (p = .627).
For SCR (Fig. 2F), a significant main effect of stimulus type (F(1,480) = 35.34, p < .001, η 2 = .07) was observed, with higher SCR to the CS+ than the CS−, but no stimulus type × group interaction (p = .384). Additionally, a significant main effect of group was observed (F(1,480) = 15.77, p < .001, η 2 = .03), reflecting overall higher SCR in children (M = .49, SD = .06) than in adults (M = .45, SD = .13).
In sum, all dependent measures indicate successful conditioning effects in both adults and children.
Generalization
With regard to valence (Fig. 3A), a significant main effect of stimulus type (F(5,2390) = 46.48, p < .001, η 2 = .09) was observed. No interactions involving the between‐subject factor group reached significance (ps ≥ .084).
For arousal ratings (Fig. 3B), a significant main effect of stimulus type (F(5,2390) = 60.47, p < .001, η 2 = .11) and a significant interaction of stimulus type × group (F(5,2390) = 4.10, p = .001, η 2 = .01) were observed, with differences in the quadratic and cubic components between groups (stimulus type × group quadratic trend: F(1,478) = 10.38, p = .001, η 2 = .02; stimulus type × group cubic trend: F(1,478) = 5.86, p = .016, η 2 = .01). Within‐group follow‐up tests revealed linear (F(1,272) = 51.16, p < .001, η 2 =.16) and quadratic (F(1,272) = 29.74, p < .001, η 2 = .10) trends in adults, and linear (F(1,206) = 78.12, p < .001, η 2 = .28), quadratic (F(1,206) = 13.12, p < .001, η 2 = .06), and cubic (F(1,206) = 3.91, p = .049, η 2 = .02) trends in children. Between‐group follow‐up tests indicated that adults rated the CS+ (t(354) = 3.33, p = .001) as more arousing than children, whereas children rated the GS2 (t(370) = −2.80, p = .005), GS4 (t(370) = −2.57, p = .010), and CS− (t(377) = −3.53, p < .001) as more arousing than adults. Group differences regarding GS3 ratings (p = .012 uncorrected) did not survive correction for multiple testing, and were not significant for GS1 ratings (t(375) =.38, p = .707).
For SCR (Fig. 3C), there was a significant main effect of stimulus type (F(5,2400) = 9.40, p < .001, η 2 = .02). A significant interaction of stimulus type × group indicated that generalization gradients differed between groups (F(5,2400) = 2.98, p =.011, η 2 =.01), with a stimulus type × group quadratic interaction (F(1,480) = 6.74, p = .010, η 2 =.01). Within‐group follow‐up tests revealed a quadratic trend in adults (F(1,273) = 7.07, p = .008, η 2 =.03) but not in children (p = .900). Between‐group follow‐up tests revealed that children were characterized by higher SCR to the GS2 (t(455) = −2.75, p = .006) and GS3 (t(480) = −3.84, p < .001) compared to adults. There were no significant differences regarding CS+, GS1, GS4 or CS− (ps ≥ .53). Again, a main effect of group emerged between adults and children (F(1,480) = 9.74, p = .002, η 2 =.02), with higher SCRs in children (M = .17, SD = .02) than adults (M = .16, SD = .03).
In sum, these analyses indicate greater generalization of arousal ratings and SCRs in children than adults.
Effects of Awareness on Acquisition and Generalization
The above analyses, except for SCR, included the between‐subject factor awareness (see Methods), but results concerning awareness are reported here for the sake of clarity since no awareness by group effects were found.
Acquisition
Valence ratings were affected by awareness (F(1,478) = 14.30, p < .001, η 2 = .03), with aware participants (children and adults) rating the CS+ as more unpleasant than unaware participants (M Aware = 3.74, SDAware = 1.69; M Unaware = 4.40, SDUnaware = 2.18). For arousal ratings, significant interactions of stimulus type × awareness (F(1,478) = 12.41, p < .001, η 2 = .03) and stimulus type × awareness × phase (F(1,478) = 7.49, p = .006, η 2 = .02) were observed. Aware participants rated the CS+ as more arousing in both phase 1 (t(246) = −2.22, p = .027) and phase 2 (t(256) = −3.15, p = .002) compared to unaware participants. Awareness differed regarding the CS− in phase 2, with lower arousal ratings in aware compared to unaware participants (t(251) = 2.66, p = .008) but not in phase 1 (p = .99).
Generalization
A significant interaction of stimulus type × awareness was observed for valence ratings (F(5,2390) = 4.40, p = .005, η 2 =.01), with more negative ratings of the CS+ (t(93) = 3.13, p = .002) and in turn a more positive evaluation of the GS3 (t(480) = −2.61, p = .009) in aware compared to unaware participants, but not for the GS1, GS2, GS4, or CS− (ps ≥ .022). Arousal generalization gradients differed as a function of awareness and phase. Two two‐way interactions (stimulus type × awareness: F(5,2390) = 6.86, p < .001, η 2 =.01 and stimulus type × phase: F(1,478) = 9.45, p = .002, η 2 = .02) were qualified by a three‐way‐interaction of stimulus type × phase × awareness (F(1,478) = 8.25, p = .004, η 2 = .02). Aware participants were characterized by steeper generalization gradients, indicating better discrimination learning: In both phases, arousal ratings for the CS+ were higher in aware than unaware participants (t Phase1(94) = −2.67, p = .009; t Phase2(480) = −2.14, p = .033). GS4 ratings were lower in aware participants in both phases (t Phase1(89) = 2.95, p = .004; t Phase2(90) = 3.49, p = .001). GS3 and CS− ratings were affected by awareness in phase 2 only, with lower ratings in aware participants (t GS3(92) = 3.16, p = .002; t CS−(92) = 4.29, p < .001).
Taken together, these results indicate that, regarding ratings, aware participants exhibited greater acquisition effects and steeper generalization gradients, however and most importantly, groups did not differ in these awareness effects.
Acquisition and Generalization Effects in Aware Participants
Since significantly more adults than children were aware of the CS‐UCS contingencies and since awareness modulated the steepness of the generalization gradients, it might be speculated that the observed stronger generalization effects in children were due to the high proportion of unaware children. Therefore, we repeated the above analyses by including only participants who were aware at generalization (263 adults and 145 children). Importantly, analyses in aware participants only yielded similar results as in the whole sample: Analysis of arousal ratings (Fig. 3E) again returned a significant interaction of stimulus type × group (F(5,406) = 7.22, p < .001, η 2 = .02), with differences in the linear, quadratic, and cubic components between groups (stimulus type × group linear trend: F(1,406) = 7.45, p = .007, η 2 = .02; stimulus type × group quadratic trend: F(1,406) = 10.01, p = .002, η 2 = .02; stimulus type × group cubic trend: F(1,406) = 8.124, p = .005, η 2 =.02). Within‐group follow‐up tests again yielded linear (F(1,262) = 559.54, p < .001, η 2 = .68) and quadratic (F(1,262) = 122.01, p < .001, η 2 = .32) trends in adults, as well as linear (F(1,144) = 108.68, p < .001, η 2 = .43), quadratic (F(1,144) = 15.61, p < .001, η 2 = .10), and cubic (F(1,144) = 9.68, p = .002, η 2 = .06) trends in children.
Likewise, the observed stimulus × group interaction for SCR data (Fig. 3F) also remained significant (F(5,406) = 3.17, p = .008, η 2 = .01), again with a stimulus type × group quadratic interaction (F(1,406) = 6.23, p = .013, η 2 = .02), as well as the main effect of group (F(1,406) = 8.20, p = .004, η 2 = .02). Within‐group follow‐ups confirmed the previous findings with a quadratic trend in adults (F(1,262) = 15.83, p < .001, η 2 = .02) but not in children (p = .133).
In sum, the stronger generalization effects we observed in children relative to adults in the complete samples were also observable in the subsamples of aware participants.
DISCUSSION
In the present study, we examined fear conditioning and its generalization in large samples of healthy children compared to adults. To our knowledge, this study constitutes the first direct comparison of children and adults regarding the acquisition and generalization of conditioned fear using the same paradigm. Most importantly, the generalization test convincingly revealed greater fear generalization in children compared to adults as indicated by explicit (verbal ratings) and implicit (SCR) measures of arousal. These far‐reaching findings have to be discussed in relation to our results from the acquisition phase indicating that cued fear conditioning in both children and adults leads to similar, robust conditioning effects with higher verbal and physiological fear responses triggered by the CS+ compared to the CS−. We also observed that contingency awareness affected both children and adults in a similar way, with aware participants showing stronger conditioning effects and less generalization. Importantly, analyses in the subgroup of aware participants only confirmed the main finding that children are characterized by enhanced fear generalization. Finally, we observed several general differences between children and adults, which we will discuss regarding their influences on the observed overgeneralization in children. Particularly, we found overall higher autonomic arousal in children compared to adults following but not prior to conditioning, and overall more children than adults did not meet criteria for contingency awareness.
The observed generalization difference between children and adults was due to the fact that most GSs triggered more arousal in children compared to adults as reflected in ratings and SCR. As a consequence, children were characterized by a more shallow generalization gradient. We conclude that children are less efficient than adults at recognizing a stimulus (CS+) that during previous learning trials predicted an aversive consequence, and at discriminating this stimulus from resembling stimuli. The only previous study on fear generalization in children used only one GS and therefore conclusions about generalization gradients could not be made (Glenn et al., 2012), although results suggest better discrimination with advancing age. Interestingly, animal research supports this conclusion. For example, Rudy and Pugh (1996) compared auditory‐cue fear conditioning in 18‐day‐old and 25‐day‐old rats and found more generalized fear in younger than in older rats. While older rats displayed a decline in response strength from the CS+ over the GS to the CS−, younger rats were less able to differentiate between the stimuli. The authors interpreted their result with brain maturation effects, suggesting that some brain structures linked with fear conditioning processes are not fully matured in 18‐day‐old rats. In addition, Campbell and Haroutunian (1983) used multiple GSs to study perceptual sharpening and showed age‐related differences in heart rate orienting response between 16‐17 days and 19‐20 days of age: older rats were much better at discriminating between the stimuli, whereas younger rats showed heightened generalization. Thus, both human and animal studies suggest that developmental progress reduces generalization and sharpens discrimination.
Importantly, no age group differences emerged with regard to the acquisition of conditioned fear. That is, children as reflected in ratings and SCR reliably discriminated between CS+ and CS− and did not differ from adults in that respect. This result corroborates previous results on fear acquisition (e.g. Craske et al., 2008; Glenn et al., 2012) also showing that children have no deficits in associating a specific cue with an aversive consequence. We conclude that children 8 years and older have no deficit in differentiating between cues predicting threat and safety as long as there are only two cues that can be differentiated easily.
The observed overgeneralization of arousal responses to stimuli resembling the CS+ in children appears to be a developmental phenomenon. The maturation of neural structures involved in the circuitry involved in fear processing may play a crucial role. Animal and human neuroimaging studies have highlighted the role of the amygdala, the prefrontal cortex (PFC), and the hippocampus in fear learning (LeDoux, 2000; Shin & Liberzon, 2010). These regions are characterized by different trajectories throughout developmental stages (Casey, Jones, & Hare, 2008), with prefrontal regions maturing later than subcortical limbic structures. The ancient subcortical brain regions are shaped by evolution, and from an evolutionary standpoint, generalization may increase survival in a generally more dangerous environment encountered by children. Cortical brain regions that evolved at later evolutionary stages and also mature later during ontogenesis might be necessary to inhibit fear responses to ambiguous safety cues (i.e. CS‐gradations). As a consequence, children exhibit normal fear conditioning but overgeneralization of fear responses with the latter effect becoming ameliorated due to PFC maturation.
An fMRI study by Lau et al. (2011) comparing adolescents and adults supports this view by revealing that fear learning in adolescents relies to a greater extent on early‐maturing subcortical regions (i.e. amygdala, hippocampus) and to a lesser extent on late‐maturing prefrontal regions such as the dorsolateral PFC. Thus, PFC involvement in the modulation of fear learning likely increases in the process of brain maturation. Although future research is needed addressing the neurobiological correlates of fear learning and generalization longitudinally across the life span from childhood over adolescence to adulthood, we think that our data suggests that, in children, subcortical regions also respond to stimuli resembling the CS+, resulting in heightened arousal to stimuli that were never associated with threat. Ergo, lack of PFC maturation may explain the increased physiological arousal in children in response to GSs. Specifically, the ventromedial PFC, one of the last brain regions to mature (Fuster, 2002), is thought to be causally involved in the regulation of SCR (Zhang et al., 2014).
Differences between children and adults in PFC maturation may also explain the observed overall enhanced SCR in children. Similar effects have been reported previously in adolescents (Lau et al., 2011). However, this overall group difference cannot account for the observed overgeneralization of arousal responses to GSs in children, but rather seems to be a non‐specific response to the experimental paradigm. First, SCR did not differ between groups prior to conditioning. Second, we found no stimulus type by group interaction during acquisition, but during generalization. Third, the shallow generalization gradient of children was due to reduced arousal in response to the CS+ but enhanced arousal to most GSs, and this effect cannot be explained by a generally enhanced arousal.
The observed group differences in generalization cannot be explained by contingency awareness either, although more children than adults were considered unaware. The latter finding again may be attributed to PFC maturation in children, which, based on the findings by Lau et al. (2011) is needed for verbal conditioning effects which prerequisite contingency awareness. Within children and adults, similar effects of awareness on conditioning were found, with stronger conditioning effects and less generalization in aware participants. Since no effect of contingency awareness was observed on group differences regarding generalization, and the same age‐group differences in generalization were observed when analyses were performed in aware participants only, we conclude that the lack of awareness in children cannot explain the observed overgeneralization effect.
Under normal circumstances, fear is a highly adaptive mechanism that allows us to react quickly and appropriately when encountering (potential) threats. Overgeneralization of the fear response to ambiguous stimuli may reflect a protective mechanism promoting cautious behavior in childhood, especially in new environments, which decreases with experience during the transitional phase of adolescence, thus leading to a reduction in generalization with advancing age. However, overgeneralization of conditioned fear in adulthood has been linked to subsequent development of anxiety symptoms (Lenaert et al., 2014) and manifest anxiety disorders (Lissek et al., 2010, 2014). Hence, the persistence of fear generalization into adulthood could have maladaptive consequences and pose a potential risk mechanism contributing to the emergence of pathological fear. However, the mechanisms involved in the development of anxiety disorders are complex and rely on the interplay of many variables, such as environmental, psychological, and neurobiological factors. Therefore, more research is needed; especially longitudinal studies of fear generalization would not only help to elucidate the mechanisms of fear learning and how fear generalization gradients change through critical developmental time periods related to the development of anxiety disorders, but would also aid the development of prevention measures.
As this is the first study comparing fear generalization in children and adults directly with the same paradigm, some limitations should be addressed. First, even though group differences were independent of awareness, the greater percentage of unaware children relative to adults might in part be due to characteristics of the scales used to assess awareness. Future studies might want to utilize different methods more adapted to children to assess contingency awareness. This may also explain why no effect of awareness on SCR emerged in children in the present or previous studies (Craske et al., 2008), contrary to what has been reported in adults (Lovibond & Shanks, 2002). Second, the present paradigm has been used repeatedly in a similar form in other studies with children, adolescents, and adults (Glenn et al., 2012; Lau et al., 2008, 2011). However, differences in age‐dependent responding to the nature of the stimuli themselves, pictures of female adults, cannot be excluded. In fact, we observed pre‐conditioning group differences with regard to overall valence ratings. Children rated both CS pictures pre‐experimentally as more pleasant than adults, while valence ratings during conditioning and generalization were similar in both groups. Finally, replication studies in clinical samples are needed to compare fear learning and generalization in children and adults with and without anxiety disorders. Additionally, longitudinal follow‐ups are required to reveal if and how generalization gradients change over time and their relationship to the development of anxiety. As a matter of fact, the present study constitutes the first step in assessing these research questions since we plan to follow up the examined samples, which can also explain the rather large sample sizes required for the prospective assessment regarding the potential onset of anxiety disorders. One consequence of assessing large sample sizes may be the detection of small magnitude effects often controversially discussed regarding their meaningfulness and replicability. However, there is a clear demand for larger sample sizes and consequently higher statistical power in psychological research in the quest for increasing replicability (Asendorpf et al., 2013). In any case our findings corroborate and advance theoretical assumptions as well as previous findings in both animals and humans.
NOTES
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB‐TRR‐58, projects B01 to PP, C02 to KD and JD, and Z02 to JD, AR, MR and PP). We are grateful to Dr. Marta Andreatta for excellent technical advice.
Miriam A. Schiele and Julia Reinhard contributed equally to this work.
Endnotes
Large sample sizes are also needed because we plan to follow‐up participants expecting an association between fear generalization and development of anxiety disorders.
REFERENCES
- Asendorpf, J. B. , Conner, M. , De Fruyt, F. , De Houwer, J. , Denissen, J. J. , Fiedler, K. , Fiedler, S. , Funder, C. F. , Kliegl, R. , Nosek, B. A. , Perugini, M. , Roberts, B. W. , Schmitt, M. , Vanaken, M. A. G. , Weber, H. , & Wicherts, J. M. ( 2013). Recommendations for increasing replicability in psychology. European Journal of Personality, 27, 108–119. [Google Scholar]
- Beesdo, K. , Knappe, S. , & Pine, D. S. ( 2009). Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM‐V. Psychiatric Clinics of North America, 32, 483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, B. A. , & Haroutunian, V. ( 1983). Perceptual sharpening in the developing rat. Journal of Comparative Psychology, 97, 3–11. [PubMed] [Google Scholar]
- Casey, B. J. , Jones, R. M. , & Hare, T. A. ( 2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske, M. G. , Waters, A. M. , Lindsey, B. R. , Naliboff, B. , Lipp, O. V. , Negoro, H. , & Ornitz, E. M. ( 2008). Is aversive learning a marker of risk for anxiety disorders in children? Behaviour Research and Therapy, 46, 954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor, J. E. , Prince, S. E. , Murty, V. P. , Kragel, P. A. , & LaBar, K. S. ( 2011). Neurobehavioral mechanisms of human fear generalization. NeuroImage, 55, 1878–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster, J. M. ( 2002). Frontal lobe and cognitive development. Journal of Neurocytology, 31, 373–385. [DOI] [PubMed] [Google Scholar]
- Ghirlanda, S. , & Enquist, M. ( 2003). A century of generalization. Animal Behaviour, 66, 15–36. [Google Scholar]
- Glenn, C. R. , Klein, D. N. , Lissek, S. , Britton, J. C. , Pine, D. S. , & Hajcak, G. ( 2012). The development of fear learning and generalization in 8–13 year‐olds. Developmental Psychobiology, 54, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn, C. R. , Lieberman, L. , & Hajcak, G. ( 2012). Comparing electric shock and a fearful screaming face as unconditioned stimuli for fear learning. International Journal of Psychophysiology, 86, 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S. G., Alpers G. W., & Pauli P., ( 2008). Phenomenology of panic and phobic disorders In Antony M. M. & Stein M. B. (Eds.), Handbook of anxiety and related disorders (pp. 34–46). New York: Oxford University Press. [Google Scholar]
- Lau, J. Y. , Britton, J. C. , Nelson, E. E. , Angold, A. , Ernst, M. , Goldwin, M. , Grillon, C. , Leibenluft, E. , Lissek, S. , Norcross, M. , Shiffrin, N. , & Pine, D. S. ( 2011). Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences of the United States of America, 108, 4500–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, J. Y. , Lissek, S. , Nelson, E. E. , Lee, Y. , Roberson‐Nay, R. , Poeth, K. , Jenness, J. , Ernst, M. , Grillon, C. , & Pine, D. S. ( 2008). Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux, J. E. ( 2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. [DOI] [PubMed] [Google Scholar]
- Lenaert, B. , Boddez, Y. , Griffith, J. W. , Vervliet, B. , Schruers, K. , & Hermans, D. ( 2014). Aversive learning and generalization predict subclinical levels of anxiety: A six‐month longitudinal study. Journal of Anxiety Disorders, 28, 747–753. [DOI] [PubMed] [Google Scholar]
- Lissek S., & Grillon C., ( 2012). Learning Models of PTSD In Beck J. G. & Sloan D. M. (Eds.), The Oxford handbook of traumatic stress disorders (pp. 175–190). New York: Oxford University Press. [Google Scholar]
- Lissek, S. , Kaczkurkin, A. N. , Rabin, S. , Geraci, M. , Pine, D. S. , & Grillon, C. ( 2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75, 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek, S. , Powers, A. S. , McClure, E. B. , Phelps, E. A. , Woldehawariat, G. , Grillon, C. , & Pine, D. S. ( 2005). Classical fear conditioning in the anxiety disorders: a meta‐analysis. Behaviour Research and Therapy, 43, 1391–1424. [DOI] [PubMed] [Google Scholar]
- Lissek, S. , Rabin, S. , Heller, R. E. , Lukenbaugh, D. , Geraci, M. , Pine, D. S. , & Grillon, C. ( 2010). Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry, 167, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond, P. F. , & Shanks, D. R. ( 2002). The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes, 28, 3–26. [PubMed] [Google Scholar]
- Rudy, J. W. , & Pugh, C. R. ( 1996). A comparison of contextual and generalized auditory‐cue fear conditioning: evidence for similar memory processes. Behavioral Neuroscience, 110, 1299–1308. [DOI] [PubMed] [Google Scholar]
- Schneider S., Unnewehr S., & Margraf J. ( 2009). Kinder‐DIPS für DSM‐IV‐TR. Diagnostisches Interview bei psychischen Störungen im Kindes‐ und Jugendalter (2. erweiterte und vollständig überarbeitete Auflage). Heidelberg: Springer. [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , Hergueta, T. , Baker, R. , & Dunbar, G. C. ( 1998). The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. Journal of Clinical Psychiatry, 59(Suppl 20), 22–33. [PubMed] [Google Scholar]
- Shin, L. M. , & Liberzon, I ( 2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35, 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco‐González, D. , Fullana, M. A. , Torrents‐Rodas, D. , Bonillo, A. , Vervliet, B. , Blasco, M. J. , Farre, M. , & Torrubia, R. ( 2015). Conditioned fear acquisition and generalization in generalized anxiety disorder. Behavior Therapy, 46, 627–639. [DOI] [PubMed] [Google Scholar]
- Tottenham, N. , Tanaka, J. W. , Leon, A. C. , McCarry, T. , Nurse, M. , Hare, T. A. , Marcus, D. J. , Westerlund, A. , Casey, B. J. , & Nelson, C. ( 2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. H. ( 2006). Grundintelligenztest Skala 2 ‐ Revision ‐ (CFT 20‐R). Göttingen: Hogrefe. [Google Scholar]
- Zhang, S. , Hu, S. , Chao, H. H. , Ide, J. S. , Luo, X. , Farr, O. M. , & Li, C. S. ( 2014). Ventromedial prefrontal cortex and the regulation of physiological arousal. Social Cognitive and Affective Neuroscience, 9, 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]


